Complete congenital heart block in a neonate with a complex congenital heart defect in Africa
Background
Complete congenital heart block (CHB) is a rare disorder. It has been estimated to occur in 20,000 live births (1). Isolated CHB frequently results from neonatal lupus erythematosus, a disease associated with transplacental passage of maternal anti-Ro/SSA and/or anti-La/SSB antibodies to the fetus (2). More rarely, it may be associated with a congenital structural heart defect (3). Although it is an uncommon disorder, CHB may be associated with a high morbidity and mortality. CHBs associated with severe structural heart disease have a poorer prognosis than infants with isolated CHB (4). As a result, it requires a high index of suspicion when there is a finding of fetal bradycardia for early diagnosis and appropriate planning of perinatal management in centers with facility for pacemaker treatment but this not often possible in low resource settings. Few cases of CHB associated with structural heart defects have been reported in Sub-Saharan Africa (SSA). One of the first cases of CHB in Africa was reported by Okoroma et al. in Nigeria in a 3-month-old infant who was successfully treated with pacemaker implantation (5).
Case presentation
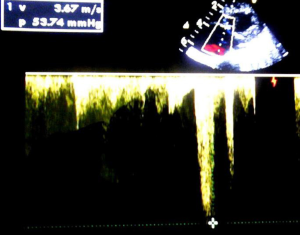
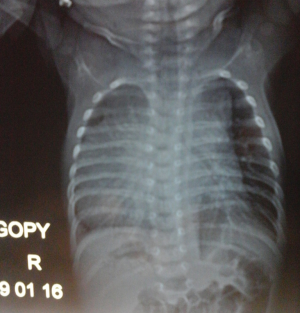
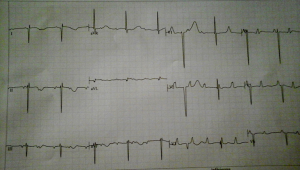
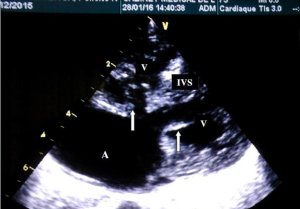
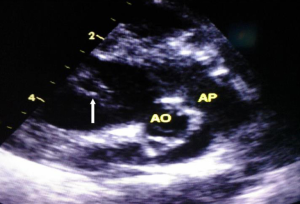
A 1-month-old neonate was brought to our center for evaluation of bradycardia. She was from a normal term pregnancy. There were no maternal infections, no maternal diabetes and no maternal alcohol consumption during pregnancy. There were no clinical signs and symptoms of maternal connective tissue disease. There was neither history of congenital heart disease in the family nor any history of recurrent pregnancy loss. Obstetrical ultrasound during pregnancy showed an inappropriately low heart rate but she was not referred for fetal echocardiography for further evaluation. There was no hydrops fetalis. At delivery birth weight was 3,540 g. At presentation, she had no dysmorphic features. There was not history of recurrent pneumonia. The heart rate was 62 beats/minute; the respiratory rate was 26 cycles/minute. Oxygen saturation was 93% at room air. She weighed 3,050 g. There was discrete cyanosis of the lips. The heart sounds S1 and S2 were normal with no audible murmurs. The femoral pulses were symmetric. The lung examination was normal. There was no hepatomegaly. Twelve lead electrocardiogram (ECG) showed a complete atrioventricular conduction block with a junctional escape rhythm at 59/minute, left axis deviation, bi-ventricular hypertrophy (Figure 1). Two-dimensional echocardiography performed demonstrated dextrocardia, situs inversus and undetermined veno-atrial concordance. There was a complete atrioventricular canal with a single atrium. The two ventricles were undifferentiated, well developed with good systolic function (Figures 2,3). The common atrioventricular valve exhibited mild regurgitation with a ventriculo-atrial outflow gradient that was estimated at 53 mmHg (Figure 4). There were two lateral cardiac outlets, side-by-side, with the aorta posterior and the pulmonary artery anterior (Figure 5). Plain chest radiography showed marked cardiomegaly and dextrocardia (Figure 6). There was no pacemaker implantation. The child was put on captopril and furosemide and was reviewed 1 week after. During the follow visit, she did not present any signs of heart failure. The parents did not show up for subsequent follow-ups.

Discussion
We have presented a rare case of complete CHB in a 1-month-old neonate with a complex congenital heart defect. To the best our knowledge, this is the first report of complete CHB associated with a complex congenital heart defect from Cameroon and SSA.
In Uganda, Namuyonga et al. reported a case of isolated CHB in an infant diagnosed at the age of 7 months and successfully treated with a permanent pacemaker (6). Echocardiography did not reveal any associated structural congenital heart defect contrary to our case. In another report, Dey et al. reported a case of isolated CHB diagnosed in utero at 24 weeks of gestation following the finding of fetal heart rate of 56–60 beats/minute as in our case (7). Our patient had a complete CHB associated with a complex congenital heart disease comprising dextrocardia, a complete atrioventricular septal defect and malposition of great arteries. Prior to delivery, the attending physician who performed the fetal echography had already noticed fetal bradycardia. The majority of cases of CHB diagnosed in utero are detected by either auscultation or routine obstetrical ultrasound with the diagnosis confirmed by fetal echocardiogram with Doppler techniques. The purpose of the fetal echocardiogram is to determine the level of block and also to rule out major associated structural defects of the heart, such as left atrial isomerism with or without atrioventricular septal defects, and ventricular inversion, which are structural diseases associated with the presence of heart block without antibodies. The fetal echocardiogram is also able to detect any associated myocarditis by looking for the presence of decreased contractility on fetal echocardiogram as well as any secondary changes of cardiomegaly, tricuspid insufficiency, pericardial effusion, or the development of hydrops fetalis. In our case, the diagnosis may have been made in-utero like that reported by Dey et al. (7) and might have dictated the attitude with respect to management of the pregnancy with serial echocardiograms and treatment after delivery. But the cardiac evaluation of fetuses with suspected CHB may be challenging most especially in resource limited settings like ours where there is scarcity of skilled personnel that can perform fetal echocardiography. CHB associated with structural heart disease is considered to be caused by failure of the atrioventricular conduction system to develop during heart development or due to displacement of the atrioventricular node. In our case we did not request to test for autoantibodies as we had a clear structural heart disease known for being associated with CHB and also because of limited resources.
CHB carries a high mortality (20–30%, primarily fetal/neonatal) and morbidity (67% require permanent pacing before adulthood) (2). The association with a structural congenital heart defect significantly increases mortality (4). According to Kertesz et al., in various series of fetal congenital complete atrioventricular block, 30% to 53% of cases have associated congenital heart defect. Of these, only 14% survived the neonatal period compared to 85% survival of the autoimmune isolated congenital complete atrioventricular block (3). The most important risk factors for death in isolated CHB are low birth weight, premature delivery, hydrops fetalis, endocardial fibroelastosis and diminished ventricular function (8). Amongst patients with CHB associated structural congenital heart defects, those with L-transposition of the great arteries and other complex structural cardiac defects have a worse prognosis unless detected and treated early (9). In our case the baby had survived intra-uterine life despite the heart block being associated with a severe structural congenital heart defect. The absence of hydrops fetalis or other signs of physiologic disturbance in cardiac function, low birth weight and prematurity may have contributed to the survival of the neonate. Children with structural heart defects may present with cyanosis, murmur, failure to thrive, or recurrent pneumonias but our patient only had discrete cyanosis on physical examination. The treatment of CHB requires pacemaker implantation as well as the treatment of any underlying structural heart defect (10). It is fairly well accepted that a mean resting heart rate below a determined number for the age group could be an indication to place a pacemaker. This is frequently quoted as a 55 bpm in the newborn period and gradually decreases with advancing age. The medical care is currently focused on identifying the optimal timing of pacemaker therapy to ensure a positive outcome. It is well accepted that symptoms referable to bradycardia are an indication to pacemaker implantation. Early intervention with pacing even in the asymptomatic patient may prevent the occurrence of significant myocardial dysfunction (10). In addition, the presence of a significant structural congenital heart defect is felt to be an indication to pace a patient with CHB (10). Significant pauses on 24-hour holter monitoring may also be an indication for pacemaker implantation. Our patient was not sent for pacing at the time we made the diagnosis due limited resources as there is no universal health coverage in the country and the parents of the neonate were poor.
Conclusions
This case reports highlights the challenges in the diagnosis and management of complex CHBs in low resource settings. A properly performed pregnancy follow-up with serial echocardiograms could aid in antenatal diagnosis and plan perinatal management when appropriate. This emphasizes the clinical value of high quality antenatal care and proper screening.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Michaëlsson M, Engle MA. Congenital complete heart block: an international study of the natural history. Cardiovasc Clin 1972;4:85-101. [PubMed]
- Buyon JP, Hiebert R, Copel J, et al. Autoimmune-associated congenital heart block: demographics, mortality, morbidity and recurrence rates obtained from a national neonatal lupus registry. J Am Coll Cardiol 1998;31:1658-66. [Crossref] [PubMed]
- Kertesz NJ, Fenrich AL, Friedman RA. Congenital complete atrioventricular block. Tex Heart Inst J 1997;24:301-7. [PubMed]
- Rosenthal E. Classification of congenital complete heart block: autoantibody-associated or isolated? Lupus 2003;12:425-6. [Crossref] [PubMed]
- Okoroma EO, Aghaji MA. Congenital complete heart block: treatment by pacemaker implantation in a 3 month old nigerian child. Cardiologie tropicale 1987;13:167-70.
- Namuyonga J, Lwabi P, Lubega S. Congenital heart block in a ugandan infant. Cardiovasc J Afr 2015;26:28.
- Dey M, Jose T, Shrivastava A, et al. Complete congenital foetal heart block: a case report. Facts Views Vis Obgyn 2014;6:39-42. [PubMed]
- Gupta M, Hamilton RM, Patnana SR, et al. Pediatric congenital atrioventricular block. Available online: http://emedicine.medscape.com/article/894703-overview#showall
- Kuleva M, Le Bidois J, Decaudin A, et al. Clinical course and outcome of antenatally detected atrioventricular block: experience of a single tertiary centre and review of the literature. Prenat Diagn 2015;35:354-61. [Crossref] [PubMed]
- Friedman D, Duncanson Lj, Glickstein J, et al. A review of congenital heart block. Images Paediatr Cardiol 2003;5:36-48. [PubMed]