Advanced imaging in acute and chronic deep vein thrombosis
Introduction
Deep venous thrombosis (DVT) is a major cause of morbidity and mortality all over the world. It commonly occurs in the lower extremity and is associated with life threatening complication of pulmonary embolism (PE) referred together as venous thrombo-embolism (VTE) (1,2). While the precise number of people affected by VTE is unknown, as per the latest CDC data and statistics published in June 2015 as many as 900,000 people could be affected (1 to 2 per 1,000) each year in the United States and about 60,000–100,000 die of VTE, one-half have long-term complications (post-thrombotic syndrome) and one-third will have a recurrence within 10 years. In comparison, upper extremity DVT has much lower incidence in the range of 4–11% (3-5).
The main cause of DVT is stasis of the blood flow. The common risk factors for upper and lower extremity DVT are trauma, malignancies and side effects of their treatment, sepsis, past DVT or PE, inherited blood clotting disorders (thrombophilias), hormone therapy (for birth control or postmenopausal symptoms), pregnancy or recent delivery and varicose veins. In addition to these, specific predisposing factors for upper extremity DVT are the use of central venous catheters, pacemakers and automated implant defibrillators (6), effort thrombosis in otherwise healthy individuals (Paget-Schroetter syndrome) and thoracic outlet obstruction related to anatomic anomalies (7).
Clinical diagnosis of DVT is unreliable and routinely used laboratory screening tests such as D dimer tend to have high sensitivity but a very low specificity (8-10). Thus there is a need for more accurate and noninvasive diagnostic tests, not only to diagnose DVT but also for proper localization and monitoring during and after treatment. Imaging tests by their virtue of readily identifying, localizing the DVT are widely used in routine clinical care.
The imaging of DVT has evolved over the past few decades from conventional contrast venography (first described in 1963) (11) and duplex sonography in 1980s to computed tomography (CT)/magnetic resonance venography (MR venography) and scintigraphy and the latest molecular imaging/nanotechnology.
The purpose of this article is to review the established modalities used for characterization and diagnosis of DVT, and further explore promising innovations and recent advances in this field, all of which when taken together may have a positive impact on the diagnosis and treatment of VTE.
Contrast venography
Historically, contrast venography was the first imaging procedure available for diagnosing DVT and is still considered the gold standard with clot being identified as a filling defect or non-opacification of the vein (12,13). However, it is an invasive procedure that requires expertise and large volume of intravenous contrast. This method is also associated with a small risk of contrast reaction and iatrogenic venous thrombosis. With advent of newer, safer and cheaper techniques like CT venography (CTV) it is seldom performed except when concurrent intervention is planned.
Ultrasound (US)
US is used in evaluation of both symptomatic and asymptomatic DVT (patients at high risk of DVT). It is useful not only in assessing DVT but can also identify other conditions causing signs and symptoms indistinguishable from DVT.
Compression US
Compression US has been procedure of choice for investigation of suspected upper and lower extremity DVT for decades (14). Other modification to this technique like two-point compression US (15), extended compression US (16) and complete compression US (17) are used in different combinations at different institutions.
Venous duplex US
Lower extremity venous duplex US combines 2 components to assess for DVT: B-mode or gray-scale imaging with transducer compression maneuvers and Doppler evaluation consisting of color-flow Doppler imaging and spectral Doppler waveform analysis. Respiratory phasicity and cessation of flow with the valsalva maneuver offer indirect evidence of patent abdominal and pelvic veins (18).
The primary diagnostic US criteria for acute DVT remains non-compressibility of the vein with secondary diagnostic criteria being echogenic thrombus within the vein lumen, venous distention, complete absence of spectral or color Doppler signal within the vein lumen, loss of flow phasicity, and loss of response to valsalva or augmentation (18). US can also be used to differentiate acute from chronic thrombus. In acute thrombosis, vein is distended by hypoechoic thrombus and shows partial or no compressibility without collaterals (Figure 1). In chronic thrombosis, the vein is incompressible, narrow and irregular and shows echogenic thrombus attached to the venous walls with development of collaterals (Figure 2).
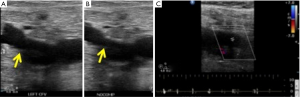
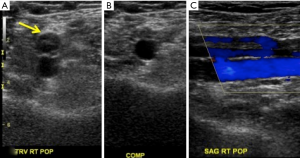
According to American College of Radiology (ACR) guidelines and technical standards, lower extremity US should include compression, color and spectral Doppler sonography with assessment of phasicity and venous flow augmentation (19,20).
Advantages of lower extremity venous Duplex US are that it is readily available, quick, cost effective, noninvasive, devoid of ionizing radiation, lacks need for intravenous contrast and can be portable for critically ill patients prone for developing DVT.
Limitations include that it is difficult and less sensitive in patients with obesity, edema, tenderness, recent hip or knee arthroplasty, cast, overlying bandages and immobilization devices. It also has limitations in patients who had previous DVT and have new symptoms shortly after the treatment. False-positive results include extrinsic compression of a vein by a pelvic mass or other perivascular pathology (21) and thrombosis in the distal popliteal vein. False-negative studies may occur in the presence of calf DVT, proximal DVT in asymptomatic (even high-risk) patients or in the presence of a thrombosed duplicated venous segment.
In a systematic review of accuracy of US in diagnosis of DVT in asymptomatic patients, Kassai et al. suggested that US was accurate in proximal veins for diagnosis of DVT in patients hospitalized for orthopaedic surgery (11) with lower sensitivity in other settings.
Sonographic elasticity imaging (SEI)
As described previously venous duplex US is considered primary noninvasive imaging for DVT. However, this method cannot assess the age and maturity of the thrombus i.e., it cannot distinguish pure post thrombotic syndrome (PTS) (which develops in 20–50% of patients after DVT) from new development of acute DVT with or without PTS (22). It is important to distinguish acute on chronic DVT from PTS as the latter doesn’t require anticoagulant therapy with highly potent fast acting anticoagulants associated with high risk of bleeding (23). Also chronic clots are treated with oral warfarin sodium, which has better safety profile than heparin.
SEI is the latest promising technique for estimating age of the thrombus. It uses tissue deformation to assess the tissue hardness and hence clot maturity (24,25). It has shown promising results in animals and in a few studies in humans. As SEI requires tissue to be deformed during imaging, it is consistent with venous duplex US which also requires compression. The degree of compression required for standard strain measurements on SEI is lesser than compression US that is beneficial for patients with swollen painful legs (26-28). Thus, even if evaluation of clot age does not work, just the use of SEI to detect thrombi may be an improvement over the present compression US technique.
SEI can hence be incorporated into standard duplex US so that thrombus can be diagnosed and presumably aged simultaneously.
SEI is in preliminary stages of investigations and there is limited data on its ability to determine the age of DVT in human subjects (26,29,30). It is highly operator dependent. Another limitation is that comparison between the two clots requires an internal standard with the same hardness in both images, as it is difficult to know the force that was used to deform the tissue in each case. Rubin et al. used the wall of the vein as standard but the hardness difference estimate was conservative due to lack of another standard reference (31). Another limitation is that it is difficult to distinguish between subacute and chronic thrombi that are closer in age (29).
CT for VTE
Rapid technological advances in the CT scanners in the past couple of decades have revolutionized the imaging of DVT and PE. CT pulmonary angiography (CTPA) has become initial modality of choice in evaluating patients with suspected PE (32). CTV of the pelvic and lower extremity veins after CTPA can be used for concurrent DVT detection. This is of added value since DVT is the most important predisposing factor for developing PE. This single stop examination offers distinct advantages and increases cost effectiveness (33,34).
Indirect CTV
Combined CTPA-CTV can depict cases of unsuspected DVT in patients suspected of having PE with studies claiming indirect CTV to be as accurate as US in diagnosing DVT (35-37). Combining CTPA with CTV has increased the sensitivity from 83% to 90% but with no change in specificity (38).
PE has a causal relationship with DVT and PE occurs in 50–60% of untreated cases of DVT with mortality rate of 25–30% (39,40). Also the prevalence of DVT in patients with acute PE has been reported to be 13–93% (13). Indirect CTV is a reasonable alternative to US in critically ill patients who have to undergo CTPA. Many studies concurred that detection of DVT in patients with PE is closely related to the success of the treatment, the follow up and the mortality rate (41). CTV may demonstrate both PE and DVT without use of additional contrast by added delayed imaging (Figure 3). It is particularly useful in detecting DVT in asymptomatic patients.
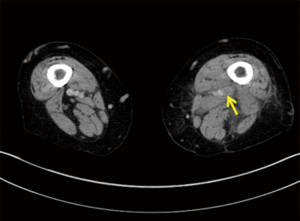
CTV offers definite advantage of evaluating the pelvic veins and inferior vena cava (IVC), difficult to assess on US. Also, other clinical conditions that simulate pain and swelling and incidental pelvic malignancy causing extrinsic venous compression can be detected concurrently on CTV (40).
One of the latest meta-analysis showed CTV has sensitivity ranging from 71% to 100% and specificity ranging from 93–100% for diagnosis of proximal DVT (42). Additionally, in 17% of patients with DVT, CTV delineated pelvic or abdominal thrombi, which are difficult or impossible to assess with US (43). This possible alternative to US is less operator-dependent and may be particularly useful in selected situations such as DVT in calf veins, in very obese patients or when pelvic or abdominal thrombus identification is desired (44).
Limitations include streak artifact from orthopaedic implants, poor venous enhancement and errors in interpretation due to adjacent pathology and reader inexperience. Other drawbacks are increased amount of contrast (approximately 150 mL of iodinated contrast) required which is more than the amount required for opacification of pulmonary arteries and can be of concern in critically ill patients with underlying renal failure (45). To improve vascular opacification use of isoosmolar contrast agent Iodixanol as opposed to nonionic monomeric contrast Iohexol (Omnipaque 300) has been evaluated (46). It showed modest but statistically significant increase in enhancement (by 7 HU) and more consistent venous enhancement but a decrease in pulmonary arterial enhancement (by 42 HU). It is suggested that since Iodixanol is expensive it can be used in patients with marginal renal function due to its lesser nephrotoxicity (46). To address the issue of streak artifacts from metallic hip prosthesis Yasaka et al. compared quality of helical CT images of the pelvis in patients with metal hip prostheses reconstructed using adaptive iterative dose reduction (AIDR) and AIDR with single-energy metal artifact reduction (SEMAR-A). They concluded that artifacts induced by metal hip prostheses were effectively reduced and the depiction of most pelvic structures was significantly improved by using SEMAR with helical pelvic CT (47).
Pelvic radiation is major concern especially in young patients whose reproductive organs are highly radiosensitive. Protocols with reformatted discontinuous images rather than helical acquisition have been recommended to reduce the radiation dose (43,48). Reports show that isolated DVT of the pelvic veins is uncommon composing 1–4% of total cases (43,49) and limiting CTV only to the lower extremity would reduce the radiation dose to the patient without significantly altering the overall detection frequency of the CT in detecting VTE (50).
To overcome these limitations, Cho et al. investigated the role of low tube voltage (100 kVp) setting in CTV in diagnosis of DVT and evaluated the feasibility of reducing the amount of intravenously administered contrast (51). They concluded that lower tube voltage CTV protocol showed significantly higher venous enhancement and contrast to noise ratio and image noise similar to the conventional 120 kVp protocol in spite of administering 18.6% less iodine by moderate concentration contrast media (300 mgI/mL) instead of high concentration contrast media (370 mgI/mL). The 100 kVp protocol had overall better diagnostic image quality for evaluating DVT by CTV and hence could provide higher diagnostic accuracy for evaluation of DVT (51).
MR imaging of VTE
In 1990s many prospective clinical trials evaluated role of MR in evaluation of VTE with interest in MR pulmonary angiography (MRPA) and MRV of the pelvic and lower extremity veins.
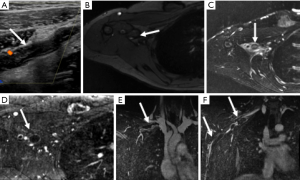
Traditionally, time of flight (TOF) techniques were used frequently for MRV with thrombus in the IVC, iliac and femoropopliteal veins seen with high accuracy (Figure 4) (52). This technique is however, limited by slow image acquisition, and flow or saturation artifacts. Contrast enhanced MRV involves the use of the same rapid, three-dimensional (3D) sequences that have been developed for arteriography. The problems of TOF techniques are overcome with contrast enhanced MRV but images require post processing to subtract arterial signal for optimizing image quality and improve interpretation.
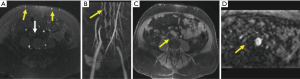
MRV of the pelvic and thigh veins (Figure 5) is less challenging than MRA as the venous flow is relatively uniform and slower and the vessel size is greater. Also the venous pathologies are usually more extensive requiring lower image resolution. It can be performed with or without intravenous contrast (TOF and phase contrast techniques). Contrast enhanced MRV has faster acquisition, better signal to noise ratio (SNR) and greater accuracy in slow flow/tortuous veins (53).
Studies have validated use of gradient echo (bright blood technique) for detection of DVT supplemented by spin echo or fast spin echo (black blood technique) as the latter is not recommended for primary diagnosis. The source images should be used for interpretation rather than the reformatted images (18). Distinguishing acute from chronic DVT is a potential advantage of MRI, with irregular wall thickening in the presence of collaterals and diminutive lumen suggestive of chronic DVT (Figure 4) (54).
Initial studies used contrast venography as gold standard and found sensitivity and/or specificity values for MRV as high as 100% in diagnosing femoro-popliteal DVT (55-58). However, it has performed less well than contrast venography in assessing the calf veins.
ACR Appropriateness Criteria has recommended MRV to be the imaging investigation of choice for evaluation of pelvic or thigh DVT if US is non-diagnostic and as an initial imaging investigation of choice for suspected central vein thrombosis in the thorax (59). Isolated pelvic vein thrombosis is uncommon accounting for 2% patients with lower extremity DVT. MRV is the modality of choice in detecting pelvic DVT for those situations in which pelvic thrombus is likely i.e., pelvic trauma, post-surgical or cryptogenic stroke or when proximal DVT is suspected despite negative US study (60).
Ono et al. prospectively assessed the diagnostic accuracy of non-contrast-enhanced MRV using both flow-refocused fresh-blood imaging (FR-FBI) and swap phase-encode arterial double-subtraction elimination (SPADE) techniques for detecting DVT, as compared to using conventional X-ray venography as the reference standard (61). The overall sensitivities and specificities for diagnosis of DVT were almost 100% (61). They concluded that non-contrast-enhanced MRV using SPADE and FR-FBI is highly accurate and reproducible for the diagnosis of DVT in symptomatic and asymptomatic patients. The SPADE and FR-FBI technique enabled depiction of the calf and thrombosed veins with slow flow and/or stationary blood to the pelvic vein and with fast flow beside the vein adjacent to metallic implants, and without the adverse effects of MR contrast agents. MRV using SPADE and FR-FBI appears especially promising for both postoperative screening and follow-up of DVT in patients undergoing major orthopedic surgery at lower field.
The ideal MRV technique for DVT would be rapid, highly accurate without need of intravenous contrast administration.
Balanced steady state free precession (SSFP) MRV may be unique in that it satisfies all of these criteria and can be readily performed on 1.5 T systems. Lindquist et al. found that in comparison with US, balanced- SSFP MRV had a sensitivity of 94.7% and specificity of 100% (62). As compared to US, the scanning time is less (20 vs. 7–10 min for the SSFP-MRV) and can be useful in patients with painful swollen legs in whom US is exceedingly painful and technically challenging. Absence of contrast administration decreases cost substantially and eliminates the risk of nephrogenic systemic fibrosis and is comparable to contrast enhanced MRV. The major limitation of this technique is susceptibility artifact and can’t be used in patients with orthopedic hardware (62).
Fraser et al. described venous enhanced subtracted peak arterial venography (VESPA), a technique of contrast enhanced MRV for diagnosis of femoral and iliac DVT (63). In this technique, contrast material is injected into a peripheral vein and serial image volume measurements are acquired. Subtraction of an early arterial phase measurement from a late arterial-venous equilibrium phase measurement yields a selective venous angiogram. This technique used double subtraction algorithm in which two early and two delayed measurements are used which increases venous signal compared to the single subtraction.
Advantages include that it allows rapid and complete visualization of the venous anatomy, especially the tortuous vessels such as collaterals and is not impaired by motion artifacts. Although subtraction and other artifacts can be seen, it usually leads to nondiagnostic rather than false positive studies and source images can help with correct diagnosis (64). As it helps in visualization of bilateral femoral and iliac venous systems simultaneously, the reporting time is reduced. Contrast enhancement of the vessel wall in cases of acute thrombosis but not in chronic thrombosis was observed in all cases which potentially can be useful to differentiate acute from chronic thrombosis in recurrent cases. Its limitation is that the use of contrast adds to the cost.
With contrast enhanced MRV unpredictable arrival of gadolinium based contrast in the more distal veins confounded by the very short transit time due to rapid redistribution into the extracellular fluid space led to development of “blood pool” contrast agent for calf veins imaging (65,66).
Li et al. (66) prospectively evaluated the use of a dual-contrast mechanism in conjunction with an iron oxide blood pool contrast agent, ferumoxytol, to depict DVT. Three techniques, including precontrast 2D TOF, ferumoxytol-enhanced bright-blood imaging, and ferumoxytol-enhanced dark-blood imaging, were applied focusing on its T1 as well as T2 relaxivity.
Bright-blood imaging is best for showing the overall anatomy of the venous system and for demonstrating venous occlusion. Dark-blood imaging is best for showing the extent of a thrombus and for detecting thrombi that are incompletely or not surrounded by blood (e.g., small calf thrombi) (66).
Stable blood pool enhancement improves the SNR and longer imaging window allows to perform multiple sequences with no loss of image quality or diagnostic accuracy (66).
Less information is available for MRI as a screening modality in asymptomatic patients. It has been suggested that silent lower extremity DVT may be demonstrated with MRI. The advantages it offers are high tissue contrast without ionizing radiation especially in pelvic DVT in young patients as the reproductive organs are in scanning field. It is particularly useful in evaluating ovarian vein thrombosis/puerperal ovarian vein thrombosis where US is technically difficult and CT not recommended (67). It helps in identification of anatomical variants like duplicated veins which are not uncommonly thrombosed (pitfall with USG and venography) and identify alternative cause for clinical signs and symptoms which are highly nonspecific in DVT (58,68).
Magnetic resonance direct thrombus imaging (MRDTI)
About 20% to 40% of patients will present with suspected recurrent DVT within 5 years of first episode (69). For these patients, accurate diagnosis of recurrent DVT is of particular importance, because patients with proven recurrent DVT are, depending on their risk profile, often subjected to indefinite anticoagulant treatment with its associated bleeding risks (70). Conversely, if they are left untreated, they are at risk for potentially fatal PE and development PTS (71). US has limited usefulness in detection of recurrent DVT and the most promising alternative is MRDTI based on assessment of shortening T1 signal. It is highly accurate for first DVT (64,72).
Tan et al. performed a prospective multicenter study to determine the sensitivity of MRDTI for the diagnosis of acute recurrent ipsilateral DVT, by comparing MRDTI scans of patients with established acute recurrent symptomatic ipsilateral DVT with MRDTI scans of patients with compression US proven residual thrombosis without suspected acute recurrent disease (73).
The sensitivity of MRDTI was 95% and specificity was 100% (73,74). MRDTI is an accurate and reproducible method for distinguishing acute ipsilateral recurrent DVT from at least 6-month-old chronic residual thrombi in the leg veins when recurrence is not suspected clinically, suggesting a high diagnostic accuracy of MRDTI for the diagnosis of acute proximal ipsilateral recurrent thrombosis when applied as a first- or second-line imaging test in the diagnostic work-up.
Advantages include that it can be performed on 1.5 T system commonly available, it is rapid as full examination of both legs can be completed in less than 5 min, it has no radiation and it has high interobserver agreement and is operator independent.
Limitations include that acute DVT can be missed in very early stages when the compression US may still detect incompletely compressible vein (74) as it needs certain amount of time for transformation of haemoglobin into sufficient amount of methemoglobin to cause T1 shortening mandatory for detection.
Recently, Phinikaridou et al. (75) used magnetic transfer (MT) and diffusion-weighted imaging (DWI) MRI to visualize and detect the thrombus protein composition, thereby allowing staging of the DVT. It demonstrated encouraging sensitivity and specificity for the identification of intermediate aged thrombi and hence estimate the age of thrombus (76).
Currently, MR is less popular than CT for evaluation of acute VTE because of technical limitations including assessment of the calf veins, higher costs, limited availability, claustrophobic environment and other logistical considerations. At present, MRI should be considered when there is a strong clinical suspicion of pelvic DVT, and in young women requiring investigation for PE with abnormal chest X-ray precluding a V/Q scan (55).
Scintigraphy
In the 1980s radionuclide venography using Tc 99m pertechnetate was first performed for diagnosing DVT (77). Various tracers used previously like Tc 99m labeled macroaggregated albumin (MAA), Tc 99m labeled red blood cell (RBC), and Tc 99m human serum albumin (HSA) blood pool venography and in vitro labeled In 111 platelet scintigraphy have shown limited utility (78).
Tc 99m recombinant tissue plasminogen activator imaging (Tc 99m-rt-PA) has high sensitivity and specificity for detection of symptomatic and asymptomatic DVT and could distinguish fresh from old thrombus which was difficult with US (79). Technetium Tc 99m-apcitide scintigraphy in another scintigraphy technique that has potential utility in suspected recurrent DVT differentiating old from acute thrombus but depends on the training and experience of the interpreters (80).
Role of other advanced imaging techniques like photoacoustic imaging (81), biotechnology/nanotechnology is being investigated (82,83) in diagnosing and estimating age and monitoring treatment of DVT (84).
Role of 18F-FDG PET/CT in evaluation of the DVT has been explored. In one of the first prospective studies, Rondina et al. showed that 18F-FDG PET/CT is a sensitive tool in detecting acute proximal symptomatic DVT (sensitivity of 87.5% and specificity of 100%). They also showed that metabolic activity in thrombosed vein segments decreases with time DVT onset, suggesting that 18F-FDG PET/CT may have utility in assessing the age of the thrombus (85).
There are numerous studies trying to evaluate the role of FDG-PET/CT in differentiating bland thrombus from tumor thrombus (Figure 6) (86-88). Though no larger studies are available due to rarity of tumor thrombi, they concluded that FDG-PET/CT is a powerful tool in differentiating bland thrombus from tumor thrombus. Also as FDG-PET/CT is routinely used in oncology, it can be useful in early detection if unsuspected/occult thrombi. Sharma et al. concluded that contrast enhanced FDG PET-CT is more suited for this purpose as it provides the best anatomic details of thrombus morphology and functional information (87).
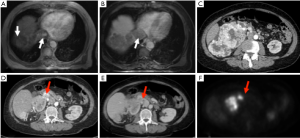
FDG-PET/CT using molecular agents also appears very promising in detecting thrombosis anywhere in the body and probably differentiate acute from chronic thrombi and hence eliminate risk of unnecessary treatment. Also accurate quantification may help in monitoring treatment. Most of these agents are under trial and will hopefully come into routine use in clinical diagnostic imaging of this potentially fatal disease (89).
Upper extremity DVT
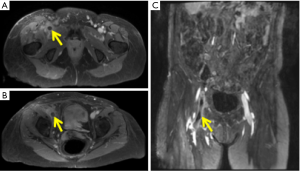
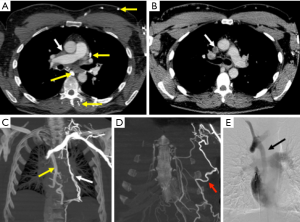
Upper extremity DVT is a thrombus in the radial, ulnar, brachial, axillary, subclavian veins, internal jugular and brachiocephalic veins and is uncommon accounting for less than 3% of total cases of DVT (14). However, the rate of PE at 12% is essentially the same (90). Secondary upper extremity DVTs are typically associated with indwelling catheters, pacemakers, and hypercoagulable states and are more common than primary upper extremity DVTs, also known as Paget-Schroetter syndrome or effort thrombosis, related to anatomical compression of vascular structures. PSS is form of venous thoracic outlet syndrome caused by compression of the subclavian vein at the level of costoclavicular space between the first rib, subclavius muscle and clavicle and causing spontaneous axillosubclavian thrombosis (Figure 7) (91). It has an incidence of 1–2 per 100,000 with average age at diagnosis being 30 years (92,93).
While US is still an initial test in diagnosing upper extremity DVT, it has limitations due to anatomical restrictions of clavicle. This makes it less reliable in thrombus detection in central portions of the subclavian vein, brachiocephalic veins and superior vena cava (Figure 8) with duplex scanning having sensitivity and specificity as low as 78% and 82% respectively (94). The multimodality imaging used for lower extremity DVT can be used for upper extremity DVT imaging as well (7). But all the noninvasive modalities have limitations and conventional catheter venography is still reference standard if the clinical suspicion for thrombosis is high as it is not only diagnostic but can also augment initial treatment by infusion of thrombolytic agent or pharmacomechanical thrombolysis (93). However, in patients with primary upper extremity DVT/venous thoracic outlet syndrome, cross sectional imaging is mandatory to delineate the abnormal anatomy, determine the structures compressed, identify fixed focal subclavian vein stenosis at the site of dynamic compression and expanded venous collaterals, exclude other causes of compression. Imaging is performed with the arm adducted and abducted (external rotation) to elicit positional vascular narrowing, as it affects surgical management by identifying the precise location of the vascular compression. Due to this while performing CT the total contrast is given in two boluses with IV cannula in the unaffected arm. For MR imaging of vascular thoracic outlet syndrome, single dose of intravascular blood pool agent (gadofesveset) or split dose of extracellular gadolinium based agent is administered intravenously via unaffected arm. Like CT, imaging is performed with the arm in adducted and abducted (external rotation). Catheter angiography still is useful in initial management in patients with acute symptoms or known thrombosis and also in postoperative patients to evaluate for residual/recurrent thrombosis (91). The advantages and disadvantages of the various modalities used for imaging DVT have been summarized (Table 1).
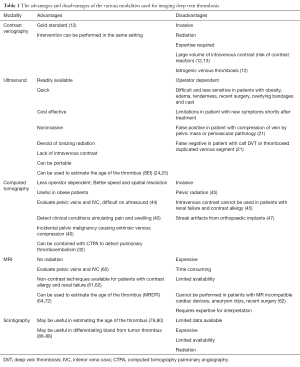
Full table
Conclusions
The incidence of DVT is increasing, not just in the lower extremity but also in upper extremity, where malignancy and central venous catheters are the major precipitating factors. While US still has advantages, it has various limitations and in such cases advanced imaging techniques such as MRI, should be considered. CTV, while sensitive, can be incorporated into CTPA in suspected PE but has the disadvantage of radiating sensitive pelvic organs especially in young patients. MRI will almost likely feature more commonly in DVT evaluation in the near future with new “blood pool” contrast agents allowing a comprehensive examination for PE and DVT in the same scan. Although advanced imaging techniques in nanotechnology/biotechnology, molecular imaging and PET are also being investigated, these may not replace the established first line modalities in diagnosis, but may be useful as adjuncts in patients who are not good candidates for structural imaging like renal disease patients with contraindication for intravenous contrast. Most of these agents are under trial and will hopefully come into routine use in diagnosing this potentially fatal disease.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Huisman MV, Klok FA. Diagnostic management of acute deep vein thrombosis and pulmonary embolism. J Thromb Haemost 2013;11:412-22. [Crossref] [PubMed]
- Galson SK. Prevention of deep vein thrombosis and pulmonary embolism. Public Health Rep 2008;123:420-1. [PubMed]
- Levy MM, Albuquerque F, Pfeifer J. Low incidence of pulmonary embolism associated with upper extremity deep vein thrombosis. Ann Vasc Surg 2012;26:964-72. [Crossref] [PubMed]
- Goldhaber SZ, Tapson V. A prospective registry of 5,451 patients with ultrasound confirmed deep vein thrombosis. Am J Cardiol 2004;93:259-62. [Crossref] [PubMed]
- Chung AS, Luk W, Lo AX, et al. Duplex sonography for detection of deep vein thrombosis of upper extremities: a 13-year experience. Hong Kong Medical Journal 2015;21:107-13. [PubMed]
- Lee Y. Current developments in imaging for deep vein thrombosis. Hong Kong Medical Journal 2015;21:96-7. [Crossref] [PubMed]
- Chin EE, Zimmeerman PT, Grant EG. Sonographic Evaluation of upper extremity deep vein thrombosis. J Ultrasound Med 2005;24:829-38. [PubMed]
- Tovey C, Wyatt S. Diagnosis, investigation, and management of deep vein thrombosis. BMJ 2003;326:1180-4. [Crossref] [PubMed]
- Kelly J, Rudd A, Lewis RR, et al. Plasma D-Dimers in the diagnosis of venous thromboembolism. Arch Intern Med 2002;162:747-56. [Crossref] [PubMed]
- Tan YK, da Silva A. Digital photoplethysmography in the diagnosis of suspected lower limb DVT: is it useful? Eur J Vasc Endovasc Surg 1999;18:71-9. [Crossref] [PubMed]
- Kassaï B, Boissel JP, Cucherat M, et al. A systematic review of the accuracy of ultrasound in the diagnosis of deep venous thrombosis in asymptomatic patients. Thromb Haemost 2004;91:655-66. [PubMed]
- de Valois JC, van Schaik CC, Verzijlbergen F, et al. Contrast venography: from gold standard to 'golden backup' in clinically suspected deep vein thrombosis. Eur J Radiol 1990;11:131-7. [Crossref] [PubMed]
- Ozbudak O, Erogullari I, Ogus C, et al. Doppler ultrasonography versus venography in the detection of deep vein thrombosis in patients with pulmonary embolism. J Thromb Thrombolysis 2006;21:159-62. [Crossref] [PubMed]
- Fraser JD, Anderson DR. Deep vein thrombosis: Recent advances and optimal investigation with US. Radiology 1999;211:9-24. [Crossref] [PubMed]
- Goodacre S, Sampson F, Thomas S, et al. Systematic review and meta-analysis of the diagnostic accuracy of ultrasonography for deep vein thrombosis. BMC Med Imaging 2005;5:6-13. [Crossref] [PubMed]
- Cogo A, Lensing AW, Prandoni P, et al. Distribution of thrombosis in patients with symptomatic deep vein thrombosis. Arch Intern Med 1993;153:2777-80. [Crossref] [PubMed]
- Elias A, Mallard L, Elias M, et al. A single complete ultrasound investigation of the venous network for the diagnostic management of patients with a clinically suspected first episode of deep venous thrombosis of the lower limbs. Thromb Haemost 2003;89:221-7. [PubMed]
- Tapson VF, Carroll B, Davidson B, et al. The diagnostic approach to acute venous thromboembolism,clinical practice guideline American thoracic society. Am J Respir Crit Care Med 1999;160:1043-66. [Crossref] [PubMed]
- American College of Radiology. Practical guidelines and standards for performance of the peripheral venous ultrasound examination. Reston (VA): 2015.
- Gottlieb RH, Widjaja J, Mehra S, et al. Clinically important pulmonary emboli: does calf vein US alter outcomes? Radiology 1999;211:25-9. [Crossref] [PubMed]
- Borgstede JP, Clagett GE. Types, frequency, and significance of alternative diagnosis found during duplex doppler venous examinations of the lower extremities. J Ultrasound Med 1992;11:85-9. [PubMed]
- Schulman S, Lindmarker P, Holmström M, et al. Post-thrombotic syndrome, recurrence, and death 10 years after the first episode of venous thromboembolism treated with warfarin for 6 weeks or 6 months. J Thromb Haemost 2006;4:734-42. [Crossref] [PubMed]
- Nayak L, Vedantham S. Multifaceted management of the postthrombotic syndrome. Semin Intervent Radiol 2012;29:16-22. [Crossref] [PubMed]
- Emelianov SY, Chen X, O’Donnell M, et al. Triplex ultrasound: elasticity imaging to age deep venous thrombosis. Ultrasound Med Biol 2002;28:757-67. [Crossref] [PubMed]
- Xie H, Kim K, Aglyamov SR, et al. Staging deep venous thrombosis using ultrasound elasticity imaging: animal model. Ultrasound Med Biol 2004;30:1385-96. [Crossref] [PubMed]
- Rubin JM, Aglyamov S, Wakefield T, et al. Clinical application of sonographic elasticity imaging for aging of deep venous thrombosis: preliminary findings. J Ultrasound Med 2003;22:443-8. [PubMed]
- Lubinski MA, Emelianov SY, O’Donnell M. Speckle tracking methods for ultrasonic elasticity imaging using short time correlation. IEEE Trans Ultrason Ferroelectr Freq Control 1999;46:82-96. [Crossref] [PubMed]
- Lubinski MA, Emelianov SY, O’Donnell M. Adaptive strain estimation using retrospective processing. IEEE Trans Ultrason Ferroelectr Freq Control 1999;46:97-107. [Crossref] [PubMed]
- Rubin JM, Xie H, Kim K, et al. Sonographic elasticity imaging of acute and chronic deep venous thrombosis in Humans. J Ultrasound Med 2006;25:1179-86. [PubMed]
- Siebers S, Geier B, Scheipers U, et al. Classification of venous thrombosis combining ultrasound elastography and tissue characterization. IEEE International Ultrasonics, Ferroelectrics, and Frequency Control Joint 50th Anniversary Conference. Germany, 2004.
- Wakefield TW, Strieter R, Prince M, et al. Pathogenesis of venous thrombosis: a new insight. Cardiovasc Surg 1997;5:6-15. [Crossref] [PubMed]
- Kanne JP, Lalani TA. Role of Computed Tomography and Magnetic Resonance Imaging for deep vein thrombosis and pulmonary embolism. Circulation 2004;12:I15-21. [Crossref] [PubMed]
- Goodman LR. 1999 plenary session: Friday imaging symposium: CT diagnosis of pulmonary embolism and deep venous thrombosis. Radiographics 2000;20:1201-5. [Crossref] [PubMed]
- Fishman EK, Horton K. CT of suspected pulmonary embolism: study design optimization. AJR Am J Roentgenol 2000;175:1002-3. [Crossref] [PubMed]
- Kim T, Murakami T, Hori M, et al. Efficacy of multislice helical CT venography for the diagnosis of deep venous thrombosis: comparison with venous sonography. Radiat Med 2004;22:77-81. [PubMed]
- Byun SS, Kim JH, Kim YJ, et al. Evaluation of deep vein thrombosis with multidetector row CT after orthopedic arthroplasty: a prospective study for comparison with doppler sonography. Korean J Radiol 2008;9:59-66. [Crossref] [PubMed]
- Duwe KM, Maria S, Budorick NE, et al. Evaluation of the lower extremity veins in patients with suspected pulmonary embolism: A retrospective comparison of helical CT venography and sonography. AJR Am J Roentgenol 2000;175:1525-31. [Crossref] [PubMed]
- Stein PD, Fowler SE, Goodman LR. Multidetector computed tomography for acute pulmonary embolism. N Engl J Med 2006;354:2317-27. [Crossref] [PubMed]
- Moser KM, Fedullo PF. LitteJohn JK. Frequent asymptomatic pulmonary embolism in patients with deep venous thrombosis. JAMA 1994;271:223-5. [Crossref] [PubMed]
- Hamper UM, DeJong MR, Scoutt LM. Ultrasound evaluation of the lower extremity Veins. Radiologic Clinics of North America 2007;45:525-47. [Crossref] [PubMed]
- Anderson FA Jr, Wheeler HB, Goldberg RJ, et al. A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study. Arch Intern Med 1991;151:933-8. [Crossref] [PubMed]
- Thomas SM, Goodacre SW, Sampson FC, et al. Diagnostic value of CT for deep vein thrombosis: results of a systematic review and meta-analysis. Clin Radiol 2008;63:299-304. [Crossref] [PubMed]
- Loud PA, Katz DS, Bruce DA, et al. Deep venous thrombosis with suspected pulmonary embolism: Detection with combined CT Venography and pulmonary angiography. Radiology 2001;219:498-502. [Crossref] [PubMed]
- Moll S. Use of combined CT venography and CT pulmonary arteriography. J Thromb Haemost 2003;1:637-9. [Crossref] [PubMed]
- Begemann PG, Bonacker M, Kemper J, et al. Evaluation of the deep venous system in patients with suspected pulmonary embolism with multi-detector CT: a prospective study in comparison to Doppler sonography. J Comput Assist Tomogr 2003;27:399-409. [Crossref] [PubMed]
- Goodman LR, Gulsun M, Nagy P, et al. CT of Deep venous thrombosis and pulmonary embolus: Does iso-osmolar contrast agent improve vascular opacification? Radiology 2005;234:923-8. [Crossref] [PubMed]
- Yasaka K, Maeda E, Hanaoka S, et al. Single-energy metal artifact reduction for helical computed tomography of the pelvis in patients with metal hip prostheses. Jpn J Radiol 2016;34:625-32. [Crossref] [PubMed]
- Goodman LR, Stein PD, Beemath A, et al. CT Venography for Deep Venous Thrombosis: Continuous Images Versus Reformatted Discontinuous Images Using PIOPED II Data. AJR 2007;189:409-12. [Crossref] [PubMed]
- Goodman LR. Venous thromboembolic disease: CT evaluation. Q J Nucl Med 2001;45:302-10. [PubMed]
- Kalva SP, Jaggannathan JP, Hahn PF, et al. Venous Thromboembolism: Indirect CT Venography during CT pulmonary angiography—should the pelvis be imaged? Radiology 2008;246:605-11. [Crossref] [PubMed]
- Cho ES, Chung JJ, Kim S, et al. CT Venography for deep vein thrombosis using a low tube voltage (100 kVp) setting could increase venous enhancement and reduce the amount of administered iodine. Korean J Radiol 2013;14:183-93. [Crossref] [PubMed]
- Carpenter JP, Holland GA, Baum RA, et al. Magnetic resonance venography for the detection of deep venous thrombosis: comparison with contrast venography and duplex Doppler ultrasonography. J Vasc Surg 1993;18:734-41. [Crossref] [PubMed]
- Prince MR, Grist TM, Debatin JF. editors. 3D Contrast MR Angiography. 3rd ed. New York: Springer Berlin Heidelberg, 2003:163-72.
- Spritzer CE, Norconk J, Sostman H, et al. Detection of deep venous thrombosis by MRI. Chest 1993;104:54-60. [Crossref] [PubMed]
- Spritzer CE, Sostman HD, Wilkes DC, et al. Deep venous thrombosis: experience with gradient echo MR imaging in 66 patients. Radiology 1990;177:235-41. [Crossref] [PubMed]
- Totterman S, Francis CW, Foster TH, et al. Diagnosis of femoropopliteal venous thrombosis with MR imaging: a comparison of four MR pulse sequences. AJR Am J Roentgenol 1990;154:175-8. [Crossref] [PubMed]
- Erdman WA, Jayson HT, Redman HC, et al. Deep venous thrombosis of extremities: role of MRI in the diagnosis. Radiology 1990;174:425-31. [Crossref] [PubMed]
- Cantwell CP, Cradock A, Bruzzi J, et al. MR venography with true fast imaging with steady-state precession for suspected lower-limb deep vein thrombosis. J Vasc Interv Radiol 2006;17:1763-69. [Crossref] [PubMed]
- Hanley M, Donahue J, Rybicki FJ, et al. American College of Radiology ACR Appropriateness Criteria® Clinical condition: suspected lower-extremity deep vein thrombosis. 2013. Available online: https://acsearch.acr.org/docs/69416/Narrative/
- Spritzer CE, Arata MA, Freed KS. Isolated pelvic deep venous thrombosis: Relative frequency as detected with MR Imaging. Radiology 2001;219:521-5. [Crossref] [PubMed]
- Ono A, Murase K, Taniguchi T, et al. Deep Venous Thrombosis: Diagnostic Value of Non-Contrast-Enhanced MR Venography Using Electrocardiography-Triggered Three-Dimensional Half-Fourier FSE. Magn Reson Med 2010;64:88-97. [Crossref] [PubMed]
- Lindquist CM, Karlicki F, Lawrence P, et al. Utility of Balanced Steady-State Free Precession MR Venography in the Diagnosis of Lower Extremity Deep Venous Thrombosis. AJR Am J Roentgenol 2010;194:1357-64. [Crossref] [PubMed]
- Fraser DG, Moody AR, Davidson IR, et al. Deep venous thrombosis: Diagnosis by using venous enhanced subtracted peak arterial MR Venography versus conventional venography. Radiology 2003;226:812-20. [Crossref] [PubMed]
- Fraser DG, Moody A, Morgan P, et al. Diagnosis of lower-limb deep venous thrombosis: a prospective blinded study of magnetic resonance direct thrombus imaging. Ann Intern Med 2002;136:89-98. [Crossref] [PubMed]
- Huang SY, Kim CY, Miller MJ, et al. Abdominopelvic and lower extremity deep venous thrombosis: Evaluation with contrast-enhanced MR Venography with a blood-pool agent. AJR Am J Roentgenol 2013;201:208-14. [Crossref] [PubMed]
- Li W, Salanitri J, Tutton S, et al. Lower extremity deep venous thrombosis: Evaluation with Ferumoxytol-enhanced MR Imaging and dual-contrast mechanism— ereliminary experience. Radiology 2007;242:873-81. [Crossref] [PubMed]
- Spritzer CE. Progress in MR Imaging of the Venous System. Perspect Vasc Surg Endovasc Ther 2009;21:105-16. [Crossref] [PubMed]
- Christie R, Andrew G, Ganapathy A, et al. Radiological Imaging and Intervention in Venous Thrombosis, Deep Vein Thrombosis. 2012. Cited 2016 July 16. Available online: http://www.intechopen.com/books/deep-vein-thrombosis/radiological-imaging-and-intervention-in-venous-thrombosis
- Baglin T, Douketis J, Tosetto A, et al. Does the clinical presentation and extent of venous thrombosis predict likelihood and type of recurrence? A patient-level meta-analysis. J Thromb Haemost 2010;8:2436-42. [Crossref] [PubMed]
- Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2016;149:315-52. [Crossref] [PubMed]
- Goldhaber SZ, Bounameaux H. Pulmonary embolism and deep vein thrombosis. Lancet 2012;379:1835-46. [Crossref] [PubMed]
- Saha P, Andia ME, Modarai B, et al. Magnetic resonance T1 relaxation time of venous thrombus is determined by iron processing and predicts susceptibility to lysis. Circulation 2013;128:729-36. [Crossref] [PubMed]
- Tan M, Mol GC, van Rooden CJ, et al. Magnetic resonance direct thrombus imaging differentiates acute recurrent ipsilateral deep vein thrombosis from residual thrombosis. Blood 2014;124:623-7. [Crossref] [PubMed]
- Westerbeek RE, Van Rooden CJ, Tan M, et al. Magnetic resonance direct thrombus imaging of the evolution of acute deep vein thrombosis of the leg. J Thromb Haemost 2008;6:1087-92. [Crossref] [PubMed]
- Phinikaridou A, Andia M, Saha P, et al. In vivo magnetization transfer and diffusion-weighted magnetic resonance imaging detects thrombus composition in a mouse model of deep vein thrombosis. Circ Cardiovasc Imaging 2013;6:433-40. [Crossref] [PubMed]
- Dharmarajah B, Sounderajah V, Rowland SP, et al. Aging techniques for deep vein thrombosis: a systematic review. Phlebology 2015;30:77-84. [Crossref] [PubMed]
- Wu CC, Jong SB. Radionuclide venography of lower limbs by subcutaneous injection: comparison with venography by intravenous injection. Ann Nucl Med 1989;3:125-33. [Crossref] [PubMed]
- Seabold JE. Radionuclide Venography and Labeled Platelets in Deep Venous Thrombosis. Semin Nucl Med 2001;31:124-8. [Crossref] [PubMed]
- Brighton T, Janssen J, Butler SP. Aging of acute deep vein thrombosis measured by Radiolabeled 99mTc-rt-PA. J Nucl Med 2007;48:873-8. [Crossref] [PubMed]
- Bates SM, Lister-James J, Julian JA, et al. Imaging characteristics of a novel technetium Tc 99m-labeled platelet glycoprotein IIb/IIIa receptor antagonist in patients With acute deep vein thrombosis or a history of deep vein thrombosis. Arch Intern Med 2003;163:452-6. [Crossref] [PubMed]
- Karpiouk AB, Aglyamov SR, Mallidi S, et al. Combined ultrasound and photoacoustic imaging to detect and stage deep vein thrombosis: phantom and ex vivo studies. J Biomed Opt 2008;13:054061. [Crossref] [PubMed]
- Wadajkar AS, Santimano S, Rahimi ME, et al. Deep vein thrombosis: Current status and nanotechnology advances. Biotechnol Adv 2013;31:504-13. [Crossref] [PubMed]
- Alonso A, Della Martina A, Stroick M, et al. Molecular imaging of human thrombus with novel abciximab immunobubbles and ultrasound. Stroke 2007;38:1508-14. [Crossref] [PubMed]
- Hara T, Bhayana B, Thompson B, et al. Molecular imaging of fibrin deposition in deep vein thrombosis using fibrin-targeted near-infrared fluorescence. JACC Cardiovasc Imaging 2012;5:607-15. [Crossref] [PubMed]
- Rondina MT, Lam U, Pendleton R, Kraiss L, et al. (18)F-FDG PET in the evaluation of acuity of deep vein thrombosis. Clin Nucl Med 2012;37:1139-45. [PubMed]
- Sharma P, Kumar R, Jeph S, et al. (18F)FDG PET-CT in the diagnosis of tumor thrombus: can it be differentiated from benign thrombus? Nucl Med Commun 2011;32:782-8. [Crossref] [PubMed]
- Sharma P, Kumar R, Singh H, et al. Imaging thrombus in cancer patients with FDG PET-CT. Jpn J Radiol 2012;30:95-104. [Crossref] [PubMed]
- Davidson T, Goitein O, Avigdor A, et al. (18F)FDG-PET/CT for the diagnosis of tumor thrombosis. Isr Med Assoc J 2009;11:69-73. [PubMed]
- Houshmand S, Salavati A, Hess S, et al. The role of molecualr imaging in diagnosis of deep vein thrombosis. Am J Nucl Med Mol Imaging 2014;4:406-25. [PubMed]
- Kooij JD, van der Zant FM, van Beek EJ, et al. Pulmonary embolism in deep venous thrombosis of upper extremity: more often in catheter-related thrombosis. Neth J Med 1997;50:238-42. [Crossref] [PubMed]
- Raptis CA, Sridhar S, Thompson RW, et al. Imaging of the Patient with Thoracic Outlet Syndrome. Radiographics 2016;36:984-1000. [Crossref] [PubMed]
- Isma N, Svensson P, Gottsäter A, et al. Upper extremity deep venous thrombosis in the population-based Malmö thrombophilia study (MATS). Epidemiology, risk factors, recurrence risk, and mortality. Thromb Res 2010;125:e335-8. [Crossref] [PubMed]
- Jourdain V, Goldenberg WD, Auten J. Paget-Schroetter syndrome: diagnostic limitations of imaging upper extremity deep vein thrombosis. Am J Emerg Med 2016;34:683.e1-3. [Crossref] [PubMed]
- Chin EE, Zimmerman P, Grant E. Sonographic evaluation of upper extremity deep venous thrombosis. J Ultrasound Med 2005;24:829-38. [PubMed]


