Cardiac surgery in Africa: a thirty-five year experience on open heart surgery in Cote d’Ivoire
Introduction
Cardio-vascular diseases (CVDs) are growing up in developing countries (DC) (1-3); they are the leading cause of deaths in the world (4-7); in low and middle income countries (LMIC) 80% of deaths are related to CVD (8) often in young population (9); it has been estimated that 8 to 9 millions deaths are attributable to CVD in developing world (10); this heavy burden of CVD death in DC is affecting negatively LMIC socio-economic development (8).
It has been also shown a recent shift in the disease pattern with an epidemiologic transition in DC where infection and malnutrition are declining while chronic diseases including CVD are increasing (3,10-12).
Despite rising rate of CVD and Atherosclerosis in DC, rheumatic heart diseases (RHD) still remain one of the most common CVD in Sub-Saharan Africa (4,12), and in developing world (13-17); it has been established that in the next 20 to 40 years DC will continue to face a double CVD burden: that of RHD and of Atherosclerosis with ischemic or hypertensive heart diseases (4).
Therefore in DC, medical and surgical CVD treatment as open heart surgical procedures will still be needed in our African countries.
Open heart surgery (OHS) has been introduced in Africa in a recent time and has a long way to go in our continent.
In Sub-Saharan Africa, OHS started in the 70’s (18), several pioneer centers were opened notably in Enugu (Nigeria), in Nairobi (Kenya), in Ibadan (Nigeria), in Khartoum (Sudan) and in Abidjan (Cote d’Ivoire) (19). In Cote d’Ivoire, Dr. H. Merle and Dr. Yangni-Angate (Figure 1), general surgeons and pioneers of cardio-thoracic surgery, had performed the first surgical procedures for chronic constrictive pericarditis, pyogenic pericarditis, pulmonary tuberculosis, mediastinal tumors, and thoracic trauma in 1961 at Treichville Hospital in Abidjan (20).

After a long period of interruption of cardio-thoracic surgery, Prof. A. Yangni-Angate, Chief General Surgeon, went to Houston (Texas-USA) in 1971 to be trained under the mentorship of Prof. Michael Debakey and Prof. Danton Cooley in OHS respectively for acquired heart diseases and congenital and pediatric heart diseases.
Back to his country, Prof. Yangni-Angate was joined by three cardio-vascular and thoracic surgeons: Prof. Metras from France, Prof. Ouezzin-Coulibaly and late Dr. Ouattara, former residents and assistants of Prof. Yangni-Angate. Subsequently, at Treichville Teaching Hospital, in the General Surgical Department, under Prof. Yangni-Angate chairmanship, cardio-thoracic surgery activities were reactivated and an OHS program was established. Afterwards, under the leadership of Prof. Edmond Bertrand, a cardiologist, an Heart Institute for Cardiology including an OHS department was commissioned in 1976 by the former President of Cote d’Ivoire with Excellency Late Dr. Felix Houphouet-Boigny (Figures 2,3). Then, the first open-heart surgery was performed with success on March 11, 1978 (Figure 4) (21) by three co-pioneers: Prof. Metras, Prof. Ouezzin-Coulibaly and Dr. Ouattara (Figure 5). From 1978, OHS is delivered to patients in Cote d’Ivoire not without several obstacles in terms of new equipments and consumables, affordability and accessibility to OHS for patients and sustainability.


The purpose of this study is to highlight Cote d’Ivoire OHS experience since 1978.
Methods
It is a retrospective study from 1978 to 2013 at our institution where 2,612 patients underwent open-heart surgery. Pathologies encountered were as follows: RHD (n=1,475), endomyocardial fibrosis (EMF) (n=126), congenital cardiopathies (n=741) and miscellaneous (n=270) which were excluded from this work. All the data were collected from patients’ case note and operating theatre register; patients’ demographics, clinical features, investigative studies and outcomes, surgical pathology findings, procedures and results, hospital mortality, late mortality and morbidity were also itemized.
Statistical analysis was done: data are expressed as mean with standard deviation or simple frequencies and percentages. X2 test or Fisher’s exact test were applied to examine differences between groups. Cox regression models were used to find univariate and multivariate predictors of mortality. The Kaplan-Meier method was used to draw survival curves and calculate 5-, 10- and 15-year survival and estimates for freedom from post-operative complications. A P value ≤0.05 was considered statistically significant.
Results
We have focused on results related to rheumatic heart valve diseases, EMF, and congenital heart diseases (CHDs).
Rheumatic heart valve diseases
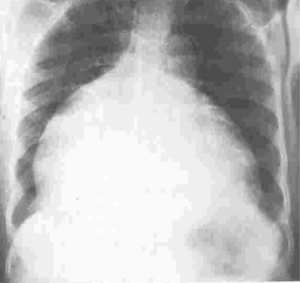
A total of 1,475 patients underwent surgery. Patients’ average age was 26±10.1 years (extremes: 4–69 years). The 60% of patients (n=885) were in functional class III or IV according to the New-York Heart Association (NYHA) classification (Figure 6). At chest X-ray, average cardiothoracic index was at 0.60 (0.40–0.80) (Figures 7,8) and diagnosis was confirmed by 2D echocardiography and/or cardiac catheterization coupled to angiocardiography. As hemodynamic parameters, in 994 patients out of 1,475, mean cardiac index, mean pulmonary blood pressure, mean left atrial pressure were respectively 2.06 L/min/m2, 32.26±1.94 mmHg, 18.33±1.38 mmHg. Monovalvular (n=1,175), bivalvular (n=280) and trivalvular (n=20) heart diseases were diagnosed (Table 1). Mitral valve diseases (n=778, 53%) were the most common.
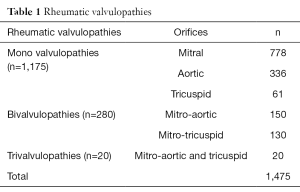
Full table
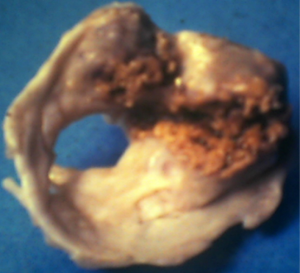
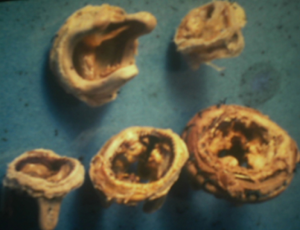
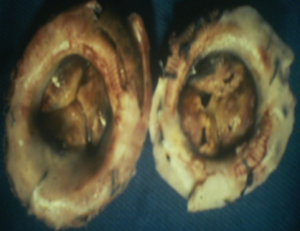
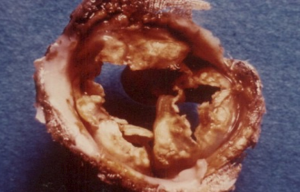
Surgery was performed under cardiopulmonary bypass (CPB) with a bubble or membrane oxygenator, partial or total hemodilution, moderate hypothermia, under aortic cross clamping and myocardial protection. Mitral and aortic rheumatic lesions were severe in most cases (77%) with valve leaflets fibrosis, stiffening, retraction, thickening even fusion of the commissures in mitral stenosis or massive calcifications and fusion, shortening of mitral chordae tendineae (Figures 9-11). A total of 1,481 valvular replacements and 445 valvular repairs were carried out. Mechanical prosthetic valves (n=992) and bioprostheses (n=489) were implanted. Valvular repairs dealt with mitral orifice (n=264), aortic orifice (n=22) and tricuspid orifice (n=159). Mitral valve repairs were performed according to Carpentier procedures (22); aortic valve repair to Trusler’s technique (23) and tricuspid valve annuloplasty to DE VEGA procedure (24) (Table 2); hospital mortality reached 6.7% (n=99) (Table 3). Low cardiac output due to cardiac failure (n=51; 52%) was the main cause of hospital deaths (Table 4). Risk factors for low cardiac output after surgery were: NYHA functional class III and IV (P=0.001), mean pulmonary arterial pressure superior to 25 mmHg (P≤0.01) and aorta cross clamping duration superior to one hour (P=0.001). Risk factors for early death after mitral valvular surgery in children are listed on Table 5.

Full table
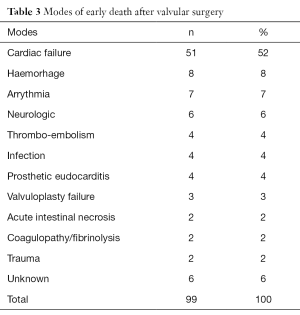
Full table

Full table
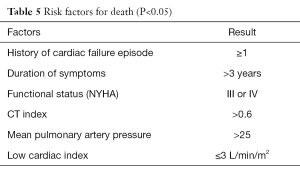
Full table
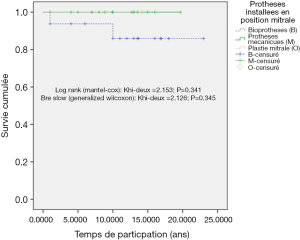
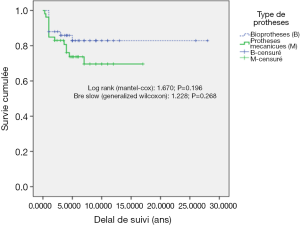
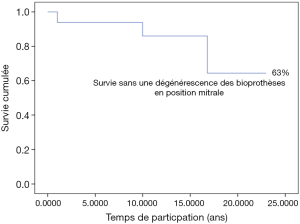
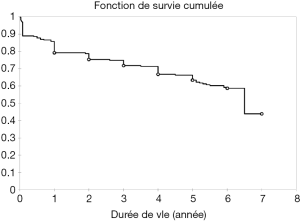
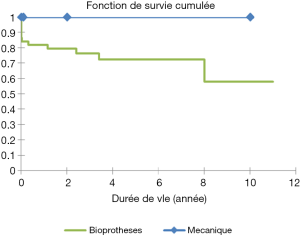
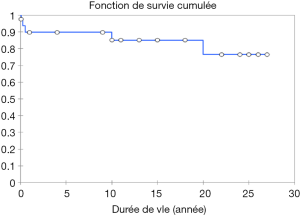
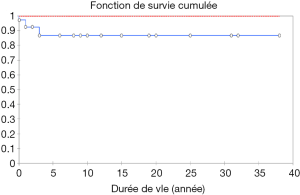
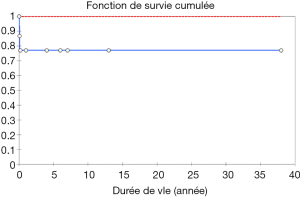
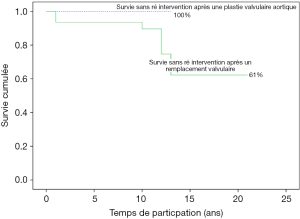
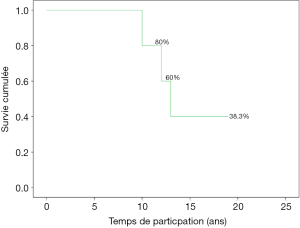
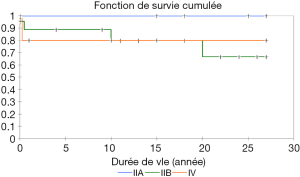
Eighty percent of survivals (n=1,100) after surgery were followed up; median follow-up time was 5 years (1 month to 20 years). Late mortality is estimated at 8.7%. For mitral valve surgery with or without DE VEGA tricuspid valve annuloplasty, survival after primary mitral valve replacement (MRV) by mechanical prosthesis or after mitral valve repair was 100%, 100%, 100% versus 95%, 95%, 85% after primary MRV with bioprosthesis at 5, 10 and 15 years respectively (Figure 12). For aortic valve replacement (AVR) by bioprosthesis, survival at 5, 10, 15 years was 85%, 82%, 82%, respectively versus 82%, 70%, 70% for AVR by mechanical prosthesis (P=0.19) versus 100% at 10 years for aortic valvuloplasty (Figure 13). Overall survival after valve replacement by bioprosthesis was 63% at 5 years versus 72% for mitral bioprosthesis, 68% for mitro-aortic bioprosthesis, 55% for aortic bioprosthesis; survival for tricuspid bioprosthesis was 68% at 5 years versus 100% for tricuspid mechanical prosthesis (Figures 14-17). Several long term complications related to the prosthesis were observed: primary bioprosthetic tissue degeneration (n=110) (Figures 18-20), infectious endocarditis on prosthesis (n=19), thrombo-embolism (n=27). Freedom from reoperation after MRV was 97%, 72.3% versus 97%, 84% after mitral valve repair (Figure 21) or versus 90%, 87% after AVR at 5 and 15 years respectively while it was 100% at 13 years after aortic valvuloplasty (Figure 22). Freedom from reoperation after tricuspid valve bioprosthesis was 70%, nil versus 100% for tricuspid mechanical prosthesis at 6 and 8 years respectively and similarly to that of bioprosthesis degeneration after tricuspid valve replacement (Figure 23). Freedom from reintervention after combined mitral and AVR was 100%, 80%, 38.3% at 5, 10, 15 years respectively (Figure 24).

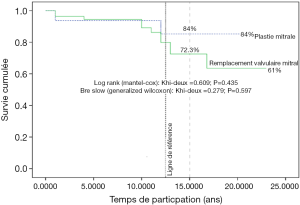
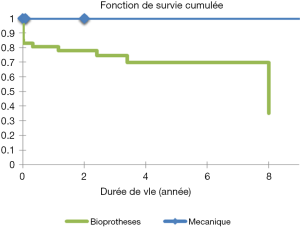
Freedom from thromboembolism following mechanical mitral valve implantation was 91% at 8 years; at 9.6 and 12 years, it was 79%, 53% respectively for combined mitral and aortic mechanical prosthesis (Figure 25).
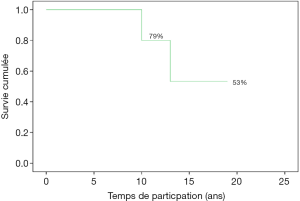
Freedom from thromboembolic complications after tricuspid valve bioprosthesis was 70%, 60% versus 100% for mechanical prosthesis at 6 and 8 years respectively (Figure 26).
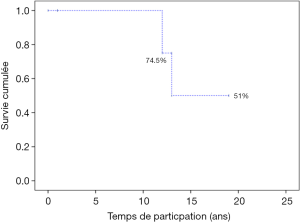
Freedom from bioprosthesis degeneration after MRV was 98%, 83%, 63% at 5, 15, 20 years respectively versus 79% at 12 years and 51% at 15 years for mitro-aortic bioprosthesis (Figure 27).
EMF
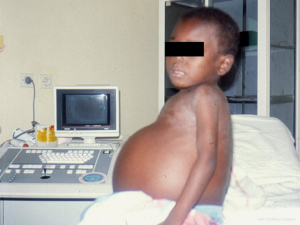
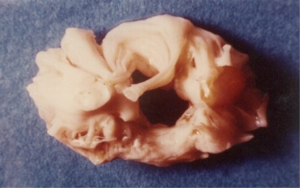
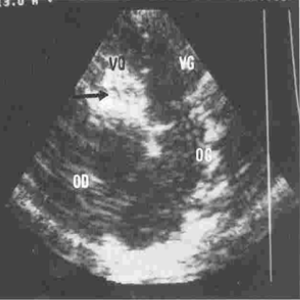
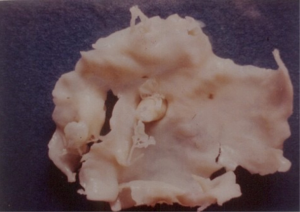
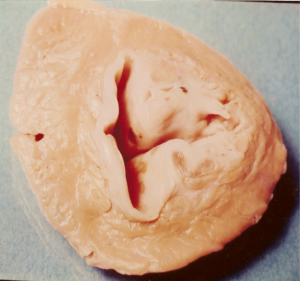
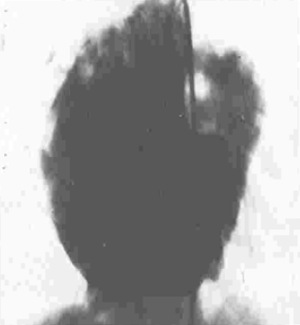
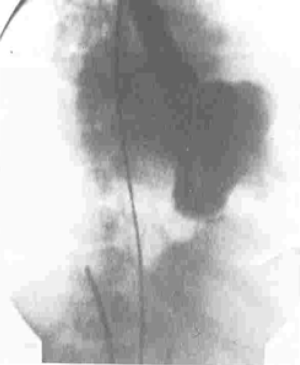
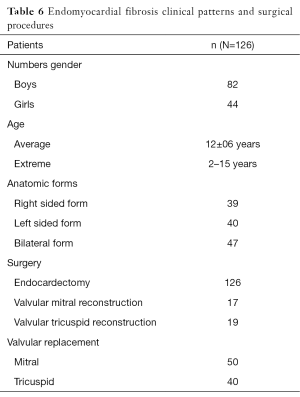
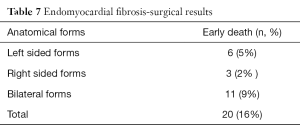
One hundred and twenty-six [126] patients were listed; average age was 12±0.6 years (extreme, 2–15 years) with 82 boys (65%) and 44 girls (35%). Anatomical types or forms were as follows: right EMF (n=39), left EMF (n=40) and bilateral or right and left EMF (n=47). Figure 28 shows a dense fibrous plaque laying on the ventricular endocardium and occluding quasi-completely the cavity of the ventricle. All the patients were coming from the same region of Cote d’Ivoire in a forest and humid area; they were all symptomatic and were all showing a late staturo-ponderal feature, a right heart failure clinical presentation with liver enlargement, ascites, peripheral edema, jugular vein distension associated with a tricuspid valve insufficiency in the right EMF and clinical signs of pulmonary congestion with dyspnea on physical exertion and a mitral valve regurgitation in the left EMF even a combination of the 2 presentations in the bilateral forms. Chest radiography and 2D echocardiography indicated characteristic findings (Figures 29,30). Typical angiocardiographic pictures were: an amputation of the right ventricle (RV) apex, a distension of the infundibulum, a right atrial distension and a tricuspid insufficiency (Figure 31), characteristics of right EMF (Figure 32). Angiocardiography of the left EMF showed an amputation more or less severe of the left ventricle apex (LV) and a mitral insufficiency (Figure 33). Right and left angiocardiographic aspects were noted in the bilateral forms. Every patient was operated under CPB according to the same modalities observed above. Surgery confirmed the diagnosis (Figure 34) and consisted in an even or bilateral retrograde endocardectomy starting by the most attacked side. It consisted by extracting the endoventricular fibrosis of the apex towards auriculo-ventricular orifice by a split identifiable between the cardiac muscle and the fibrotic plaque (Figure 31). In the right EMF a blade of fibrosis is left under the tricuspid septal valve in order to avoid the post-surgical atrioventricular blocks. Any endocardectomy was followed by either a mitral valvular repair surgery according to Carpentier’s procedures or by a tricuspid annuloplasty of De Vega i.e., a valvular replacement by a bioprostheses or a mechanical prosthesis (Table 6). Figure 35 demonstrates a monobloc extracted fibrous plaque. In early post-operative period, 20 deaths were reported (16%) caused by an acute cardiac irreversible failure (Table 7).
Full table
Full table
Congenital cardiopathies (Table 5)
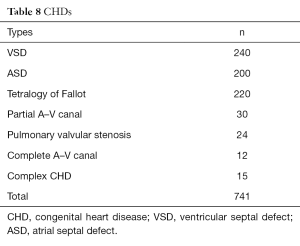
Ventricular and atrial septal defects (ASD), ostium primum ASD or partial atrio-ventricular septal defect (partial AVSD) and tetralogy of Fallot (T4F) were the most frequent (Table 8).
Full table
VSD
Two hundred-forty [240] cases were operated (Table 8) on; average age was 9±1.2 years (9 months–32 years). There were 144 male and 96 female patients i.e., a gender ratio of 144/96=1.5. Mean weight was 25±2 kg. In all patients, symptoms suggested left to right shunt clinical feature with a systolic murmur heard in the fifth left interspace coexisting with a loud pulmonary component of the second sound of the heart; mean cardio-thoracic index was 0.61±0.01 (0.47–0.81) at radiograph; in most cases either left or bilateral ventricular hypertrophy at electrocardiogram were visualized. Diagnosis, morphology, hemodynamic profile according to Nadas and Fyler classification (25) of VSD had been confirmed by both 2D echocardiography coupled with Doppler colour flow evaluation and/or Cardiac catheterization with angiography (n=100) for all patients. Four main anatomical forms were described: peri-membranous VSD (76%), infundibular or sub arterial VSD (21%), inlet VSD (2%) and muscular VSD (1%). Mean systolic pulmonary arterial pressure, mean pulmonary arterial pressure, mean Qp/Qs, pulmonary vascular resistance and cardiac index were 51.7±18 mmHg, 34.7±2.40 mmHg, 3.1±0.2, 3.46±0.5 IU, 6.2±2.97 L/min/m2 respectively. Nadas and Flyer classification type IIa (n=120, 50%), type IIb (n=96, 40%) and type IV (n=24, 10%) were listed. VSD were repaired on conventional cardio-pulmonary by-pass through right atrium (n=187; 78%) and right ventricular (n=53; 22%) approaches. VSD closure was done by direct suture (n=8; 3.3%) or with autologous or synthetic patch (n=232, 96.7%). Hospital mortality was 4.1% (n=10) due to acute right ventricular heart failure in 7 cases, ventricular arrhythmias (2 cases), and hemorrhage (1 case). Postoperative acute cardiac failure was a predictable risk factors for early death (P=0.001). Average follow up duration was 15 years (1 month–35 years). Survival was 90%, 87%, 78% at 5, 15 and 25 years respectively (Figures 36,37).
ASD
Two hundred [200] patients with ASD underwent surgery (Table 8); they were 113 males and 87 females; average age was 17±2.3 years (2.5–58 years) and a mean weight at 38±3.4 kg (5–73 kg). All patients were symptomatic with clinical patterns related to a large left to right shunt.
Pulmonary hypertension was attested (n=180, 90%) by an accentuation of the second heart sound ostium secundum (n=180, 80%). One hundred and seventy-eight (89%) patients were in NYHA class II and 22 (11%) in NYHA class III or IV. Chest radiograph and electrocardiography reflected in all patients the large left to right shunt at atrial level confirmed by both echocardiography, cardiac catheterization and angiocardiography. Hemodynamics parameters were as follows: mean systolic pulmonary arterial pressure at 42.05±1.6 mmHg; mean pulmonary arterial pressure at 17.2±0.87 mmHg; mean QP/QS at 3.3±0.34 (1.5–9.9). Fossa ovalis defect (80%, n=160); superior caval type of ASD or sinus venosus defect (13%, n=26); low fossa ovalis ASD (7%, n=14) were the anatomic forms described. Associated cardiac lesions as left superior vena cava (n=2), mitral valve stenosis (n=2), tricuspid valve regurgitation (n=2), VSD (n=4), pulmonary arterial stenosis (n=7), mitral valve regurgitation (n=9), anomalous connection of right pulmonary veins to right atrium (n=30). Under CPB with or without aortic clamping a direct closure was carried out (n=54; 27%) or by a synthetic or pericardial patch (n=146; 73%). Early death was 3.3% (n=8) due to severe arterial pulmonary hypertension (n=2), ventricular arrhythmia (n=1), cardiac failure (n=4) and haemorrage (n=1). No incremental risk factor for death was found. Actuarial survival at 25 years was 97% (Figure 38).
Partial AVSD
Thirty [30] cases (Table 8) were recorded among 11 boys and 19 girls with an average age of 17±2.8 years (9 months–47 years) and a mean weight at 33±5.9 kg (extreme, 8–73). Clinical presentation, chest radiograph, electrocardiogram and echocardiography shown typical signs of partial ASD; cardiac catheterization and angiocardiography were performed on 16 patients; mean systolic pulmonary artery pressure, mean pulmonary arterial pressure, mean cardiac index, QP/QS mean value, pulmonary arterial resistance were: 33.6±3.9 mmHg (17–49 mmHg), 21±2.62 mmHg (10–32 mmHg), 2.69±0.62 (1.66–4.47), 2.9±0.29 (1.2–4.42), 4.9±1.6 UIR (1.92–10.6 UIR) respectively. “Swan neck” aspect, in accordance to an absence of the atrio-ventricular septum with an elongation of the left ventricular outflow track, was the angiographic characteristic image combined with a mitral leak due to a valvular split. Complete repair was carried out (n=30) under CPB: closure of the mitral split and implementation of a synthetic or pericardial patch for closing the atrio-VSD. Early mortality occurred in 4 patients attributable to complete atrio-ventricular block (n=1), low cardiac output (n=1), unknown cause (n=2). Median follow-up reached 10 years (1 month–30 years); 4 were lost to follow-up; no late mortality was noted; actuarial survival was 78% at 5 and 20 years (Figure 39).
T4F
Two hundred and twenty [220] cases (Table 8) were recorded; there were 139 boys and 81 girls, average age of patients was 5.8 years (2 months–32 years); average weight was at 19.5 kg (4–57 kg). Cyanosis, effort dyspnea was present in all patients; both of them appear smaller and less heavy than expected for age. 20% of patients presented hypoxic spells. A systolic murmur sound was always audible along the left sternal border. Chest radiograph revealed classic boot-shaped heart (n=200); electrocardiography shown right ventricular hypertrophy in all patients; 2D echocardiography, cardiac catheterization and angiocardiography confirmed the diagnosis of T4F marked by four fundamental lesions: a malignment VSD; an anterior shift of the aorta over the VSD (overriding aorta); an obstruction of the right ventricular outflow tract; a right ventricular hypertrophy. Angiocardiography provided the accurate anatomic features according to the morphology and sites of the right ventricular outflow obstruction: group 1 (n=132) called “regular form” composed of T4F with isolated infundibular stenosis (n=132) and group 2 (n=88) called “irregular form” composed of T4F combined with infundibular plus valvar stenosis or infundibular plus valvar plus anular stenosis or infundibular stenosis plus diffuse hypoplasia. Hundred patients underwent palliative surgery as classic Blalock-Taussig operation or modified Blalock-Taussig procedure followed by a complete repair under CPB; primary one stage complete repair was performed in 120 patients. Early death was 10% (n=22) due mainly to hemorrhage (n=8), right ventricular failure (n=6), low cardiac output (n=4) and others (n=4). Average follow-up was 8.4 years (1 month–20 years). Two late deaths, secondary to infectious endocarditis (n=1) and gastro-intestinal infection (n=1), were observed.
Discussion
Rheumatic heart diseases
In Africa, patients concerned by valvular heart diseases are young (9), and as mentioned by several authors (14-17) rheumatic fever is the first etiology in those diseases; patients with rheumatic heart diseases used to come late to hospital; in such situation, surgery becomes often necessary.
In our experience, most of the time mitral or aortic rheumatic lesions were severe, massive, with valvular retraction, thickness, rigidity or, calcification coexisting with shortened, thick, retracted, fused sub-valvular apparatus including chordates and papillary muscles; such advanced lesions made any repair difficult even impossible with valve replacement as the only choice. At the first years, we preferred bioprosthes is implantation rather than mechanical valves because most of our young patients could not have regular access to anticoagulation drugs (21). Meanwhile, at follow-up, we observed heavy bioprosthetic deteriorations after an average time of 4 years (26); this very short post-operative interval dictated us a new option in favour of valve repair; if this wasn’t not achievable, then mechanical prosthesis was our choice.
When possible, it has been demonstrated that vavuloplasty is the best option; Antunes (27) in 1990 has reported a comparative study between valve reconstruction and rheumatic mitral valve disease; he analysed three groups: patients with mitral vavuloplasty (n=241); MRV by mechanical prosthesis (n=386) and bioprosthesis (n=289). Early mortality was significantly better after plasty 3% than mechanical valve replacement 7.8% or bioprothesis 6.6%; actuarial global survival and survival free from valve related complication were far better for vavuloplasty than replacement 90%±4% and 70%±6% for vavuloplasty versus 76%±3% and 71%±5% for mechanical valve replacement and 62%±7% and 30%±7% for bioprosthesis valve replacement; in another study the same author (28) has demonstrated that repair of mitral regurgitation can be performed in most cases with a repair rate of nearly 81% with excellent immediate, medium term results and in a well-controlled situation a survival of 89% and a survival free from reoperation of 85% at 15 years. Similar results have been shown by Kumar et al. (29) in India with a 90% survival at the same follow-up duration. In the same line, Finucane and Wilson (30) in Australia noted that 50% of his patients with MRV had a significant hemorragic, thrombotic or embolic complication within 11 years versus 100% freedom from embolic, thrombotic or hemorragic events in those with mitral valve repair. Those results are not different from Remenyi et al. (31) experience with a significant advantage of valve repair as he has demonstrated an actuarial freedom from valve related complications at 10 and 14 years at 63% and 45% for patients with valvular replacement compared to 100% and 100% for patients with valve repair. Even if valvuloplasty provides better long term results, we also noted in our study that valve replacement can be useful as reminded by Cardoso et al. (32) who reported an excellent rate of freedom from reintervention for primary valve replacement at 6 months, 5 years, 10 years at 100%, 91.7% and 91.7% respectively versus 96.4%, 72%, 44% at 6 months, 5 years, 10 years respectively after primary valve repair. To avoid valve replacement and to get greater opportunity for valve repair and percutaneous balloon valvuloplasty, Russell (33) in her review of valve surgery for RHD in Australia on patients has recommended earlier referral prior to the establishment of valvular fibrosis and calcifications and earlier surgical management.
Our experience on aortic valve repair is lower than that of AVR; this is based on the fact that in most of our cases repair was not feasible due to the severity of RHD lesions; when done aortic repair technique we used was Trusler procedure; however other techniques have been described in literature (34,35). In his article on repair options in rheumatic aortic valve disease in young patients like ours d’Udekem (36) has highlighted arguments against cusp extension for different reasons: patients are usually old enough for valve replacement as adults; lack of antibioprophyaxis compliance; repair within a short postoperative period 4 to 5 years and other argument such as myocardial ischemic complications. On contrary to d’Udekem, Polimenakos (37) and Myers (38) had concluded in their studies that aortic repair by cusp extension for rheumatic aortic insufficiency in children gives satisfactory long term results Myers (38) has found a rate of freedom from reoperation at 96%±2.3% at 1 year, 87.5%±3.9% at 5 years, 80.7%±4.9% at 10 years and 75.3%±6% at 15 years. In addition, Karamlou (35) in his study on 61 patients who underwent rheumatic aortic valvuloplasty, reported a low early death at 4.9% and excellent actuarial and reoperation-free survival at 106 months at 95%±2.8% and 85.4%±6.7% respectively. Based on analysis of papers published on the choice between aortic repair or replacement by bioprosthetic valve. Tourmousoglou (39) had asserted that aortic valve repair for severe aortic regurgitation offers good early and mid-term results; and for this author aortic valve repair is an acceptable alternative to aortic replacement with a bioprosthesis. However, in children, because there is no ideal valve prosthesis, the best option for aortic valve surgery, repair or replacement, still be pending: valve replacement: mechanical valve or biologic valve? or Ross procedure or bioprosthetic valve; in our experience, in case of severe rheumatic aortic valvular lesions, replacement with bioprosthetic valves or mechanical prosthetis was our first option; no Ross procedure had been done by our team; we had similar short and long term results for AVR as those in literature (35,40). We have noted in a personal study not yet published on isolated AVR for aortic regurgitation, at early, mid and long terms a mortality at 10%, 9.4%, 1.3% respectively with significant risk factors for early death such as preoperative NYHA functional class (P=0.00002), cardio thoracic index (P=0.0057), left ventricular end diastolic diameter >60 mm (P=0.000000). Karamlou (35) has reported an early death at 8% after AVR in 160 children with associated significant risk factors for death without a second AVR as lower weight, younger age at AVR, performance of aortic arch reconstruction together with AVR and non autograft use. Alsoufi (41) in his series of 123 valve replacements including 8 MRV and 36 aortic valve found an early death after AVR at 5.5% and outlined the fact that patients with AVR had lower freedom from reoperation compared to those with MVR and has reported that bioprosthetic valves in mitral position had a higher longevity compared to bioprosthesis in aortic position. The same author (42) has reported a lower operative mortality for Ross procedure (2.3%) than for mechanical valves (6.1%) and a better survival for patients with Ross procedure without late mortality in comparison to mechanical group. In a similar line, Ruzmetov (43) showed an overall and late mortality at 7.5% in 147 children who underwent AVR and recommended Ross procedure as first option.
We performed more tricuspid valve repair (n=159) than tricuspid valve replacement (n=70). Underlying indication for tricuspid repair was mainly functional tricuspid regurgitation (FTR) combined with left sided rheumatic valve diseases; De Vega annuloplasty was the procedure we used to treat FTR (n=159); it is safe, not time consuming, not costly, easy to do, efficient and durable. In a previous study (44), we analyzed our early and long term outcomes after De Vega tricuspid annuloplasty on 42 patients with mitral valve diseases coexisting with FTR. Hospital mortality was 4.76%; at early post-operative period, right ventricular failure symptoms decreased significantly in 22 patients (P<0.05); at echocardiography one year later, the same observation was made for patients with a left ventricular systolic ejection fraction (LVSEF) ≥45% versus LVSEF <45% (P=0.046) and freedom from complications after De Vega tricuspid annuloplasty was 100%, 58%±2.4% at 10 and 15 years respectively. Tettey (45) in Ghana from his study on 64 patients noted that 90.6% of patients who underwent modified De Vega annuloplasty for tricuspid valve regurgitation had grade I or no regurgitation after surgery and after an average follow-up of 61.3 months, 64.1% of patients had no tricuspid valve regurgitation. Abdul (46) from Pakistan in a prospective study on 160 patients with De Vega tricuspid annuloplasty reported an early mortality of 3.7% and at 6-month follow-up majority of his patients improved their NYHA functional class and freedom from tricuspid regurgitation was 81.3% in MRV group. Naqshband (47) also from Pakistan in another series of 106 patients with FTR in rheumatic heart disease had an overall early mortality at 2.8% and freedom from tricuspid regurgitation after modified tricuspid De Vega annuloplasty along with Mitral valve or mitro-AVR, at 100% at 7 years. Akhter (48) in North America from his series of 32 patients with De Vega tricuspid annuloplasty for severe tricuspid regurgitation preoperatively showed a significant improvement to an absence of tricuspid regurgitation after De Vega procedure persistent even at 1 year post operatively. Our results are similar to others mentioned above who concluded that De Vega tricuspid annuloplasty, modified or not, is safe, not time consuming, not costly, easy to do, efficient and durable.
Indications for tricuspid valve annuloplasty had been well set up by Tornos Mas (49) and Naqshband (47). According to Pillar’s recommendations: severe secondary tricuspid regurgitation requires operation during mitral or aortic valve surgery; surgery should done in patients with minor or moderate FTR with dilated annulus, tricuspid valve repair should be also considered in case of moderate FTR associated to pulmonary artery hypertension when left side valve surgery is planned. From Salik’s experience, FTR having no significant annular dilatation can be left without any dilatation and those with annular dilatation can be cured by modified De Vega annuloplasty.
Risk factors for tricuspid valve reoperation can exist; Revuelta (50) has demonstrated some as follows: presence of residual tricuspid valve incompetence; pulmonary artery systolic pressure superior to55 mmHg or ignored functional tricuspid insufficiency. In our experience (51) on 30 patients with neglected minimal or moderate FTR, risk factors for worsening regurgitation were: preoperative heart failure episodes (P=0.02); mitral valve surgery (P=0.0045); moderate FTR (P=0.046).
When not reparable tricuspid valve needs replacement by mechanical or biological prosthesis; that situation often appears with EMF in our daily practice.
EMF
EMF is a mysterious disease (52-54): its etiology remains unknown; it is a restrictive cardiomyopathy (52) including occlusive, thick and fibrous tissue into heart ventricular cavities, this fibrous tissue compromises heart ventricular function and atrio-ventricular valves movement of the heart (55).
It usually affects children and young population (56) and most often occurs in hot, humid, forest and tropical regions in Africa (55-57): Uganda, Côte d’Ivoire, Nigeria, Mozambique, Gabon, Cameroon.
Côte d’Ivoire team has one of the largest series published in the world with more than 100 cases diagnosed (54). EMF is also discovered in inter-tropical countries in South and central America (Colombia, Brazil, Venezuela, Mexico), in India and in North America or in Asia (52,56,57). Several causes of EMF have been shown and none of them was validated; they are as follows (52-57): eosinophilia, but endomyocardial biopsies made were negative; Infections such as toxoplasmosis, rheumatic fever, malaria, and helminthic parasites, toxic agents: cassava, serotonin; malnutrition; autoimmunity and ethnicity. Clinical patterns (52,54,58) include right ventricular failure symptoms like liver enlargement, ascites, peripheral edema, splenomegaly and tricuspid regurgitation; or left ventricular failure characterized by pulmonary hypertension and mitral regurgitation. Diagnosis is confirmed by echocardiography, cardiac catheterization and angiocardiography which demonstrate obliteration of the apex of one or both ventricles (52,54,59). Surgery is indicated in patients with NYHA functional class III or IV (59). EMF extended most frequently to all the endocardium, sticking the chordaes, the papillary muscles and the valvular tissues; what justifies the predominance of valvular replacement rather than valve repair in our study. Hospital mortality was high in the literature: 20% for Moraes (60-62), 16% for Metras (63); in our study on bilateral EMF in children we reported an early mortality after surgery at 29.4% (64). Fibrosis is not recurring as evocated by Tharakan (59); it indicates the end of a pathological process and the proof was given during an autopsy carried out in Abidjan with a patient 4 years after a right EMF surgery (65).
Congenital cardiopathies
Pattern of CHD is similar among countries in Sub-Saharan Africa where commonest CHD found are: VSD, ASD, patent ductus arteriosus (PDA), T4F as observed in South Africa (66), Malawi (67), Senegal (68), Cameroon (69), Côte d’Ivoire (70), Nigeria (71) and Ghana (72).
In Côte d’Ivoire, looking at the incidence of congenital cardiopathies between 5 and 8 for 1,000 births in the literature and the number of 360,000 births per year, between 1,800 and 2,800 congenital anomalies per year should be discovered (70). We are still far from the account here as well as in African regions where the number of children and adults affected with CHD may be underestimated (73,74).
Sani (75) in Kano, Nigeria, through an echocardiography study on 122 patients among 1,312 patients with abnormal echocardiography, set up the spectrum of CHD in his area; according to his work, VSD was the commonest (N=56, 45.9%) followed by T4F (N=32, 26.2%), ASD (N=15, 12.3%), endocardial cushion defect (N=10, 8%) and 9 (7.4%) other heart anomalies. Based on the fact that the incidence of CHD in Nigeria in Nigeria is estimated at 3.5 per thousand births (71) and 8 per thousand births worldwide (74), Zühlke (9) has concluded that in his environment only a minority of cases are detected while a large number of CHD still be undetected.
In Mozambique, same findings have been published by Marijon (76) where with a very low prevalence of 2.3 per thousand has been noted, this prevalence remains lower than 6.9, 8.2; 9.3 per thousand live births respectively in North America, Europe and Asia (73,74).
In Sub-Saharan Africa, congenital heart surgery are performed by pediatric heart surgeons in a very few cardiac centres in Sub-Saharan Africa; those most active and running by local teams are located in South Africa, Côte d’Ivoire and Ghana. Humanitarian services from developed countries or establishment of new OHS programs in Africa attempt to fill the gap in some countries like Cameroon (77), Tanzania (78), Uganda (79), Edwin (80) in Ghana has demonstrated that early mortality rate after surgery for CHD in children is low and comparable to outcomes in the world. Aliku in Uganda (79) reported on 124 patients with an average age at 9.93 years (3 months–52 years) who underwent OHS for CHD: VSD 34.7%, ASD 34.7%, T4F 10.5%, and an overall early mortality at 3.2% (n=4). Nyangasa (81) in Tanzania published a one year experience of OHS on 105 patients with a mean age at 19.4±12.3; surgery concerned mainly Rheumatic valve disease 47.6% and CHD 35.2% and an early mortality at 13.3% (n=14). Those results after surgery for CHD are comparable to ours and others found in literature (82).
Perspectives
Based on the epidemiological results of this study, it urges for Sub-Saharan African countries including Cote d’Ivoire to have more and well established data registry centers on acquired and congenital CVDs; and, to encourage where it doesn’t exist, institutional review boards for supervising and approving research projects in cardio-vascular field.
It urges for the next future to screen more CHD, to build more infrastructures for OHS, to train more qualified medical and nurses staff in more cardiac centers to fit with African population needs for these reasons. So, in Côte d’Ivoire, as a supplement to the Institut de Cardiologie of Abidjan in the south of the country, the construction of a second center of cardiology started in Bouake, a town located in the center of our country (Figure 40).
Conclusions
OHS in Cote d’Ivoire was successfully performed in most of our patients, the spectrum of acquired valvular heart diseases and CHDs in our country is similar to others in sub Saharan Africa. Rheumatic heart valvular diseases and EMF were the main acquired valvular heart diseases concerned by surgery; unfortunately, because of late presentation of our patients, valve replacement rather than valve repair was indicated in majority of cases. For CHDs, complete correction was done with satisfactory results. Therefore, OHS can be provided safely in our country and so far, we should recommend our government to assist more our OHS program for a significant increase of OHS cases annually.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Sliwa K, Acquah L, Gersh BJ, et al. Impact of Socioeconomic Status, Ethnicity, and Urbanization on Risk Factor Profiles of Cardiovascular Disease in Africa. Circulation 2016;133:1199-208. [Crossref] [PubMed]
- Livesay JJ. Cardiovascular disease in Africa. Tex Heart Inst J 2007;34:6-7. [PubMed]
- Jamison DT, Feachem RG, Makgoba MW, et al. editors. Disease and Mortality in Sub-Saharan Africa. 2nd edition. Washington (DC): World Bank, 2016.
- The Current Burden of Cardiovascular Diseases in Developing countries. In: Howson CP, Reddy KS, Ryan TJ, et al. editors. Control of Cardiovascular Diseases in Developing countries: Research, Development and Institutional Strengthening. Washington (DC): National Academy Press, 1998:11-23.
- Deaton C, Froelicher ES, Wu LH, et al. The global burden of cardiovascular disease. Eur J Cardiovasc Nurs 2011;10 Suppl 2:S5-13. [Crossref] [PubMed]
- Lim GB. Global burden of cardiovascular disease. Nat Rev Cardiol 2013;10:59. [Crossref] [PubMed]
- O’Rourke K, Vander Zanden A, Shepard D, et al. Cardiovascular Disease Worldwide, 1990-2013. JAMA 2015;314:1905. [Crossref]
- Kelly BB, Narula J, Fuster V. Recognizing global burden of cardiovascular disease and related chronic diseases. Mt Sinai J Med 2012;79:632-40. [Crossref] [PubMed]
- Zühlke L, Mirabel M, Marijon E. Congenital heart disease and rheumatic heart disease in Africa: recent advances and current priorities. Heart 2013;99:1554-61. [Crossref] [PubMed]
- Reddy KS, Yusuf S. Emerging epidemic of cardiovascular disease in developing countries. Circulation 1998;97:596-601. [Crossref] [PubMed]
- Gaziano TA, Bitton A, Anand S, et al. Growing epidemic of coronary heart disease in low- and middle-income countries. Curr Probl Cardiol 2010;35:72-115. [Crossref] [PubMed]
- Mocumbi AO. Lack of focus on cardiovascular disease in sub-Saharan Africa. Cardiovasc Diagn Ther 2012;2:74-7. [PubMed]
- WHO. Rheumatic Fever and Rheumatic Heart Disease. 2004. Available online: http://www.who.int/cardiovascular_diseases/resources/en/cvd_trs923.pdf
- Seckeler MD, Hoke TR. The worldwide epidemiology of acute rheumatic fever and rheumatic heart disease. Clin Epidemiol 2011;3:67-84. [Crossref] [PubMed]
- Chin TK, Patnana SR. Pediatric Rheumatic Heart Disease. Available online: http://emedicine.medscape.com/article/891897-overview
- Kumar RK, Tandon R. Rheumatic fever & rheumatic heart disease: the last 50 years. Indian J Med Res 2013;137:643-58. [PubMed]
- Watson G, Jallow B, Le Doare K, et al. Acute rheumatic fever and rheumatic heart disease in resource-limited settings. Arch Dis Child 2015;100:370-5. [Crossref] [PubMed]
- Adebo O. A History of Open Heart Surgery at the University College Hospital. Ibadan Nigerian Journal of Cardiology 2005;2:6-9.
- Anyanwu C, Ihenacho H, Okoroma E, et al. Initial Experience with Open Heart Surgery in Nigeria. Tropical Cardiology 1982;8:123-7.
- Merle H, Yangni-Angate A, Delormas P, et al. Thoracic Surgery in Côte d’Ivoire. Medical Africa 1961;1:25-33.
- Nogueira EO. Genome sequencing analysis of Brazilian chicken anemia virus isolates that lack MSB-1 cell culture tropism. Comp Immunol Microbiol Infect Dis 2007;30:81-96. [Crossref] [PubMed]
- Acquired Valvular Heart Disease Congenital Heart Disease. In: Kirklin JW, Barratt-Boyes BG. editors. Cardiac Surgery. Second Edition. Churchill Livingstone Inc., 1993:425-606; 609-1596.
- Trusler GA, Moes CA, Kidd BS. Repair of ventricular septal defect with aortic insufficiency. J Thorac Cardiovasc Surg 1973;66:394-403. [PubMed]
- De Vega NG. Selective, adjustable and permanent annuloplasty. An original technic for the treatment of tricuspid insufficiency. Rev Esp Cardiol 1972;25:555-6. [PubMed]
- Fyler DC, Rudolph AM, Wittenborg MH, et al. Ventricular septal defect in infants and children; a correlation of clinical, physiologic, and autopsy data. Circulation 1958;18:833-51. [Crossref] [PubMed]
- Yangni-Angate H, Tanauh Y, Yapobi Y, et al. The Heart valvular bioprosthesis reintervention at Abidjan - Early outcomes - Indications and surgical risk factors. Tropical Cardiology 1990;16:87-92.
- Antunes MJ. Mitral valvuloplasty, a better alternative. Comparative study between valve reconstruction and replacement for rheumatic mitral valve disease. Eur J Cardiothorac Surg 1990;4:257-62; discussion 263-4. [Crossref] [PubMed]
- Antunes MJ. Challenges in rheumatic valvular disease: Surgical strategies for mitral valve preservation. Glob Cardiol Sci Pract 2015;2015:9.
- Kumar AS, Rao PN, Saxena A. Results of mitral valve reconstruction in children with rheumatic heart disease. Ann Thorac Surg 1995;60:1044-7. [Crossref] [PubMed]
- Finucane K, Wilson N. Priorities in cardiac surgery for rheumatic heart disease. Glob Heart 2013;8:213-20. [Crossref] [PubMed]
- Remenyi B, Webb R, Gentles T, et al. Improved long-term survival for rheumatic mitral valve repair compared to replacement in the young. World J Pediatr Congenit Heart Surg 2013;4:155-64. [Crossref] [PubMed]
- Cardoso B, Loureiro P, Gomes I, et al. Mitral Valve Surgery for Rheumatic Lesions in Young Patients. World J Pediatr Congenit Heart Surg 2016;7:321-8. [Crossref] [PubMed]
- Russell EA, Tran L, Baker RA, et al. A review of valve surgery for rheumatic heart disease in Australia. BMC Cardiovasc Disord 2014;14:134. [Crossref] [PubMed]
- Talwar S, Saikrishna C, Saxena A, et al. Aortic valve repair for rheumatic aortic valve disease. Ann Thorac Surg 2005;79:1921-5. [Crossref] [PubMed]
- Karamlou T, Jang K, Williams WG, et al. Outcomes and associated risk factors for aortic valve replacement in 160 children: a competing-risks analysis. Circulation 2005;112:3462-9. [Crossref] [PubMed]
- d'Udekem Y, Sharma V. Repair options in rheumatic aortic valve disease in young patients: potential problems with pericardial cusp extension. World J Pediatr Congenit Heart Surg 2013;4:392-6. [Crossref] [PubMed]
- Polimenakos AC, Sathanandam S, Elzein C, et al. Aortic cusp extension valvuloplasty with or without tricuspidization in children and adolescents: long-term results and freedom from aortic valve replacement. J Thorac Cardiovasc Surg 2010;139:933-41; discussion 941. [Crossref] [PubMed]
- Myers PO, Tissot C, Christenson JT, et al. Aortic valve repair by cusp extension for rheumatic aortic insufficiency in children: Long-term results and impact of extension material. J Thorac Cardiovasc Surg 2010;140:836-44. [Crossref] [PubMed]
- Tourmousoglou C, Lalos S, Dougenis D. Is aortic valve repair or replacement with a bioprosthetic valve the best option for a patient with severe aortic regurgitation? Interact Cardiovasc Thorac Surg 2014;18:211-8. [Crossref] [PubMed]
- Singh AK, Ungerleider RM, Law YM. The Impact of Aortic Valve Replacement on Left Ventricular Remodeling in Children. Pediatr Cardiol 2016;37:1022-7. [Crossref] [PubMed]
- Alsoufi B, Manlhiot C, McCrindle BW, et al. Aortic and mitral valve replacement in children: is there any role for biologic and bioprosthetic substitutes? Eur J Cardiothorac Surg 2009;36:84-90; discussion 90. [Crossref] [PubMed]
- Alsoufi B, Al-Halees Z, Manlhiot C, et al. Mechanical valves versus the Ross procedure for aortic valve replacement in children: propensity-adjusted comparison of long-term outcomes. J Thorac Cardiovasc Surg 2009;137:362-370.e9. [Crossref] [PubMed]
- Ruzmetov M, Vijay P, Rodefeld MD, et al. Evolution of aortic valve replacement in children: a single center experience. Int J Cardiol 2006;113:194-200. [Crossref] [PubMed]
- Yangni-Angate KH, Ayegnon KG, Meneas C, et al. Annuloplastie tricuspidienne de De Vega au cours du remplacement valvulaire mitral: experience chirurgicale en Cote d’Ivoire. Ann Afr Chir Thor Cardiovasc 2014;9:85-92.
- Tettey L, Sereboe L, Edwin F, et al. Modified De Vega annuloplasty for functional tricuspid valve regurgitation. Ann Afr Chir Thor Cardiovasc 2009;4:14-8.
- Abdul M, Khalil IK, Ali SM, et al. De Vega's ttricuspid annuloplasty for severe tricuspid regurgitation - early and midterm follow up. Pak Heart J 2012;45:11-6.
- Naqshband MS, Abid AR, Akhtar RP, et al. Functional tricuspid regurgitation in rheumatic heart disease: surgical options. Ann Thorac Cardiovasc Surg 2010;16:417-25. [PubMed]
- Akhter SA, Salabat MR, Philip JL, et al. Durability of De Vega tricuspid valve annuloplasty for severe tricuspid regurgitation during left ventricular assist device implantation. Ann Thorac Surg 2014;98:81-3. [Crossref] [PubMed]
- Tornos Mas P, Rodríguez-Palomares JF, Antunes MJ. Secondary tricuspid valve regurgitation: a forgotten entity. Heart 2015;101:1840-8. [Crossref] [PubMed]
- Revuelta JM. The forgotten functional tricuspid valve insufficiency: Is valve repair necessary? Available online: www.ctsnet.org/sections/innovation/valvetechnology/articles/article-9.html
- Yangni-Angate KH, Ayegnon KG, Diby F, et al. Neglected tricuspid insufficiency during mitral valve. Ann Afr Chir Thor Cardiovasc 2014;9:17-22.
- Mocumbi AO. Endomyocardial fibrosis: a form of endemic restrictive cardiomyopathy. Glob Cardiol Sci Pract 2012;2012:11.
- Bukhman G, Ziegler J, Parry E. Endomyocardial fibrosis: still a mystery after 60 years. PLoS Negl Trop Dis 2008;2:e97. [Crossref] [PubMed]
- Bertrand E. Endomyocardial Fibrosis Heart and Vessels. Med and Surg Encycl 11008 A10, 10-1985, 10p.
- Grimaldi A, Mocumbi AO, Freers J, et al. Tropical Endomyocardial Fibrosis: Natural History, Challenges, and Perspectives. Circulation 2016;133:2503-15. [Crossref] [PubMed]
- Verma VK, Zafar KS. Tropical endomyocardial fibrosis: an overview. Int J Res Med Sci 2014;2:1267-77. [Crossref]
- Mocumbi AO, Ferreira MB, Sidi D, et al. A population study of endomyocardial fibrosis in a rural area of Mozambique. N Engl J Med 2008;359:43-9. [Crossref] [PubMed]
- Vijayaraghavan G, Sivasankaran S. Tropical endomyocardial fibrosis in India: a vanishing disease! Indian J Med Res 2012;136:729-38. [PubMed]
- Tharakan J, Bohora S. Current perspective on endomyocardial fibrosis. Current Science 2009;3:405-10.
- Moraes CR, Buffolo E, Lima R, et al. Surgical treatment of endomyocardial fibrosis. J Thorac Cardiovasc Surg 1983;85:738-45. [PubMed]
- Moraes CR, Escobar M, Lima R, et al. Technical aspects in surgery for endomyocardial fibrosis: experience with 37 patients. Tex Heart Inst J 1983;10:115-8. [PubMed]
- Da Costa FD, Moraes CR, Rodriques JV, et al. Early surgical results in the treatment of endomyocardial fibrosis. A Brazilian cooperative study. Eur J Cardiothorac Surg 1989;3:408-13. [Crossref] [PubMed]
- Metras D, Coulibaly AO, Ouattara K. The surgical treatment of endomyocardial fibrosis: results in 55 patients. Circulation 1985;72:II274-9. [PubMed]
- Yangni-Angate H, Ayegnon G, Diby F, et al. Bilateral Endomyocardial Fibrosis In Children: An Ivorian Surgical Experience. African Journal of Paediatric Surgery 2007;4:64-7.
- Metras D, Ouattara K, Coulibaly AO, et al. Left endomyocardial fibrosis with severe mitral insufficiency; the case for mitral valve repair. A report of 4 cases. Thorac Cardiovasc Surg 1983;31:297-300. [Crossref] [PubMed]
- Hewitson J, Zilla P. Children’s heart disease in sub-Saharan Africa: Challenging the burden of disease. Sa Heart 2010;7:18-29.
- Kennedy N, Miller P. The spectrum of paediatric cardiac disease presenting to an outpatient clinic in Malawi. BMC Res Notes 2013;6:53. [Crossref] [PubMed]
- Diop IB, Ba SA, Ba K, et al. Congenital cardiopathies: anatomo-clinical, prognostic, and therapeutic features apropos of 103 cases seen at the Cardiology Clinic of the Dakar University Hospital Center. Dakar Med 1995;40:181-6. [PubMed]
- Tantchou Tchoumi JC, Butera G. Profile of cardiac disease in Cameroon and impact on health care services. Cardiovasc Diagn Ther 2013;3:236-43. [PubMed]
- Chauvet J, Kakou GM, Andoh J, et al. Consultation in Pediatric Cadiology. Tropical Cardiology 1985;13-17.
- Thomas MO, Olusoji O, Awolola N. Spectrum of congenital heart diseases in an African population: A necropsy study. World Journal of Cardiovascular Diseases 2013;3:34-9. [Crossref]
- Amoah AG. Spectrum of cardiovascular disorders in a national referral centre, Ghana. East African Medical Journal 2000;77:648-53.
- Chinawa JM, Eze JC, Obi I, et al. Synopsis of congenital cardiac disease among children attending University of Nigeria Teaching Hospital Ituku Ozalla, Enugu. BMC Res Notes 2013;6:475. [Crossref] [PubMed]
- van der Linde D, Konings EE, Slager MA, et al. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol 2011;58:2241-7. [Crossref] [PubMed]
- Sani MU, Mukhtar-Yola M, Karaye KM. Spectrum of congenital heart disease in a tropical environment: an echocardiography study. J Natl Med Assoc 2007;99:665-9. [PubMed]
- Marijon E, Tivane A, Voicu S, et al. Prevalence of congenital heart disease in schoolchildren of sub-Saharan Africa, Mozambique. Int J Cardiol 2006;113:440-1. [Crossref] [PubMed]
- Budzee A, Tantchou Tchoumi JC, Ambassa JC, et al. The Cardiac Center of Shisong Hospital: the first cardio-surgical center in West and Central Africa is inaugurated in Cameroon. Pan Afr Med J 2010;4:4. [PubMed]
- Medical Missions in Tanzania: Creating a Sustainable Health Care Infrastructure. Available online: http://www.acc.org/latest-in-cardiology/articles/2015/11/13/13/14/medical-missions-in-tanzania-creating-a-sustainable-health-care-infrastructure#sthash.dIu9q5sd.dpuf
- Aliku TO, Lubega S, Lwabi P, et al. Outcome of patients undergoing open heart surgery at the Uganda heart institute, Mulago hospital complex. Afr Health Sci 2014;14:946-52. [Crossref] [PubMed]
- Edwin F, Sereboe LA, Tettey MM, et al. Experience from a single centre concerning the surgical spectrum and outcome of adolescents and adults with congenitally malformed hearts in West Africa. Cardiol Young 2010;20:159-64. [Crossref] [PubMed]
- Nyangasa B, Bgoya J, Mahalu WC. One year experience of cry at muhimbili national hospital, dar es salaam, tanzania east and central. African Journal of Surgery 2010;15:111-8.
- Acquired Valvular Heart Disease, Congenital Heart Disease; and Other Cardiac conditions. In: Kirklin JW, Barratt-Boyes BG. editors. Cardiac Surgery. Third Edition. Churchill Livingstone Inc., 2003:483-711; 715-1625; 1724-1731.