Transcatheter aortic valve replacement with the SAPIEN 3 valve: preparing the field for the final expansion
The very first transcatheter aortic valve replacement (TAVR) procedure was performed in 2002 (1), and this treatment has now been established as the best option for inoperable patients with symptomatic severe aortic stenosis (2), and as a good alternative for high-risk individuals (3). To date, >200,000 TAVR procedures have already been performed in more than 65 countries (4). As shown in recent publications (5-8), improved transcatheter devices, increasing operator experience, and the very low incidence of significant structural transcatheter heart valve (THV) failure at 5-year follow-up, have provided the rationale for a worldwide shift towards treating lower surgical risk patients with a catheter-based approach. In the 2,032 intermediate risk patients with severe aortic stenosis randomized in the PARTNER 2A trial (9), TAVR was similar to surgical aortic valve replacement (AVR) with respect to the 2-year primary endpoint of death or disabling stroke (19.3% in the TAVR group and 21.1% in the surgery group; HR 0.89; 95% confidence interval: 0.73–1.09; P=0.25). Meanwhile, the results of the prospective randomized all-comers NOTION trial were recently published, showing no significant difference between TAVR and SAVR for the composite outcome of death from any cause, stroke, or myocardial infarction after 1 year in 280 low risk [mean Society of Thoracic Surgeons (STS) score: 3%] patients with aortic stenosis (7). However, the expansion of TAVR towards the treatment of lower risk and younger patients, especially with newer generation devices, definitely mandates continued surveillance and thorough clinical-trial validation.
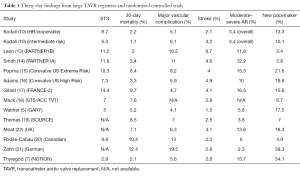
Kodali et al. (10) recently reported the early outcomes of a large, multicenter registry of inoperable, high-risk, and intermediate-risk patients with severe, symptomatic aortic stenosis who underwent TAVR with the SAPIEN 3 THV at 57 sites in the United States (US) and Canada. The vast majority of patients received their treatment through a transfemoral approach (n=1,443, 86.9%), and the alternative approaches (transapical or transaortic) were used in a minority of patients (n=218, 13.1%). The rate of 30-day all-cause mortality was as low as 2.2% and 1.1% in inoperable/high-risk and intermediate-risk patients respectively. TAVR with the new SAPIEN 3 valve was also associated with low rates of stroke, repeat hospitalization, and procedural complications including coronary obstruction, valve embolization, and annulus rupture. Meaningful improvements in quality of life and functional status were also noted following treatment with the SAPIEN 3 THV. Overall, moderate or severe paravalvular regurgitation was observed in only 3.4% of patients. Nevertheless, the rates of new permanent pacemaker implantation were 13.3% in inoperable/high-risk patients and 10.1% in intermediate-risk patients, which are higher than previously reported rates of pacemaker post-TAVR with balloon-expandable THVs. Interestingly, similar results have been obtained in other trials evaluating the SAPIEN 3 valve. In a nationwide prospective multicenter cohort study (Swiss Transcatheter Aortic Valve Implantation Registry), the use of the SAPIEN 3 valve reduced the risk of more than mild paravalvular regurgitation and vascular complications, but was linked with an increased risk of permanent pacemaker compared with the implantation of the SAPIEN XT valve (11). In a meta-analysis of 6 observational cohort studies comparing the SAPIEN 3 and the SAPIEN XT valves, the use of the SAPIEN 3 valve was associated with a lower rate of moderate to severe paravalvular regurgitation, major vascular complications and need for ballon post-dilation (12). Nonetheless, compared to the results of prior randomized controlled trials and other large registries (5,7,13-21), the early rate of mortality observed in the Kodali’s study (10) is the lowest ever reported in the TAVR field. Table 1 summarizes some of the most important 30-day outcomes in major TAVR studies and registries published to date.
Full table
As mentioned by the authors, factors including increased operator experience, better patient selection, the systematic use of multidetector computed tomography combined with some particular features of the SAPIEN 3 device might explain the low rates of paravalvular regurgitation, bleeding, and major vascular complications found in the study by Kodali et al. (10). These factors, in addition to improved patient selection, probably contributed to the very low mortality rates observed in this study. The SAPIEN 3 valve has an external polyethelene terephthalate cuff in order to enhance paravalvular sealing. This newer generation valve holds an improved open cell geometry of the stent frame to allow a very low delivery profile (14 or 16 Fr). Also, a better distal flex of the delivery system provides an enhanced positioning process and better coaxiality. Finally, compared to previous generation transcatheter ballon-expandable valves, the stent frame of the SAPIEN 3 is longer and foreshortens more during deployment. These latter features associated with different sizing and positioning guidelines can partially elucidate the marginally higher rate of new pacemaker implantation reported in this study. Of note, the SAPIEN 3 THV received CE (Communauté européenne) mark and US Food and Drug Administration approval in January 2014 and June 2015, respectively.
The early results obtained with this newer generation transcatheter valve represent a major step forward in the TAVR field. However, some limitations of the manuscript by Kodali et al. (10) should be acknowledged. Intrinsic limitations associated with this type of industry-sponsored registries include potential selection bias due to the non-randomized distribution of the therapies, and the presence of other unmeasured confounders. Another limitation is the inclusion of 2 cohorts of very different patients. For example, the intermediate risk patients were less symptomatic and had lower rates of coronary artery disease, peripheral vascular disease, chronic obstructive pulmonary disease, chronic renal insufficiency, atrial fibrillation, and severe pulmonary hypertension than the high-risk individuals. Additionally, the patients of the intermediate-risk cohort were enrolled after the high-risk cohort, which implies greater operator experience, and potentially improved patient screening and pre-procedural planning. Therefore, any direct comparison between the 2 cohorts would be certainly biased and difficult to interpret. Furthermore, it is important to emphasize that only the early (30 days) clinical and echocardiographic results were reported. Thus, longer term follow-up data will be needed before drawing any definitive conclusions.
Another aspect of the Kodali’s work relates to the evaluation of the procedural risk. The operative risk was determined by the heart team, which included one cardiac surgeon and one interventional cardiologist. If the STS risk score was >8% or if the heart team thought that the patient was at extreme or high surgical risk for other clinical motives, the patient was deemed high-risk or inoperable. On the other hand, patients were classified in the intermediate risk cohort if the STS was between 4% and 8% or based on the evaluation of the heart team. Although the “STS or Euroscore based” methodology to stratify TAVR candidates has been applied in the vast majority of studies, its validity is questionable. In fact, these surgical risk scores have not been designed or validated to assess the risk of mortality after transcatheter interventions. Even after refinement in the Euroscore II and the recent recalibration of the STS score, these tools tend to overestimate mortality risk after TAVR (23). Thus, the development of a TAVR dedicated risk score with superior calibration to predict outcome and to optimize the selection of TAVR patient still constitutes an unmet clinical need (24).
The current American College of Cardiology (ACC)/American Heart Association (AHA) guidelines (25) state that TAVR is recommended in patients who meet an indication for AVR who have a prohibitive risk for surgical AVR and a predicted post-TAVR survival greater than 12 months (class I, level of evidence: B). In high surgical risk patients the guidelines stipulate that TAVR is a reasonable alternative to surgical AVR (class IIa, level of evidence: B). However, in our opinion, TAVR should probably be elevated to a class I recommendation for high surgical risk patients who are good candidates for TAVR based on the available evidence including the data from Kodali’s work (10). On the other hand, surgical AVR is still recommended in patients who meet an indication for AVR with low or intermediate surgical risk (class I). The results of the PARTNER 2A (9) trial and those reported by Kodali et al. (10) provide the basis for including TAVR as a valid alternative for intermediate surgical risk patients. However, it would be important to continue gathering solid clinical data in this group of patients. Another trial including individuals at intermediate risk, the Safety and Efficacy of the Medtronic Corevalve System in the Treatment of Severe, Symptomatic Aortic Stenosis in Intermediate Risk Subjects who need Aortic Valve Replacement (SURTAVI) trial (NCT01586910), has recently completed its recruitment and will definitely provide useful information for the ever expanding TAVR data bank. In a step forward, the PARTNER 3 trial (NCT02675114) has recently been launched with the objective to demonstrate the non-inferiority of TAVR with the SAPIEN 3 valve vs. surgical AVR for the treatment of low risk (STS <4%) patients with severe aortic stenosis. This trial is of major importance for the expansion of the TAVR technology towards the treatment of the entire spectrum of aortic stenosis patients.
The future of TAVR is bright and full expansion of this technology for the treatment of most patients with aortic stenosis is likely to occur within the next few years. However, some economic issues regarding this expansion should be considered. Previous studies have demonstrated the cost-effectiveness of TAVR in inoperable and high-risk patients (26-28), but in a lower risk population where the cost of conventional surgical AVR is noticeably less, it is unclear whether this finding will still hold true (29). The costs of TAVR can be reduced by improvements in peri-procedural care and “minimalist” approach with earlier discharge (30), but a decrease in transcatheter valve costs will definitely be needed in order to make this therapy available and cost-effective worldwide. Hopefully, with an increasing number of transcatheter valves being manufactured, market forces will probably reduce the price of the devices, similar to the trend already observed with coronary stents (31).
In conclusion, Kodali et al. (10) showed that TAVR with the new SAPIEN 3 valve is associated with a low rate of major peri-procedural complications and mortality in patients at prohibitive, high and intermediate surgical risk. While a better understanding of the predictors of new pacemaker implantation after TAVR with the SAPIEN 3 valve is mandatory, the excellent results of this transcatheter valve will undoubtedly lead previous generation devices to fall into disuse. Also, future studies will need to confirm these outstanding early mortality results as well as the durability of the numerous THVs in intermediate risk patients. Nevertheless, the study by Kodali et al. (10) reinforces the rationale for implementing TAVR as a first-line treatment in high risk patients and as an alternative to surgery in those at intermediate risk. Furthermore, it certainly provides the basis for the final expansion of TAVR towards the treatment of lower risk patients.
Acknowledgements
Dr. Rodés-Cabau has received research grants from Edwards Lifesciences, Medtronic and St Jude Medical. Dr. Paradis has received research grant from St Jude Medical.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Cribier A, Eltchaninoff H, Bash A, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation 2002;106:3006-8. [Crossref] [PubMed]
- Makkar RR, Fontana GP, Jilaihawi H, et al. Transcatheter aortic-valve replacement for inoperable severe aortic stenosis. N Engl J Med 2012;366:1696-704. [Crossref] [PubMed]
- Kodali SK, Williams MR, Smith CR, et al. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med 2012;366:1686-95. [Crossref] [PubMed]
- Vahl TP, Kodali SK, Leon MB. Transcatheter Aortic Valve Replacement 2016: A Modern-Day "Through the Looking-Glass" Adventure. J Am Coll Cardiol 2016;67:1472-87. [Crossref] [PubMed]
- Walther T, Hamm CW, Schuler G, et al. Perioperative Results and Complications in 15,964 Transcatheter Aortic Valve Replacements: Prospective Data From the GARY Registry. J Am Coll Cardiol 2015;65:2173-80. [Crossref] [PubMed]
- Piazza N, Kalesan B, van Mieghem N, et al. A 3-center comparison of 1-year mortality outcomes between transcatheter aortic valve implantation and surgical aortic valve replacement on the basis of propensity score matching among intermediate-risk surgical patients. JACC Cardiovasc Interv 2013;6:443-51. [Crossref] [PubMed]
- Thyregod HG, Steinbrüchel DA, Ihlemann N, et al. Transcatheter Versus Surgical Aortic Valve Replacement in Patients With Severe Aortic Valve Stenosis: 1-Year Results From the All-Comers NOTION Randomized Clinical Trial. J Am Coll Cardiol 2015;65:2184-94. [Crossref] [PubMed]
- Mack MJ, Leon MB, Smith CR, et al. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet 2015;385:2477-84. [Crossref] [PubMed]
- Leon MB, Smith CR, Mack MJ, et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med 2016;374:1609-20. [Crossref] [PubMed]
- Kodali S, Thourani VH, White J, et al. Early clinical and echocardiographic outcomes after SAPIEN 3 transcatheter aortic valve replacement in inoperable, high-risk and intermediate-risk patients with aortic stenosis. Eur Heart J 2016;37:2252-62. [Crossref] [PubMed]
- Binder RK, Stortecky S, Heg D, et al. Procedural Results and Clinical Outcomes of Transcatheter Aortic Valve Implantation in Switzerland: An Observational Cohort Study of Sapien 3 Versus Sapien XT Transcatheter Heart Valves. Circ Cardiovasc Interv 2015;8:e002653. [Crossref] [PubMed]
- Ando T, Briasoulis A, Holmes AA, et al. Sapien 3 versus Sapien XT prosthetic valves in transcatheter aortic valve implantation: A meta-analysis. Int J Cardiol 2016;220:472-8. [Crossref] [PubMed]
- Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010;363:1597-607. [Crossref] [PubMed]
- Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011;364:2187-98. [Crossref] [PubMed]
- Popma JJ, Adams DH, Reardon MJ, et al. Transcatheter aortic valve replacement using a self-expanding bioprosthesis in patients with severe aortic stenosis at extreme risk for surgery. J Am Coll Cardiol 2014;63:1972-81. [Crossref] [PubMed]
- Adams DH, Popma JJ, Reardon MJ, et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med 2014;370:1790-8. [Crossref] [PubMed]
- Gilard M, Eltchaninoff H, Iung B, et al. Registry of transcatheter aortic-valve implantation in high-risk patients. N Engl J Med 2012;366:1705-15. [Crossref] [PubMed]
- Mack MJ, Brennan JM, Brindis R, et al. Outcomes following transcatheter aortic valve replacement in the United States. JAMA 2013;310:2069-77. [Crossref] [PubMed]
- Thomas M, Schymik G, Walther T, et al. Thirty-day results of the SAPIEN aortic Bioprosthesis European Outcome (SOURCE) Registry: A European registry of transcatheter aortic valve implantation using the Edwards SAPIEN valve. Circulation 2010;122:62-9. [Crossref] [PubMed]
- Rodés-Cabau J, Webb JG, Cheung A, et al. Transcatheter aortic valve implantation for the treatment of severe symptomatic aortic stenosis in patients at very high or prohibitive surgical risk: acute and late outcomes of the multicenter Canadian experience. J Am Coll Cardiol 2010;55:1080-90. [Crossref] [PubMed]
- Zahn R, Gerckens U, Grube E, et al. Transcatheter aortic valve implantation: first results from a multi-centre real-world registry. Eur Heart J 2011;32:198-204. [Crossref] [PubMed]
- Moat NE, Ludman P, de Belder MA, et al. Long-term outcomes after transcatheter aortic valve implantation in high-risk patients with severe aortic stenosis: the U.K. TAVI (United Kingdom Transcatheter Aortic Valve Implantation) Registry. J Am Coll Cardiol 2011;58:2130-8. [Crossref] [PubMed]
- Seiffert M, Sinning JM, Meyer A, et al. Development of a risk score for outcome after transcatheter aortic valve implantation. Clin Res Cardiol 2014;103:631-40. [Crossref] [PubMed]
- Puri R, Iung B, Cohen DJ, et al. TAVI or No TAVI: identifying patients unlikely to benefit from transcatheter aortic valve implantation. Eur Heart J 2016;37:2217-25. [Crossref] [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Thorac Cardiovasc Surg 2014;148:e1-e132. [Crossref] [PubMed]
- Reynolds MR, Magnuson EA, Wang K, et al. Cost-effectiveness of transcatheter aortic valve replacement compared with standard care among inoperable patients with severe aortic stenosis: results from the placement of aortic transcatheter valves (PARTNER) trial (Cohort B). Circulation 2012;125:1102-9. [Crossref] [PubMed]
- Reynolds MR, Magnuson EA, Lei Y, et al. Cost-effectiveness of transcatheter aortic valve replacement compared with surgical aortic valve replacement in high-risk patients with severe aortic stenosis: results of the PARTNER (Placement of Aortic Transcatheter Valves) trial (Cohort A). J Am Coll Cardiol 2012;60:2683-92. [Crossref] [PubMed]
- Fairbairn TA, Meads DM, Hulme C, et al. The cost-effectiveness of transcatheter aortic valve implantation versus surgical aortic valve replacement in patients with severe aortic stenosis at high operative risk. Heart 2013;99:914-20. [Crossref] [PubMed]
- Osnabrugge RL, Head SJ, Genders TS, et al. Costs of transcatheter versus surgical aortic valve replacement in intermediate-risk patients. Ann Thorac Surg 2012;94:1954-60. [Crossref] [PubMed]
- Noad RL, Johnston N, McKinley A, et al. A pathway to earlier discharge following TAVI: Assessment of safety and resource utilization. Catheter Cardiovasc Interv 2016;87:134-42. [Crossref] [PubMed]
- Brown A, Meenan BJ, Young TP. Marketing innovation: medical device prices follow the experience curve. J Med Market 2007;7:203-12. [Crossref]


