Use of novel oral anticoagulant agents in venous thromboembolism
Introduction
Venous thromboembolic (VTE) disease imposes a significant disease burden associated with marked morbidity and mortality in contemporary healthcare (1). VTE occurs in approximately 100 persons per 100,000 each year in the United States, with an exponential increase to 500 persons per 100,000 at the age of 80 (2). Of those patients with VTE, approximately two-thirds will have deep venous thromboses (DVT) and one-third will have pulmonary embolism (PE) at the time of diagnosis. The first line standard of care for treatment of VTE relies on a parenteral anticoagulant such as heparin or low molecular weight heparin (LMWH) with overlapping initiation of administration of a vitamin K antagonist to reach the goal international normalized ratio (INR) (3). Warfarin has been used as an approved blood thinner for over 60 years and still is the most widely prescribed oral anticoagulant in the United States (4). Warfarin functions as a vitamin K antagonist by inhibiting vitamin K epoxide reductase, limiting the production of vitamin K-dependent coagulation factors (II, V, IX, X) (5,6). However, it can be cumbersome and a strain on the healthcare system due to multiple drug interactions, essential dietary counseling, and routine monitoring of blood coagulation parameters in an outpatient setting (6). Further, warfarin is associated with major and minor bleeding complications.
In VTE, the goal INR for patients is 2–3 with an objective of maintaining patients in that therapeutic window. A measured marker of the efficacy of this drug is percentage time in therapeutic range (TTR). The United States Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) examined TTR in more than 5,000 patients and found that overall 59% of measured INR values were between 2.0 and 3.0 (7). In the ORBIT-AF cohort, patients at the highest predicted risks of stroke and bleeding were least likely to be in the TTR. The initial phase of warfarin initiation in sub-therapeutic patients contains a window of hypercoagulability associated with increased incidence of embolic events. As such, some patients receive treatment with unfractionated or LMWH as a “bridge” until the goal INR is reached. This practice can result in increased hospitalization days as well as significant increased costs to the healthcare system incurred by coagulation monitoring (8).
Given multiple drawbacks to the standard of care for treatment of VTE with warfarin, several new drugs have been developed, including factor Xa inhibitors (apixaban, rivaroxaban, and edoxaban) and direct thrombin inhibitors (dabigatran) (9). These drugs have all been approved for treatment of DVT and PE (10-13). In this paper, a systematic review of the literature has been performed including landmark trials describing the role of new oral anticoagulants (NOAC) in patients with VTE disease.
Dabigatran
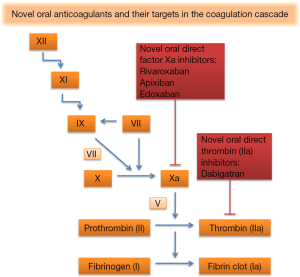
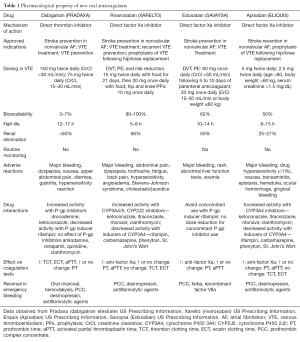
Dabigatran is a direct thrombin inhibitor with a serum half-life of 12 to 17 hours (Figure 1) (14). Like all NOACs, dabigatran does not require any routine monitoring (15). It has been approved for the treatment of nonvalvular atrial fibrillation (AF), the treatment for acute DVT and PE in patients who have been treated with a parenteral anticoagulant for 5 to 10 days, and for risk reduction in the setting of prevention for recurrent DVT and PE in patients who have been previously treated (10). Current recommended dosing is 150 mg twice daily for patients with preserved renal function, i.e., a creatinine clearance (CrCl) greater than 30 mL/min (10). Table 1 summarizes the pharmacological properties and drug monitoring requirements of dabigatran and the other NOACs.
Full table
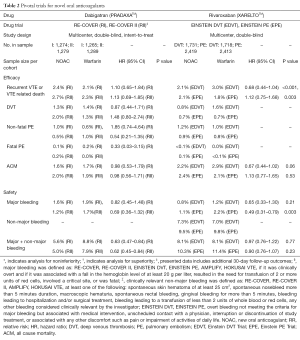
The RE-COVER trial was a noninferiority study that compared the efficacy and safety of dabigatran versus warfarin in patients with acute venous thromboembolism for a six-month treatment periods who had received initial parenteral anticoagulation therapy for a median of nine days (16). The trial randomly assigned 2,539 patients to one of two cohorts—adjustable dose warfarin to goal INR 2–3 or dabigatran 150 mg twice daily. Recurrent VTE occurred in 2.4% of patients in the dabigatran cohort versus 2.0% in the warfarin group demonstrating an absolute risk reduction of 0.4% [95% confidence interval (CI), −0.8 to 1.5] and a hazard ratio of 1.10 (95% CI, 0.65–1.84) with a P<0.001 for a prespecified noninferiority margin. Investigators concluded that dabigatran was non-inferior to warfarin with respect to 6-month recurrence of VTE and had a similar safety profile with respect to major and non-major bleeding events (16). The safety findings were consistent with those demonstrated by the RE-LY trial (17). Table 2 reviews the RE-COVER trial study design and major results.
Full table
RE-COVER-II was a randomized, double-blinded, multicenter trial that extended findings from its predecessor study and was performed with the intention of confirming results from RE-COVER trial and to allow for more precise subgroup analyses (18). In RE-COVER-II, 2,589 patients were treated with parenteral anticoagulation for 5 to 11 days and then switched to either dabigatran 150 mg twice daily or adjustable dose warfarin to goal INR 2–3 for a 6-month treatment period. Primary efficacy and safety outcomes were identical to those found in RE-COVER. Pooled analysis of both studies showed similar rates of recurrent VTE and safety outcomes and superiority of dabigatran with regards to composite bleeding but not major bleeding alone. Table 2 highlights these data. At this time, dabigatran has not been evaluated as a monotherapy agent without initial 5–10 days parenteral anticoagulation.
In addition to treatment for acute VTE, dabigatran was compared with subcutaneous enoxaparin for prophylaxis against VTE in patients who underwent total knee arthroplasty in the RE-MODEL trial (19). RE-MODEL was a double blinded study that randomized 2,076 patients into either receiving dabigatran 150 or 220 mg once daily versus subcutaneous enoxaparin 40 mg once daily for 6 to 10 days, starting on the evening before the planned total knee arthroplasty. Primary outcomes included occurrence of VTE and mortality as well as incidence of bleeding. Study findings demonstrated similar efficacy and safety profiles and the investigators concluded dabigatran to be non-inferior to subcutaneous enoxaparin for VTE prevention in this patient population.
RE-MOBILIZE was a randomized double-blinded, multicenter trial in which 2,596 patients who had receive unilateral total knee arthroplasty were randomized to receive either dabigatran 220 mg once daily, dabigatran 150 mg once daily, or enoxaparin 30 mg subcutaneously twice daily beginning 12 hours after surgery and continued for a total of 12 to 15 days (20). The primary efficacy outcome was a composite of total VTE events and all-cause mortality and occurred in 31.1% of patients in the 220 mg dabigatran cohort, 33.7% of the 150 mg dabigatran cohort, and 25.3% of the enoxaparin cohort. Both dabigatran dosages failed to show noninferiority to enoxaparin. The primary safety outcome was the incidence of major bleeding and clinically relevant non-major bleeding events and occurred in 0.6% of patients in the 220 mg dabigatran cohort, 0.6% of the 150 mg dabigatran cohort, and 1.4% of the enoxaparin cohort—events were concluded to be uncommon and statistically insignificant among the groups.
Rivaroxaban
Rivaroxaban selectively and competitively inhibits free and prothrombinase/clot-associated factor Xa through reversible interactions thereby inhibiting thrombin formation and decreasing fibrin clot formation (21-23). It is indicated for the treatment of DVT, PE, and for preventive reduction in their risk of recurrence (12). It has also been approved for DVT prophylaxis in patients undergoing hip and knee replacement surgery. Current recommended dosing is 15 mg twice daily for the first 21 days followed by 20 mg daily for the remaining treatment period. For DVT prophylaxis following hip and knee replacement surgery the recommendation is 10 mg daily for 35 and 12 days, respectively (12).
The EINSTEIN investigators conducted an open-label, randomized, event driven, noninferiority study that compared rivaroxaban with the current standard therapy consisting of subcutaneous enoxaparin followed by a vitamin K antagonist for treatment of acute DVT (Table 2) (24). One arm received rivaroxaban 15 mg twice daily for three weeks, followed by 20 mg once daily, while the other arm received subcutaneous enoxaparin followed by a vitamin K antagonist. The investigators also conducted a double blind, randomized, parallel extension study that compared rivaroxaban with placebo 6 or 12 months beyond the 6 or 12-month treatment period. The primary outcome of the trial was recurrent VTE and the principle safety outcome was again clinically significant bleeding. Noninferiority was demonstrated with respect to the primary outcome. There were 32 events in the rivaroxaban group versus 51 events in the enoxaparin-vitamin K antagonist group (2.1% vs. 3.0%; HR: 0.68; 95% CI, 0.44–1.04; P<0.001). Major bleeding was observed in 139 patients treated with rivaroxaban and 138 patients randomized to the standard therapy (8.1% vs. 8.1%; HR: 0.97; 95% CI, 0.76–1.22; P=0.77). For the extension study, 8 primary outcome events were reported in the rivaroxaban group and 41 events in the placebo group (1.3% vs. 7.1%; HR: 0.18; 95% CI, 0.09–0.39; P<0.001) with major bleeding occurring in 4 patients in the rivaroxaban group and in none in the placebo group (0.7% vs. 0.0%, P=0.11). This demonstrates that rivaroxaban is similar in efficacy when compared to standard therapy for the treatment of acute DVT and appears to be an attractive treatment regimen for preventing recurrences with an acceptable bleeding risk profile.
Previous studies have shown rivaroxaban to be non-inferior to standard therapy in the initial and long-term treatment of PE with a better bleeding risk profile (25). The EINSTEIN-PE investigators conducted a randomized, open label, noninferiority trial in a similar fashion to the aforementioned trial (Table 2). The treatment protocol, primary efficacy outcome, and principle safety outcome were similar to the prior study. Venous thromboembolism occurred in 50 patients in the rivaroxaban group and in 44 patients in the standard therapy arm (2.1% vs. 1.8%; HR: 1.12; 95% CI, 0.75–1.68; P=0.003). Major bleeding occurred in 26 patients treated with rivaroxaban and 52 patients in the standard therapy group (1.1% vs. 2.2%; HR: 0.49; 95% CI, 0.31–0.79; P=0.003). This study suggests a beneficial safety profile of rivaroxaban with regard to major bleeding.
In both of the above trials, subgroup analyses demonstrated similar primary efficacy and safety outcomes regardless of ages, sex, weight, and renal function. Unlike for treatment of nonvalvular AF, dosages need not be adjusted for CrCl less than 50 mL/min (EINSTEIN, EINSTEIN PE). Furthermore, the standard of therapy arm in both trials had acceptable values in the TTR for INR—58% in the DVT trial and 63% in the PE trial. These values are similar to those in other DVT and PE trials.
In addition to DVT and PE, rivaroxaban has been approved for thromboprophylaxis in patients undergoing total hip and knee arthroplasty. In the “Rivaroxaban versus Enoxaparin for Thromboprophylaxis after Hip Arthroplasty” (RECORD 1) trial patients received either 10 mg of oral rivaroxaban daily after surgery or 40 mg of subcutaneous enoxaparin the evening before surgery (26). The primary efficacy outcome was development of DVT, nonfatal PE, or death from any cause at 36 days. This primary outcome occurred in 1.1% of patients in the rivaroxaban group and 3.7% of patients in the enoxaparin group (ARR 2.6%; 95% CI, 1.3–3.7; P<0.001). The secondary efficacy outcome of major VTE defined as proximal DVT, nonfatal PE, or death form VTE, occurred in 0.2% of patients in the rivaroxaban group and 2.0% of patients in the enoxaparin group (ARR 1.7%; 95% CI, 1.0–2.5; P<0.001). Major bleeding occurred in 0.3% of patient in the rivaroaxaban group and 0.1% of patients in the enoxaparin group (P=0.18).
The RECORD 3 study had the same dosages of medications, primary efficacy, secondary efficacy, and safety outcomes but evaluated patients undergoing knee arthroplasty (27). The primary outcome occurred in 9.6% of patients in the rivaroxaban group and 18.9% of patients in the enoxaparin group (ARR 9.2%; 95% CI, 5.9–12.4; P<0.001). Major VTE occurred in 1.0% versus 2.6% in the enoxaparin group (ARR 1.6%; 95% CI, 0.4–2.8; P=0.01). Bleeding rates were similar, 0.6% in the rivaroxaban group and 0.5% in the enoxaparin group (P=0.77). There were no PE or deaths in patients receiving rivaroxaban, however the enoxaparin group did have four patients with PE and two of those died. Notably, only 67% of patients that were randomized were included in this modified intention-to-treat population.
Both RECORD 1 and RECORD 3 used a modified intention-to-treat population—only 67% of patient that were randomized were included in the study. Most of the patients were excluded because the number of valid venograms was lower than expected. Both studies increased recruitment to ensure their studies maintained statistical power.
Apixaban
Apixaban, another direct factor Xa inhibitor, has also been approved for prophylaxis of DVT following hip or knee arthroplasty, treatment of acute DVT or PE, and risk reduction for recurrent DVT and PE following initial therapy (11). Current recommended dosing for treatment of DVT and PE is 10 mg twice daily for 7 days followed by 5 mg twice daily for patients with preserved renal function. Recommended dosing for prophylaxis of DVT following hip or knee arthroplasty is 2.5 mg twice daily starting the day before the surgery and continued for 35 days for hip surgery and 12 days for knee surgery.
The Apixaban Dose Orally Vs ANtiCoagulation with Enoxaparin 1 (ADVANCE-1) trial aimed to assess apixaban for noninferiority to enoxaparin for thrombophylaxis in patients undergoing knee replacement (28). ADVANCE-1 was a double-blinded randomized controlled trial in which 3,195 patients undergoing total knee replacement were randomized to either apixaban 2.5 mg twice daily or 30 mg enoxaparin subcutaneously every 12 hours to be started 12–24 hours after surgery and continued for 10–14 days. A primary composite efficacy outcome of symptomatic DVT, nonfatal PE, and all-cause mortality occurred in 9.0% in the apixaban cohort versus 8.8% in the enoxaparin group (RR: 1.02; 95% CI, 0.78–1.32; P=0.06 for noninferiority). In this study, apixaban approached but did not meet the noninferiority threshold compared to enoxaparin for thrombophylaxis. However, a secondary outcome of major VTE based on a composite of proximal DVT, nonfatal PE and VTE-related deaths occurred in 2.1% of patients in the apixaban group versus 1.6% in the enoxaparin cohort (RR: 1.25; 95% CI, 0.70–2.23). With regards to safety outcomes, a composite endpoint of major bleeding and clinically relevant non-major bleeding was 2.9% with apixaban and 4.3% with enoxaparin.
ADVANCE-2 was the second of three phase 3 study trials to evaluate the safety and efficacy of apixaban in prevention of VTE after elective total knee or hip replacement (29). Study design was grossly identical to that of the ADVANCE-1 trial. However, subcutaneous enoxaparin was dosed at 40 mg daily and the first dose was delivered 12 hours prior to planned surgery. Apixaban was administered 12 hours after wound closure, similar to ADVANCE-1. The primary outcome was the composite incidence of symptomatic and asymptomatic DVTs assessed by bilateral ascending venography scheduled 10–14 days after the surgery when study drugs were discontinued, non-fatal PE, and all-cause mortality during the treatment period. In contrast to ADVANCE-1, ADVANCE-2 demonstrated increased efficacy with use of apixaban compared to enoxaparin. Incidence of the primary outcome occurred in 15.1% of apixaban patients compared to 24.4% of enoxaparin patients (RR: 0.62; 95% CI, 0.51–74, P<0.001). Major bleeding, adapted from International Society of Thrombosis and Haemostasis (ISTH) criteria, occurred in 0.6% of apixaban patients versus 0.9% of enoxaparin patients (ARD: −0.33%; 95% CI, −0.95 to 0.29; P=0.3014). A composite outcome of major bleeding and clinical relevant non-major bleeding was also found in 3.5% in the apixaban group versus 4.8% in the enoxaparin group (ARD: −1.24%; 95% CI, −2.66 to 0.18; P=0.0881). Study investigators concluded that apixaban was superior to enoxaparin for prevention of the primary efficacy outcome and that the incidence of major bleeding or clinically relevant non-major bleeding did not differ significantly between the two groups.
Finally, ADVANCE-3 was a double-blind randomized controlled trial in which 5,407 patients undergoing total hip arthroplasty were given either apixaban 2.5 mg daily or enoxaparin 40 mg subcutaneously every 24 hours with initial dosage identical to ADVANCE-2 (30). Primary efficacy and safety endpoints were also identical to those described in ADVANCE-2. The composite of symptomatic and asymptomatic DVT, nonfatal PE, and all-cause mortality occurred in 1.4% of apixaban patients and 3.9% of enoxaparin patients (RR: 0.36; 95% CI, 0.22–0.54; P<0.001 for both noninferiority and superiority). The composite of major bleeding and clinically relevant nonmajor bleeding occurred in 4.8% of apixaban patients and 5.0% of enoxaparin patients (ARD: −0.2; 95% CI, −1.4 to 1.0; P=0.72). Similar to ADVANCE-2, investigators concluded apixaban to be superior to enoxaparin with similar bleeding risks.
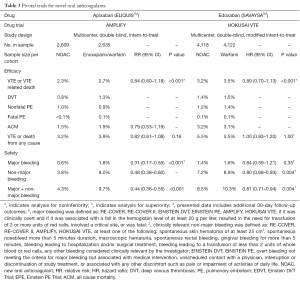
After all ADVANCE trials had been published, apixaban was evaluated for use in treatment of acute VTE in the Apixiban for the Initial Management of Pulmonary Embolism and Deep-Venous Thrombosis as First-Line Therapy (AMPLIFY) trial (Table 3) (31). In the AMPLIFY study, investigators randomized 5,935 patients with acute VTE to either conventional therapy with subcutaneous enoxaparin followed by adjustable dose warfarin or fixed dose apixaban. Apixaban was dosed at 10 mg twice daily for 7 days, followed by 5 mg twice daily for 6 months. The primary outcome was defined as a composite incidence of recurrent symptomatic VTE or death related to VTE and occurred in 2.3% of patients in the apixaban cohort versus 2.7% of patients receiving conventional therapy (RR: 0.84; 95% CI, 0.60–1.18; P<0.001 for noninferiority). Safety was assessed as a composite endpoint of incidence of major bleeding (per ISTH criteria) and clinically relevant non-major bleeding. The primary safety endpoint occurred in 4.3% of patients on apixaban versus 9.7% of patients receiving enoxaparin followed by warfarin (RR: 0.44; 95% CI, 0.36–0.55; P<0.001). All-cause mortality did not differ between the two groups, but apixaban was superior to conventional therapy when evaluating a composite incidence of recurrent VTE, VTE-related death, and major bleeding. Researchers concluded that apixaban was noninferior to enoxaparin followed by warfarin for treatment of acute VTE with an additional benefit of major bleeding risk reduction when using apixaban.
Full table
AMPLIFY-EXT was a follow-up study to evaluate use of apixaban as an option for extended treatment of VTE (32). Apixaban dosing was determined based on results from the ARISTOTLE and ADVANCE trials. A total of 2,486 patients were randomized to receive either apixaban 5 mg twice daily or 2.5 mg twice daily or placebo in a 1:1:1 ratio. Patients were included if they had been treated for 6–12 months with warfarin or were members of the AMPLIFY trial and there was clinical equipoise about cessation or continuation of anticoagulation after completion of acute therapy. The primary efficacy outcome was the composite incidence of symptomatic recurrent VTE and all-cause mortality and occurred in 3.8% of patients in the apixaban 2.5 mg cohort, 4.2% of patients in the apixaban 5 mg cohort, and 11.6% of patients receiving placebo. Both doses of apixaban were superior to placebo based on an intention-to-treat analysis. The primary safety outcome was incidence of major bleeding, once again defined by ISTH criteria; a secondary outcome of composite incidence of major bleeding and clinically relevant non-major bleeding was also assessed. Major bleeding occurred in 0.2% of patients in the apixaban 2.5 mg cohort, 0.1% of patients in the apixaban 5 mg cohort, and 0.5% of patients receiving placebo. The RR in the rate of major bleeding with the 2.5 mg dose and the 5 mg dose was 1.93 (95% CI, 0.18–21.25). The composite incidence of major and clinically relevant non-major bleeding occurred in 3.2% of patients in the apixaban 2.5 mg cohort, 4.3% of patients in the apixaban 5 mg cohort, and 2.7% of patients receiving placebo. Study investigators concluded that both doses of apixaban reduced the risk of recurrent VTE with similar rates of major bleeding compared with placebo. Additionally, for patients with VTE for whom continued anticoagulation beyond a standard treatment period is controversial, AMPLIFY-EXT suggests continuing anticoagulation therapy with apixaban for an additional 12 months would be a safe and effective approach.
Edoxaban
Edoxaban has also been approved for the treatment of both DVT and PE, but it has not been approved for thromboprophylaxis in total hip and knee arthroplasty (13). Current dosing guidelines recommend 60 mg once daily and 30 mg once daily for patients with CrCl 15 to 50 mL/min or body weight less than or equal to 60 kg or who use the P-gp inhibitor rifampin. This medication should be started 5–10 days after initiation of a parenteral anticoagulant. Similar to rivaroxaban, edoxaban is a selective inhibitor of factor Xa (33,34). The peak plasma concentration is achieved in 1 to 2 hours and steady state is achieved in 3 days. Edoxaban is eliminated primarily through the urine with a half-life of 5 to 11 hours. As with rivaroxaban, drug levels correlate with anti-Xa activity, prothrombin time (PT), and activated partial thromboplastin time (aPTT).
The Hokusai-VTE investigators conducted a randomized, double-blinded, noninferiority trial that compared heparin followed by edoxaban to heparin followed by warfarin for treatment of PE, DVT, or both (Table 3) (35). The treatment dose for edoxaban was adjusted from 60 mg once daily to 30 mg once daily for those with CrCl between 30 to 50 mL/min or body weight less than 60 kg. The primary efficacy outcome and principle safety outcome were recurrent systemic VTE (defined as composite of DVT or nonfatal or fatal PE) and major or clinically relevant non-major bleeding, respectively. Systemic VTE occurred in 3.2% of patients in the edoxaban group and 3.5% of patients in the warfarin group (HR: 0.89; 95% CI, 0.70–1.13; P<0.001). The principle safety outcome occurred in 8.5% of patients in the edoxaban arm and 10.3% of patients in the warfarin arm (HR: 0.81; 95% CI, 0.71–0.94; P=0.004). In the edoxaban group there were only two (<0.1%) fatal major bleeding events versus 10 (0.2%) in the warfarin group and no intracranial or retroperitoneal bleeding was notified in the edoxaban group. This study further measured right ventricular dysfunction and NT-proBNP levels to assess the severity of PE. The rate of recurrence of VTE in those with right ventricular dysfunction or NT-proBNP >500 pg/mL was 3.3% in the edoxaban arm versus 6.2% in the warfarin arm (HR: 0.52; 95% CI, 0.28–0.98). Thus edoxaban was found to be noninferior to standard of therapy for treatment of VTE and superior with respect to its bleeding risk.
As with the other factor Xa inhibitors, reversal in the setting of major bleeding has been an ongoing challenge. Current studies indicate that prothrombin complex concentrate, activated prothrombin complex concentrate, and recombinant factor VIIa as well as PER977 are all promising for reversal of anticoagulation with edoxaban (36,37). A novel recombinant protein (r-Antidote, PRT064445) that binds to factor Xa inhibitors and reverses the anticoagulant effect also has potential for factor Xa inhibitor reversal (38). At this time, however, there are no FDA approved measures for reversal of edoxaban or other factor Xa inhibitors.
Reversal agents
Until recently, a common disadvantage for all four NOACs was the lack of reversal agents in cases of serious bleeding or need for rapid reversal of anticoagulation, such as in the case of emergent surgery. Treatment during medical emergencies has typically consisted of resuscitation with blood products as well as non-specific procoagulant agents, prothrombin complex concentrates and recombinant factor VIIa. Idarucizumab (Praxbind) is a reversal agent for dabigatran that received FDA approval in October 2015. Idarucizumab is a monoclonal antibody fragment that neutralizes dabigatran by binding both free and thrombin-bound dabigatran with high affinity (39). The RE-VERSE AD trial is an ongoing prospective cohort study that is evaluating the ability of idarucizumab to reverse the anticoagulant effect of dabigatran in patients with either serious bleeding events while on dabigatran (group A) or in patients on dabigatran who require emergent surgery that necessitates immediate anticoagulation reversal (group B), with the primary end point being the maximum percentage reversal of the anticoagulant effect of dabigatran within 4 hours of giving idarucizumab as measured by the dilute thrombin time or ecarin clotting time (39). Restoration of hemostasis is a secondary end point in this study. RE-VERSE AD has already published data on a cohort of 90 subjects who received 5 g of IV idarucizumab and were followed for either 1 month or until death. Of these 90 subjects, 68 could be assessed by the dilute thrombin time test and 81 could be assessed by the ecarin clotting time test; 98% of subjects in group A and 93% of subjects in group B had normalization of the direct thrombin time, and 89% and 88% of subjects, in their respective groups, had normalization of the ecarin clotting time. The median maximum percentage reversal in both groups was 100% (95% CI, 100–100). There were a total of 18 deaths, nine in each study group; five deaths were attributed to fatal bleeding. There were thrombotic events (DVT, PE, or left atrial thrombus) in five subjects. The RE-VERSE AD trial is still an ongoing, multicenter study, currently recruiting more participants. Initial data suggests idarucizumab as an effective reversal agent for dabigatran and this reversal agent should be used when deemed clinically indicated.
Adexanet alfa is currently being studied as a reversal agent for indirect and direct Xa inhibitors, including apixaban, rivaroxaban, and edoxaban (40). It is a recombinant modified human factor Xa decoy protein that can bind Xa inhibitors with high affinity. Adexanet is currently being evaluated in ANNEXA-4, a phase 4 trial, with its use as an antidote in patients with severe bleeding (41). Aripazine (PER977) has been developed as a reversal agent for ultra-fractionated heparin and low-molecular weight heparin, but also appears to effectively bind edoxaban, rivaroxaban, apixaban, and dabigatran (37).
The most worrisome complication of all oral anticoagulation use is the risk of intracranial hemorrhage. Despite the lack of widely available reversal agents for NOACs, with regard to intracranial bleeding, the NOACs seem to be safer than warfarin. In a meta-analysis of the efficacy and harms of the NOACs by Sharma et al., there was a significant risk reduction of intracranial bleeding for all four NOACs when compared to warfarin (42). For dabigatran (both 150 and 110 mg dosing), rivaroxaban, and edoxaban 60 mg, there was an increased risk of gastrointestinal bleeding when compared to warfarin. However, intracranial bleeding arguably poses a greater risk of morbidity and mortality towards patients than gastrointestinal bleeding; NOACs should be considered favorably when compared to warfarin in terms of the overall safety profile.
Discussion
In the last decade, the medical community has markedly increased utilization of NOACs in the United States (43). This increased trend in prescription volume was recently explored in a recent Canadian health system audit (44). Reasons for increased NOAC use likely include growing physician experience and familiarity as well as emerging indications (first for nonvalvular AF and now from treatment and prophylaxis of VTE).
The rise in NOAC use can lead to provider discomfort in choosing exactly which NOAC to prescribe, particularly when adjudicating the plethora of numerical data available in the landmark trials. Unfortunately, there are no head-to-head trials to date comparing the different NOACs to one another in terms of safety and efficacy. However, a meta-analysis performed by Hirschl et al. aimed to provide indirect comparisons of NOACs (45). The meta-analysis found no differences between NOACs and warfarin with regard to recurrent VTE and death. Major bleeding was significantly reduced by rivaroxaban and apixaban; composite major bleeding and clinically relevant non-major bleeding was significantly reduced by dabigatran, apixaban, and edoxaban. No differences were found between the NOACs with regards to efficacy, but apixaban appeared to reduce the incidence of major bleeding more than dabigatran and edoxaban. With regards to the composite bleeding outcome, apixaban appeared to fare better than all other NOACs. Although indirect comparison analyses have inherent limitations (for example, the different baseline demographics in various analyses), review of each trial independently seems to suggest that NOACs are at least as effective as warfarin in the treatment and prevention of recurrent VTE. Moreover, trials also suggest each NOAC has an advantage over warfarin with regards to safety outcomes. Apixaban demonstrated a superior bleeding profile when compared to warfarin, and edoxaban demonstrated superiority in major bleeding and composite major bleeding plus clinically relevant non-major bleeding when compared to warfarin. The practicality and ease of use for NOACs combined in the setting of this array of favorable clinical data makes use of these drugs appealing. Additionally, the development of reversal agents may also lead to improve comfort for both providers and patients.
The American College of Chest Physicians (ACCP) has provided guidelines for the evidenced-based treatment and management of VTE for many years and has recently released an updated set of recommendations. In these recommendations, all four NOACs are recommended for use over warfarin for long-term anticoagulation (first 3 months) in patients without cancer with DVT of the leg or PE (grade 2B). This is considered a weak recommendation based on moderate quality evidence but still represents a departure from longstanding guidelines (46).
A perceived downside of NOAC use is the cost of individual NOACs for the patient when compared to warfarin, yet several studies have suggested that individual NOACs are a more cost-effective option for the health care system, offer more value in quality-adjusted measures for users, reduce length-of-stay for hospitalization for VTE when compared to enoxaparin plus warfarin, and reduce the utilization of the inpatient health care system due to fewer major bleeding events than warfarin (47-50).
Ultimately, the introduction of NOACs as an alternative to warfarin improves the potential for individualized therapy moving forward. Prescribers should undertake a comprehensive discussion regarding risks and benefits of the NOACs when selecting the agent which best suits each patient’s comorbidities and personal preferences. In general, patients who have excellent medication adherence are better candidates for NOACs, as there is no routine monitoring nor definitive markers to verify compliance. Many potential indications for NOACs remain to be investigated—treatment or prevention for VTE in oncology patients as well as for patients with adult congenital heart disease. As usage patterns for NOACs continue to increase, a clear understanding of the evolving field of oral anticoagulants will be important for both patients and physicians.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Luo H, Li J. The specialty of pulmonary vascular medicine in China: historical development and future directions. Cardiovasc Diagn Ther 2012;2:240-5. [PubMed]
- White RH. The epidemiology of venous thromboembolism. Circulation 2003;107:I4-8. [Crossref] [PubMed]
- Yeh CH, Gross PL, Weitz JI. Evolving use of new oral anticoagulants for treatment of venous thromboembolism. Blood 2014;124:1020-8. [Crossref] [PubMed]
- Holbrook AM, Pereira JA, Labiris R, et al. Systematic overview of warfarin and its drug and food interactions. Arch Intern Med 2005;165:1095-106. [Crossref] [PubMed]
- Freedman MD. Oral anticoagulants: pharmacodynamics, clinical indications and adverse effects. J Clin Pharmacol 1992;32:196-209. [Crossref] [PubMed]
- Ansell J, Hirsh J, Hylek E, et al. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008;133:160S-198S.
- Pokorney SD, Simon DN, Thomas L, et al. Patients' time in therapeutic range on warfarin among US patients with atrial fibrillation: Results from ORBIT-AF registry. Am Heart J 2015;170:141-8,148.e1.
- Bauer KA. Pros and cons of new oral anticoagulants. Hematology Am Soc Hematol Educ Program 2013;2013:464-70.
- Madan S, Shah S, Partovi S, et al. Use of novel oral anticoagulant agents in atrial fibrillation: current evidence and future perspective. Cardiovasc Diagn Ther 2014;4:314-23. [PubMed]
- Ingelheim B. Pradaxa (dabigatran etexilate): US prescribing information. Available online: http://docs.boehringer-ingelheim.com/Prescribing Information/PIs/Pradaxa/Pradaxa.pdf. Accessed on 22 August 2016
- Squibb BM. Eliquis (apixaban): US prescribing information. Available online: http://packageinserts.bms.com/pi/pi_eliquis.pdf. Accessed on 22 August 2016
- Pharmaceuticals J. Xarelto (rivaroxaban): US prescribing information. Available online: https://www.xareltohcp.com/shared/product/xarelto/prescribing-information.pdf. Accessed on 22 August 2016.
- Sankyo D. Savaysa (edoxaban): US prescribing information. Available online: http://dsi.com/prescribing-informationportlet/getPIContent?productName+Savaysa&inline=true . Accessed on 22 August 2016.
- Stangier J. Clinical pharmacokinetics and pharmacodynamics of the oral direct thrombin inhibitor dabigatran etexilate. Clin Pharmacokinet 2008;47:285-95. [Crossref] [PubMed]
- Madan S, Muthusamy P, Mowers KL, et al. Safety of anticoagulation with uninterrupted warfarin vs. interrupted dabigatran in patients requiring an implantable cardiac device. Cardiovasc Diagn Ther 2016;6:3-9. [PubMed]
- Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009;361:2342-52. [Crossref] [PubMed]
- Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139-51. [Crossref] [PubMed]
- Schulman S, Kakkar AK, Goldhaber SZ, et al. Treatment of acute venous thromboembolism with dabigatran or warfarin and pooled analysis. Circulation 2014;129:764-72. [Crossref] [PubMed]
- Eriksson BI, Dahl OE, Rosencher N, et al. Oral dabigatran etexilate vs. subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: the RE-MODEL randomized trial. J Thromb Haemost 2007;5:2178-85. [Crossref] [PubMed]
- RE-MOBILIZE Writing Committee, Ginsberg JS, Davidson BL, et al. Oral thrombin inhibitor dabigatran etexilate vs North American enoxaparin regimen for prevention of venous thromboembolism after knee arthroplasty surgery. J Arthroplasty 2009;24:1-9. [Crossref] [PubMed]
- Laux V, Perzborn E, Kubitza D, et al. Preclinical and clinical characteristics of rivaroxaban: a novel, oral, direct factor Xa inhibitor. Semin Thromb Hemost 2007;33:515-23. [Crossref] [PubMed]
- Perzborn E, Strassburger J, Wilmen A, et al. In vitro and in vivo studies of the novel antithrombotic agent BAY 59-7939--an oral, direct Factor Xa inhibitor. J Thromb Haemost 2005;3:514-21. [Crossref] [PubMed]
- Kubitza D, Becka M, Wensing G, et al. Safety, pharmacodynamics, and pharmacokinetics of BAY 59-7939--an oral, direct Factor Xa inhibitor--after multiple dosing in healthy male subjects. Eur J Clin Pharmacol 2005;61:873-80. [Crossref] [PubMed]
- EINSTEIN Investigators, Bauersachs R, Berkowitz SD, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010;363:2499-510. [Crossref] [PubMed]
- EINSTEIN–PE Investigators, Büller HR, Prins MH, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med 2012;366:1287-97. [Crossref] [PubMed]
- Eriksson BI, Borris LC, Friedman RJ, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med 2008;358:2765-75. [Crossref] [PubMed]
- Lassen MR, Ageno W, Borris LC, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med 2008;358:2776-86. [Crossref] [PubMed]
- Lassen MR, Raskob GE, Gallus A, et al. Apixaban or enoxaparin for thromboprophylaxis after knee replacement. N Engl J Med 2009;361:594-604. [Crossref] [PubMed]
- Lassen MR, Raskob GE, Gallus A, et al. Apixaban versus enoxaparin for thromboprophylaxis after knee replacement (ADVANCE-2): a randomised double-blind trial. Lancet 2010;375:807-15. [Crossref] [PubMed]
- Lassen MR, Gallus A, Raskob GE, et al. Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. N Engl J Med 2010;363:2487-98. [Crossref] [PubMed]
- Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med 2013;369:799-808. [Crossref] [PubMed]
- Agnelli G, Buller HR, Cohen A, et al. Apixaban for extended treatment of venous thromboembolism. N Engl J Med 2013;368:699-708. [Crossref] [PubMed]
- Ogata K, Mendell-Harary J, Tachibana M, et al. Clinical safety, tolerability, pharmacokinetics, and pharmacodynamics of the novel factor Xa inhibitor edoxaban in healthy volunteers. J Clin Pharmacol 2010;50:743-53. [Crossref] [PubMed]
- Furugohri T, Isobe K, Honda Y, et al. DU-176b, a potent and orally active factor Xa inhibitor: in vitro and in vivo pharmacological profiles. J Thromb Haemost 2008;6:1542-9. [PubMed]
- Hokusai-VTE Investigators, Büller HR, Décousus H, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med 2013;369:1406-15. [Crossref] [PubMed]
- Fukuda T, Honda Y, Kamisato C, et al. Reversal of anticoagulant effects of edoxaban, an oral, direct factor Xa inhibitor, with haemostatic agents. Thromb Haemost 2012;107:253-9. [Crossref] [PubMed]
- Ansell JE, Bakhru SH, Laulicht BE, et al. Use of PER977 to reverse the anticoagulant effect of edoxaban. N Engl J Med 2014;371:2141-2. [Crossref] [PubMed]
- Lu G, DeGuzman FR, Hollenbach SJ, et al. A specific antidote for reversal of anticoagulation by direct and indirect inhibitors of coagulation factor Xa. Nat Med 2013;19:446-51. [Crossref] [PubMed]
- Pollack CV Jr, Reilly PA, Eikelboom J, et al. Idarucizumab for Dabigatran Reversal. N Engl J Med 2015;373:511-20. [Crossref] [PubMed]
- Siegal DM, Curnutte JT, Connolly SJ, et al. Andexanet Alfa for the Reversal of Factor Xa Inhibitor Activity. N Engl J Med 2015;373:2413-24. [Crossref] [PubMed]
- Tummala R, Kavtaradze A, Gupta A, et al. Specific antidotes against direct oral anticoagulants: A comprehensive review of clinical trials data. Int J Cardiol 2016;214:292-8. [Crossref] [PubMed]
- Sharma M, Cornelius VR, Patel JP, et al. Efficacy and Harms of Direct Oral Anticoagulants in the Elderly for Stroke Prevention in Atrial Fibrillation and Secondary Prevention of Venous Thromboembolism: Systematic Review and Meta-Analysis. Circulation 2015;132:194-204. [Crossref] [PubMed]
- Desai NR, Krumme AA, Schneeweiss S, et al. Patterns of initiation of oral anticoagulants in patients with atrial fibrillation- quality and cost implications. Am J Med 2014;127:1075-82.e1. [Crossref] [PubMed]
- Weitz JI, Semchuk W, Turpie AG, et al. Trends in Prescribing Oral Anticoagulants in Canada, 2008-2014. Clin Ther 2015;37:2506-2514.e4. [Crossref] [PubMed]
- Hirschl M, Kundi M. New oral anticoagulants in the treatment of acute venous thromboembolism - a systematic review with indirect comparisons. Vasa 2014;43:353-64. [Crossref] [PubMed]
- Kearon C, Akl EA, Ornelas J, et al. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest 2016;149:315-52. [Crossref] [PubMed]
- Patel AA, Ogden K, Mody SH, et al. The economic implications of switching to rivaroxaban from enoxaparin plus vitamin K antagonist in the treatment of venous thromboembolism. J Med Econ 2015;18:323-32. [Crossref] [PubMed]
- Bookhart BK, Haskell L, Bamber L, et al. Length of stay and economic consequences with rivaroxaban vs enoxaparin/vitamin K antagonist in patients with DVT and PE: findings from the North American EINSTEIN clinical trial program. J Med Econ 2014;17:691-5. [Crossref] [PubMed]
- Lefebvre P, Coleman CI, Bookhart BK, et al. Cost-effectiveness of rivaroxaban compared with enoxaparin plus a vitamin K antagonist for the treatment of venous thromboembolism. J Med Econ 2014;17:52-64. [Crossref] [PubMed]
- Preblick R, Kwong WJ, White RH, et al. Cost-effectiveness of edoxaban for the treatment of venous thromboembolism based on the Hokusai-VTE study. Hosp Pract (1995) 2015;43:249-57. [PubMed]


