Angiotensin II type-2 receptor-specific effects on the cardiovascular system
Introduction
The renin-angiotensin system (RAS) plays a critical role in cardiovascular homeostasis. The principle effector of this system is angiotensin II (Ang II), which acts at least four different receptors subtypes (ATIIR 1-4). AT1R and AT2R receptors cDNAs have been cloned successfully almost two decade ago (1). Most of the classic cardiovascular effects of the Ang II, including blood pressure regulation, promotion of inflammatory responses, arterial wall thickening, and myocardial fibrosis, have been demonstrated to be mediated by the AT1R .The AT2R is clearly distinct from the AT1R in molecular weight, tissue-specific expression, and signaling mechanisms. Past studies have demonstrated that AT2R may counter-regulate AT1R function. However, the specific role of the AT2R is incompletely understood, with contradictory results in the various studies. As there is an increasing number of drugs with effect on the RAS, including angiotension converting enzyme inhibitors (ACEI) and angiotension receptor blocker (ARB), used in clinical practice, it becomes essential to clarify the specific function of the AT2R.
Gene location and gene polymorphisms
The genes encoding the AT2R are localized on human chromosome Xq22-q2 (1). The genomic DNA of the human AT2R consists of three exons with an uninterrupted coding region being confined to the third exon (2). Both AT1R and AT2R belong to the seven-transmembrane domain superfamily of receptors, and they share 34% of their nucleic acid sequence. Human AT2R molecular weight is 41 kDa, with 363 amino-acids (3). Mouse and human AT2R both have more than 92% homology with the rat homolog (4). Several single nucleotide polymorphic (SNP) variants of human AT2R are known. A study about genetic variants of AT2R (1675A>G and 3123C>A) and cardiovascular risk in 2579 subjects suggests there is no association between coronary heart disease (CHD) risk and 1675A versus 1675G SNP variants. However, the 1675A genotype is associated with a higher risk of CHD in subjects with systolic hypertension, whereas 1675G carriers were protected from the effect of hypertension, with no association between increasing risk and increasing SBP. Furthermore, individuals carrying both 3123A and 1675A SNPs have a tenfold greater risk of CHD than 3123A and 1675G haplotypes (5). Other studies have focused on the association between polymorphism of AT2R 1332A>G SNPs and cardiovascular disease and conclude that 1332G SNP variants have a higher prevalence of hypertension (6) and premature coronary artery disease (7).
AT2R expression
The AT2 receptors are highly expressed in fetal tissues, although their expression dramatically decreases after birth, being restricted to a few organs, including the cardiovascular system (8), suggesting it might play an important role in fetal cardiovascular development. But recent data using Western blot technique to measure total AT2R protein in tissue, showed adult rats exhibited a higher AT2R protein level compared with foetus or neonates in the brainstem, liver and kidney tissue (9). In the cardiovascular system, AT2R expresses in the heart (cardiomyocytes (10), cardiac fibroblasts (11)), and vessels (aorta (12), coronary artery (13), resistant artery (14)). In pathological conditions, including inflammation, congestive heart failure, hypertension, myocardial ischemia and vascular injury, AT2R can be regulated (8). For example, a previous study (12) demonstrated that AT2R expression was increased in segments from diseased compared with control aortas, whereas AT2R was down-regulated in resistance arteries of hypertensive subjects compared with adult SHR and WKY rats. After RAS specific and nonspecific antihypertensive treatment, AT2R expression and vasodilator functions could be reversed (14). These data suggest complex local regulation of AT2R expression, which may medicate vascular growth, development, and repair (Figure 1).
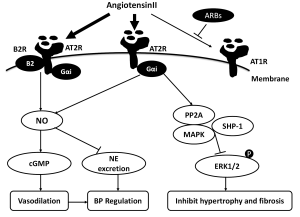
Vasodilation
It is generally accepted that Ang II mediate vasodilation through the AT2R pathway, opposing the vasoconstrictor action of AT1R. Studies in the mid to late 1990s indicated links between AT2R and vasodilators including bradykinin(BK), nitric oxide(NO), and guanosine cyclic 3', 5'-monoghosphate (cGMP) (15-17). Recently, the AT2 receptor and bradykinin B2 receptor were shown to form a stable functional heterodimer, which leads to increased NO and cGMP production (18). These discoveries turned attention to the possibility that the AT2R may medicate vasodilation. However, it is difficult to elicit a vasodilatory response directly in animal models, because AT2R expression is extremely lower than AT1R. To unmask the vasodilator action of AT2R, experiments have focused on eliminating the vasoconstrictor action of AT1R with an AT1R blocker before and during AT2R stimulation. Most studies demonstrated that the AT2R mediate vasodilator response in animal models and human resistance vessels when AT1R were blocked in acute and chronic experiments. For instant, in the presence of AT1R blockade, AngII induced endothelial AT2R cause vasodilation through local production of BK in resistant arteries of rat mesentery in a flow-dependent manner (19). In hypertensive rats, chronic AT1R blockade is also associated with an reversed vasomotor response to Ang II via AT2R mediated NO production (20). In a study by Savoia et al., AT2R expression was up-regulated and mediated vasodilation during selective AT1R blockade for 1 year in resistance vessels of high-risk patients (21). Batenburg et al. (13), studying human coronary microarteries, demonstrated that Ang II-induced contraction was potentiated by AT2 receptor blockade and this phenomenon was abolished by icatibant, L-NAME, or removal of the endothelium. This study demonstrated functional AT2 vasodilator receptors in the coronary microcirculation that use a BK-NO-cGMP signaling pathway. In addition to resistance vessels, the vasolilator role of AT2R in capacitance vessels was confirmed in recent studies. Yayama et al. tested the contractile response of thoracic aorta to AngII under pressure-overload by abdominal aortic banding in rats. An AT2 receptor antagonist could increase the Ang II responsiveness (22). Subsequent studies used an AT2 receptor activator to directly investigate the role of AT2R in vitro. Compound21, a newly created non-peptide AT2R agonist, evoked dose-dependent vasorelaxation in aortic and mesenteric vessels, abolished by PD123319 (23). Moltzer et al. found that PD123319 enhanced the constrictive effects of Ang II and III in coronary arteries in Wistar rats, but did not alter the responses in SHRs. In fact, the coronary constrictor effects of Ang II and III in SHRs in the absence of PD123319 were as large as their coronary constrictor effects in Wistar rats in the presence of PD123319. This suggests that the main reason for the enhanced coronary constrictor effects in SHRs is the lack of counterregulatory AT2 receptor-mediated coronary vasodilation (24). In contrast a study by You et al. showed that AT2R stimulation induces a vasoconstriction in untreated SHR resistance arteries. Specific or non-specific anti-hypertensive treatments for four weeks may restore vasodilator function (14).
Blood pressure regulation
In the mid 1990s, Scheuer et al. (25) found that during AT1R blockade, Ang II and AngIII cause a hypotensive response, which was eliminated by AT2R antagonist. This observation demonstrated that the AT2 receptors mediate a depressor response to Angiotensin. AT2R knockout models (AT2R-KO) developed normal blood pressure, but showed an increased vasopressor respond after injection of Ang II (26), while chronic infusion of Ang II into mice with overexpression of AT2-TG mice completely abolished the AT1-mediated pressor effect (27). A recent study demonstrated that low dose of Ang II may significantly reduce BP in female rats through the AT2R pathway (28) and PD123319 treatment of obese Zucker rats raised mean arterial pressure by 13 mmHg (29), while Compund21 may reduce blood pressure in SHR when combined with the AT1R antagonist, candesartan (23). It is generally accepted that AT2R contribute to maintenance of blood pressure by controlling the vascular tone through vasodilation. Recently, studies suggested a potential inhibitory effect of stimulating central AT2Rs on blood pressure, which is likely mediated by sympatho-inhibition. As mentioned before, AT2R shows high expression in brainstem, which may mediate AngII function in central nervous system. Gao et al. (30) demonstrated that overexpression of AT2R protein in the rostral ventrolateral medulla (RVLM; a primary brainstem nucleus related to the control of sympathetic outflow) suppressed norepinephrine excretion and reduced arterial blood pressure in normal rats. In addition, chronic infusion of Compund 21 decreased nocturnal norepinephrine (NE) excretion and blood pressure via a nNOS/NO signaling pathway within PVN (Paraventricular nucleus) and RVLM. In summary, AT2R is involved in blood pressure regulation, but the detailed mechanism are incompletely understood.
Inhibition of myocardial hypertrophy and fibrosis
AngII mediate cell proliferation and ACEI inhibit left ventricular hypertrophy independent of the effect on blood pressure. Previous studies have examined the role of AT1R and AT2R in myocardial hypertrophy and fibrosis. In early studies, Booz et al. have shown that angiotensin II stimulates increased hypertrophy in isolated neonatal rat cardiac myocytes, measured by an increasing protein/DNA ratio. This action was blocked by specific AT1 receptor antagonists, while AT2 antagonists enhanced Ang II stimulation of protein synthesis (10). Similar results were shown in aged rats: AT1R antagonists significantly reduced cardiac hypertrophy and fibrosis while these structural changes were reversed by concomitant AT2 antagonist administration (31). Persistent cardiac overexpression of the AT2R resulted in an 85% attenuation of left ventricular wall thickness, 91% attenuation of heart weight to body weight ratios, and a 43% decrease in myocardial fibrosis induced by angiotensin infusion in rats (32). Aortic banding in mice with overexpression of AT2R-TG resulted in reduction of left ventricular hypertrophy as demonstrated by decreased cardiomyocyte diameter and collagen content (33). These studies clearly demonstrate that AT2R plays a functional role in the cardiac hypertrophic and fibrosis process in vivo, and AT2R may mediate these functions by selectively regulating the expression of growth-promoting and growth-inhibiting factors. However, contradictory results were demonstrated in AT2R-KO mice. Ang II elevated systolic blood pressure to comparable levels in AT2R-KO and WT mice. WT mice developed prominent concentric cardiac hypertrophy and prominent fibrosis while there was no significant change in AT2R-KO mice (34). D’Amore et al. used recombinant adenoviruses expressing different ratios of rat AT1R and AT2R to infect cardiomyocytes cultured from neonatal rat hearts to study their combined effects on hypertrophy of the cells in response to Ang II. The results showed that basal and Ang II-mediated hypertrophy was increased with the amplified expression of the AT2R and this was unaffected by Ang II stimulation or the classic AT2 antagonists, and Ang II stimulation promoted hypertrophy via activation of mitogen-activated protein kinases (MAPKs) (35). The authors concluded that the AT2R could play a deleterious role in cardiac hypertrophy. Recently, Ha et al. found septal hypertrophy and upregulated AT2R in young rats with early obesity. However, it is not clear whether septal hypertrophy was associated with an increase of AT2R, although the author regarded AT2R as a cardioprotective effector (36).
Inhibition of arterial hypertrophy and fibrosis
As mentioned before, AT2R is highly expressed in the fetal heart and vessel system, declines rapidly after birth, but may increases after vascular injury. This suggests an important role in the remodeling of vascular tissue. In 1996, Levy et al. showed that long and chronic blockade of AT2R in Ang II-induced hypertensive rats had no effect on arterial pressure, but antagonized the effect of Ang II on arterial hypertrophy and fibrosis, suggesting AT2R could play a deleterious role in the process of vascular remodeling (37). However, subsequent data showed AT2R may protect the function of vascular tissue. Brede et al. compared isolated femoral arteries from AT2R-KO mice and WT mice, and demonstrated enhanced vasoconstriction of femoral arteries from AT2R-KO mice to angiotensin II. Morphometric analysis of large and small femoral arteries revealed significant hypertrophy of smooth muscle cells in the media (38). Similar results were also found in aged rats. Jones et al. showed that cardiac hypertrophy and fibrosis, and aortic hypertrophy were all significantly reduced by candesartan cilexetil and these structural changes were reversed by concomitant PD123319 administration, which demonstrate AT2R may inhibit cardiovascular hypertrophy and fibrosis in the ageing heart and vasculature (31). Okumura et al. found that arterial AT2R expression and plasma estrogen level were lower in aged than young female WT mice, and no significant change of AT1R between aged female mice and young female mice (39). In addition, estrogen supplementation and ARB treatment showed synergistic inhibition of atherosclerosis (40). It has therefore been suggested that AT2R may be involved in the response to estrogen and improvement of vascular remodeling in the aged female group. Recently, Habashi et al. showed that loss of AT2 expression accelerates the aberrant growth and rupture of the aorta in a mouse model of Marfan syndrome (MFS). ARB was more effective than ACEI to arrest aneurysm progression in the mice. Those data highlight the protective role of AT2R with potential impact on future choice of therapies in MFS (41). The signal pathway for antiproliferation and antigrowth function of AT2R is not clear. A lot of evidence demonstrated that extracellular signal-regulated kinases 1 and 2 (ERK1/2) were desphosphorylated when AT2R activation (42), and Src homology region 2 domaincontaining phosphatase (SHP-1), MAPKs, and protein phosphatase 2A (PP2A) may involved in the process of activation (43-45). But D’Amore et al.’s study showed showed that adenovirus-mediated over-expression of AT2 receptors in neonatal cardiomyocytes increased growth in a constitutive and ERK1/2-independent manner (35).
Cardiac protective function after myocardial infarction
In an early study, Jalowy et al. found that the AT1R antagonist candesartan reduced the infarct size in pigs. Surprisingly, this protective function could be abolished after pretreatment with an AT2R antagonist (46). The author suggested that AT2R activation may be involved in the therapeutic effect of the candesartan. Yang et al. studied cardiac function at baseline and after myocardial infarction (MI) in mice with overexpression of AT2-TG compared with WT mice. The study demonstrated that AT2-TG mice developed a significantly smaller end-systolic volume index (ESVI) and higher ejection (EF) fraction than WT mice. There were no differences on heart rate, blood pressure and stroke volume after MI, but ESVI remained lower and EF higher at each time point up to day 28 post-MI. At day 28, LV wall thickness, filling pressures, and contractile function were higher in AT2-TG than WT mice (47). In contrast to overexpression of AT2R, Brede et al. studied AT2R-KO mice after myocardial infarction, which led to increases in heart: body weight ratios and myocardial cross-sectional areas compared with WT mice. The AT2R-KO mice had down-regulation of myocardial endothelial nitric oxide synthase and reduced cGMP levels (48). Adachi et al. also demonstrated that AT2 receptor deficiency exacerbates short-term death rates and heart failure after experimental AMI in AT2-KO mice (49). Oishi et al. (50) demonstrated that AT2R-KO mice have greater cardiac remodeling/hypertrophy, systolic and diastolic dysfunction, and increased mortality after MI compared with WT mice. In contrast to the previous study, the cardioprotective role of the AT2 receptor was independent of myocyte hypertrophy. Furthermore, Compound 21, given for 7 days, can improve the ventricular function and reduce the scar area by anti-inflammation way after MI in rats (51).
Conclusions
A large number of studies have shown that the AT2 receptor plays a major role as a AT1 receptor antagonist, but other studies challenge this observation. Diverse expression and function of AT2R in different individuals, tissue, and pathological conditions may explain these contradictory results. ARB is widely used for patients with hypertension and other cardiovascular pathologies. It predominantly blocks the Ang II AT1R to achieve its clinical effect. Obviously, in the setting of AT1R blockage by ARB molecules, increasing amount of Ang II will combine with the AT2R, with incompletely understood effect on cardiovascular homeostasis. Therefore to understand the pharmacological effects of ARB, further studies are necessary to clarify the cardiovascular effects of the AT2R.
Acknowledgments
This work was partly supported by the National Basic Research Program of China (2011CB512001), and the International Scientific and Technological Cooperation Project of Hunan (2010WK2006).
Disclosure: The authors declare no conflict of interest.
References
- Koike G, Horiuchi M, Yamada T, et al. Human type 2 angiotensin II receptor gene: cloned, mapped to the X chromosome, and its mRNA is expressed in the human lung. Biochem Biophys Res Commun 1994;203:1842-50.
- de Gasparo M, Catt KJ, Inagami T, et al. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev 2000;52:415-72.
- Kambayashi Y, Bardhan S, Takahashi K, et al. Molecular cloning of a novel angiotensin II receptor isoform involved in phosphotyrosine phosphatase inhibition. J Biol Chem 1993;268:24543-6.
- Mukoyama M, Nakajima M, Horiuchi M, et al. Expression cloning of type 2 angiotensin II receptor reveals a unique class of seven-transmembrane receptors. J Biol Chem 1993;268(33):24539-42.
- Jones A, Dhamrait SS, Payne JR, et al. Genetic variants of angiotensin II receptors and cardiovascular risk in hypertension. Hypertension 2003;42:500-6.
- Zivković M, Djurić T, Stancić O, et al. X-linked angiotensin II type 2 receptor gene polymorphism -1332A/G in male patients with essential hypertension. Clin Chim Acta 2007;386:110-3.
- Alfakih K, Brown B, Lawrance RA, et al. Effect of a common X-linked angiotensin II type 2-receptor gene polymorphism (-1332 G/A) on the occurrence of premature myocardial infarction and stenotic atherosclerosis requiring revascularization. Atherosclerosis 2007;195:e32-8.
- Levy BI. How to explain the differences between renin angiotensin system modulators. Am J Hypertens 2005;18:134S-141S.
- Yu L, Zheng M, Wang W, et al. Developmental changes in AT1 and AT2 receptor-protein expression in rats. J Renin Angiotensin Aldosterone Syst 2010;11:214-21.
- Booz GW, Baker KM. Role of type 1 and type 2 angiotensin receptors in angiotensin II-induced cardiomyocyte hypertrophy. Hypertension 1996;28:635-40.
- Tsutsumi Y, Matsubara H, Ohkubo N, et al. Angiotensin II type 2 receptor is upregulated in human heart with interstitial fibrosis, and cardiac fibroblasts are the major cell type for its expression. Circ Res 1998;83:1035-46.
- Baños M, Arellano-Mendoza MG, Vargas-Robles H, et al. Relationship between angiotensin II receptor expression and cardiovascular risk factors in Mexican patients with coronary occlusive disease. Exp Mol Pathol 2011;91(1):478-83.
- Batenburg WW, Garrelds IM, Bernasconi CC, et al. Angiotensin II type 2 receptor-mediated vasodilation in human coronary microarteries. Circulation 2004;109:2296-301.
- You D, Loufrani L, Baron C, et al. High blood pressure reduction reverses angiotensin II type 2 receptor-mediated vasoconstriction into vasodilation in spontaneously hypertensive rats. Circulation 2005;111:1006-11.
- Siragy HM, Carey RM. The subtype-2 (AT2) angiotensin receptor regulates renal cyclic guanosine 3', 5'-monophosphate and AT1 receptor-mediated prostaglandin E2 production in conscious rats. J Clin Invest 1996;97:1978-82.
- Siragy HM, Jaffa AA, Margolius HS, et al. Renin-angiotensin system modulates renal bradykinin production. Am J Physiol 1996;271:R1090-5.
- Siragy HM, Carey RM. The subtype 2 (AT2) angiotensin receptor mediates renal production of nitric oxide in conscious rats. J Clin Invest 1997;100:264-9.
- Abadir PM, Periasamy A, Carey RM, et al. Angiotensin II type 2 receptor-bradykinin B2 receptor functional heterodimerization. Hypertension 2006;48:316-22.
- Katada J, Majima M. AT(2) receptor-dependent vasodilation is mediated by activation of vascular kinin generation under flow conditions. Br J Pharmacol 2002;136:484-91.
- Cosentino F, Savoia C, De Paolis P, et al. Angiotensin II type 2 receptors contribute to vascular responses in spontaneously hypertensive rats treated with angiotensin II type 1 receptor antagonists. Am J Hypertens 2005;18:493-9.
- Savoia C, Touyz RM, Volpe M, et al. Angiotensin type 2 receptor in resistance arteries of type 2 diabetic hypertensive patients. Hypertension 2007;49:341-6.
- Yayama K, Horii M, Hiyoshi H, et al. Up-regulation of angiotensin II type 2 receptor in rat thoracic aorta by pressure-overload. J Pharmacol Exp Ther 2004;308:736-43.
- Bosnyak S, Welungoda IK, Hallberg A, et al. Stimulation of angiotensin AT2 receptors by the non-peptide agonist, Compound 21, evokes vasodepressor effects in conscious spontaneously hypertensive rats. Br J Pharmacol 2010;159:709-16.
- Moltzer E, Verkuil AV, van Veghel R, et al. Effects of angiotensin metabolites in the coronary vascular bed of the spontaneously hypertensive rat: loss of angiotensin II type 2 receptor-mediated vasodilation. Hypertension 2010;55:516-22.
- Scheuer DA, Perrone MH. Angiotensin type 2 receptors mediate depressor phase of biphasic pressure response to angiotensin. Am J Physiol 1993;264:R917-23.
- Hein L, Barsh GS, Pratt RE, et al. Behavioural and cardiovascular effects of disrupting the angiotensin II type-2 receptor in mice. Nature 1995;377:744-7.
- Tsutsumi Y, Matsubara H, Masaki H, et al. Angiotensin II type 2 receptor overexpression activates the vascular kinin system and causes vasodilation. J Clin Invest 1999;104:925-35.
- Sampson AK, Moritz KM, Jones ES, et al. Enhanced angiotensin II type 2 receptor mechanisms mediate decreases in arterial pressure attributable to chronic low-dose angiotensin II in female rats. Hypertension 2008;52:666-71.
- Siddiqui AH, Ali Q, Hussain T. Protective role of angiotensin II subtype 2 receptor in blood pressure increase in obese Zucker rats. Hypertension 2009;53:256-61.
- Gao L, Zucker IH. AT2 receptor signaling and sympathetic regulation. Curr Opin Pharmacol 2011;11:124-30.
- Jones ES, Black MJ, Widdop RE. Angiotensin AT2 receptor contributes to cardiovascular remodelling of aged rats during chronic AT1 receptor blockade. J Mol Cell Cardiol 2004;37:1023-30.
- Falcón BL, Stewart JM, Bourassa E, et al. Angiotensin II type 2 receptor gene transfer elicits cardioprotective effects in an angiotensin II infusion rat model of hypertension. Physiol Genomics 2004;19:255-61.
- Yan X, Schuldt AJ, Price RL, et al. Pressure overload-induced hypertrophy in transgenic mice selectively overexpressing AT2 receptors in ventricular myocytes. Am J Physiol Heart Circ Physiol 2008;294:H1274-81.
- Ichihara S, Senbonmatsu T, Price E Jr, et al. Angiotensin II type 2 receptor is essential for left ventricular hypertrophy and cardiac fibrosis in chronic angiotensin II-induced hypertension. Circulation 2001;104:346-51.
- D'Amore A, Black MJ, Thomas WG. The angiotensin II type 2 receptor causes constitutive growth of cardiomyocytes and does not antagonize angiotensin II type 1 receptor-mediated hypertrophy. Hypertension 2005;46:1347-54.
- Ha KS, Yoo KH, Yim HE, et al. Cellular and RAS changes in the hearts of young obese rats. Pediatr Cardiol 2011;32:659-66.
- Levy BI, Benessiano J, Henrion D, et al. Chronic blockade of AT2-subtype receptors prevents the effect of angiotensin II on the rat vascular structure. J Clin Invest 1996;98:418-25.
- Brede M, Hadamek K, Meinel L, et al. Vascular hypertrophy and increased P70S6 kinase in mice lacking the angiotensin II AT(2) receptor. Circulation 2001;104:2602-7.
- Okumura M, Iwai M, Nakaoka H, et al. Possible involvement of AT2 receptor dysfunction in age-related gender difference in vascular remodeling. J Am Soc Hypertens 2011;5:76-84.
- Tsuda M, Iwai M, Li JM, et al. Inhibitory effects of AT1 receptor blocker, olmesartan, and estrogen on atherosclerosis via anti-oxidative stress. Hypertension 2005;45:545-51.
- Habashi JP, Doyle JJ, Holm TM, et al. Angiotensin II type 2 receptor signaling attenuates aortic aneurysm in mice through ERK antagonism. Science 2011;332:361-5.
- Porrello ER, Delbridge LM, Thomas WG. The angiotensin II type 2 (AT2) receptor: an enigmatic seven transmembrane receptor. Front Biosci 2009;14:958-72.
- Bedecs K, Elbaz N, Sutren M, et al. Angiotensin II type 2 receptors mediate inhibition of mitogen-activated protein kinase cascade and functional activation of SHP-1 tyrosine phosphatase. Biochem J 1997;325:449-54.
- Fischer TA, Singh K, O'Hara DS, et al. Role of AT1 and AT2 receptors in regulation of MAPKs and MKP-1 by ANG II in adult cardiac myocytes. Am J Physiol 1998;275:H906-16.
- Huang XC, Richards EM, Sumners C. Angiotensin II type 2 receptor-mediated stimulation of protein phosphatase 2A in rat hypothalamic/brainstem neuronal cocultures. J Neurochem 1995;65:2131-7.
- Jalowy A, Schulz R, Dörge H, et al. Infarct size reduction by AT1-receptor blockade through a signal cascade of AT2-receptor activation, bradykinin and prostaglandins in pigs. J Am Coll Cardiol 1998;32:1787-96.
- Yang Z, Bove CM, French BA, et al. Angiotensin II type 2 receptor overexpression preserves left ventricular function after myocardial infarction. Circulation 2002;106:106-11.
- Brede M, Roell W, Ritter O, et al. Cardiac hypertrophy is associated with decreased eNOS expression in angiotensin AT2 receptor-deficient mice. Hypertension 2003;42:1177-82.
- Adachi Y, Saito Y, Kishimoto I, et al. Angiotensin II type 2 receptor deficiency exacerbates heart failure and reduces survival after acute myocardial infarction in mice. Circulation 2003;107:2406-8.
- Oishi Y, Ozono R, Yoshizumi M, et al. AT2 receptor mediates the cardioprotective effects of AT1 receptor antagonist in post-myocardial infarction remodeling. Life Sci 2006;80:82-8.
- Kaschina E, Grzesiak A, Li J, et al. Angiotensin II type 2 receptor stimulation: a novel option of therapeutic interference with the renin-angiotensin system in myocardial infarction? Circulation 2008;118:2523-32.


