Coronary microvascular dysfunction in women with nonobstructive ischemic heart disease as assessed by positron emission tomography
Introduction
From 2003 to 2013, rates of death attributable to cardiovascular diseases have declined in the United States, but the burden is still high. In fact, in 2013 cardiovascular diseases accounted for 1 of every 3 deaths, and coronary artery disease (CAD) alone for 1 of every 7 deaths (1,2). Moreover, CAD is the most common single cause of death in Europe, resulting in 19% of deaths in men and 20% of deaths in women (3).
It is important to mention that mortality in women is still significantly affected by delayed detection of ischemic heart disease (IHD) (4). This can be related to the fact that women are not alert about their cardiovascular risk and the clinicians often underestimate women´s symptoms of IHD, with the consequent failure or delay for implementing gender-specific diagnosis (5).
Traditional approaches for risk assessment of IHD are based on the physiological consequences of an epicardial coronary stenosis. Noteworthy, normal coronary arteries or nonobstructive CAD evaluated by cardiac computed tomography (CT) or invasive coronary angiography is a common finding in women with symptoms of ischemia (6-10). Therefore, assessment of risk based on a coronary stenosis approach may fail in women. However, the evaluation of coronary microcirculation may uncover other mechanisms, such as endothelial and vascular smooth muscle dysfunction responsible for the disease (10).
Current evidence shows that women presenting signs and symptoms of myocardial ischemia in the absence of obstructive CAD do not have a good prognosis (5). In fact, the Women’s Ischemia Syndrome Evaluation (WISE) (11) has shown that after 10 years of follow up, cardiovascular death or myocardial infarction occurred in 6.7% of women with no evident CAD, and in 12.8% of those with nonobstructive CAD. Furthermore, it is quite common persistent chest pain or worsening of the symptoms over the years (12).
Despite that widespread imaging techniques for the functional evaluation of CAD are single photon emission computed tomography (SPECT) and stress echocardiography, positron emission tomography (PET) has unique features that may help to reveal IHD in women: (I) the ability of quantify myocardial blood flow (MBF) in absolute units and to calculate coronary flow reserve (CFR); (II) a routine attenuation correction; and (III) a uniform independent resolution (13). These capabilities of PET can be useful because IHD in women may result from the interactions of many variables, such as focal stenosis, diffuse epicardial coronary narrowing, impaired endothelial shear stress and microvascular dysfunction, which difficult the diagnosis of the disease (14).
The present review will focus on assessment of coronary microvascular dysfunction, diagnosis and prognosis of IHD in women in absence of obstructive CAD as assessed by PET.
Assessing of coronary microvascular function by PET
CFR is a noninvasive measure of coronary vasomotor function that integrates the hemodynamic effects of epicardial coronary stenosis, diffuse atherosclerosis and microvascular dysfunction on myocardial tissue perfusion (13) (see Figure 1). CFR can be measured noninvasively by PET, transthoracic Doppler echocardiography and cardiovascular MRI (15). Dynamic PET imaging affords robust and reproducible measurements of absolute MBF in ml/min/g at rest and during pharmacological stress which allows the calculation of CFR (defined as the ratio between MBF at stress and MBF at rest) (13,16). Thresholds of MBF at rest in healthy individuals may vary between 0.4 to 1.2 mL/g/min and hyperemic MBF between 1.8 to 2.3 mL/g/min (depending on the PET flow tracer). For N-13 Ammonia and Rb-82, CFR less than 2 is considered abnormal (17). Of note, since CFR is a ratio, factors affecting measurements of resting and hyperemic MBFs may influence its calculation (16). Automated analysis programs are commercially available to calculate absolute MBF. In hybrid PET/CT scanners, list-mode acquisitions allow evaluation of MBF, myocardial perfusion and left ventricular function with a single injection of a tracer (18). Routine signal attenuation correction is performed with this technique both at rest and during stress. This is an important issue, because the breast attenuation on myocardial perfusion images is a common methodological problem in women that can cause artifacts in the anterior wall of the left ventricle. In the case of hybrid PET/CT instrumentation, a low dose CT is used for attenuation correction purposes and also for calculating coronary artery calcium score (19). Other important advantages for PET imaging are: (I) the low radiation dose for the patient as compared to conventional myocardial perfusion SPECT imaging (either with N-13 Ammonia or Rubidium-82 as flow tracers) (20); and (II) the short time of the study completion (between 30 to 90 minutes total, depending of the PET-tracer).
It is important to remark that the assessment of the coronary microcirculatory function by PET requires of certain pharmacological interventions. Typically, the flow responses to the application of smooth muscle vasodilators such as dipyridamole, adenosine or selective adenosine receptor agonists like regadenoson and binodenoson (21,22) maximally reduce the vascular resistance to flow in the microcirculation, and induce maximum or near-maximum hyperemia. All these compounds are direct coronary vasodilators, activating the adenosine (A2) receptors in the coronary arterial wall. In patients without CAD, adenosine and dipyridamole infusion increase coronary blood flow three to five times above baseline levels (23,24). On the other hand, in patients with obstructive and significant epicardial CAD, the MBF response to these pharmacological agents is diminished with the consequent reduction in CFR (25). MBF responses to these agents provide information ‘predominantly’ on the vascular smooth muscle function, but also on the integrated function of the coronary circulation. In fact, 20–40% of the maximal vasodilator response caused by dipyridamole or adenosine is related to the release of nitric oxide from intact endothelium due to increased shear stress on endothelial cells caused by the hyperemic response (26). Clinically, these vasodilators are used to assess global and regional CFR in patients with and without epicardial CAD (15).
There is some variability of MBF values throughout the spectrum of coronary atherosclerosis. This notion has been recently reviewed by Gould and collaborators (13). According to these authors, a possible explanation of such variability may be attributed to many factors such as the wide prevalence of subclinical respect to clinical CAD, the presence of risk factors (hypertension, diabetes, cigarette smoking, hyperlipidemia) and also technical issues as the variability inherent to the technique itself (which is around 14% for normal PET MBF) (27-29). Therefore, clinical interpretation of MBF and derived CFR requires understanding range values (normal limits), the limitations of the technique and the biological variables that can affect these measurements (13,16).
Endothelium-mediated coronary vasomotor function can be determined by the cold pressor test (CPT) (30,31). Several reports have been published using CPT as a sympathetic stimulus in healthy volunteers and patients with cardiovascular risk factors, yielding a good correlation of angiographically established flow-dependent dilation and MBF measured by PET (32). This test involves immersion of a patient’s hand or foot in icy water for 90 s while the injection of a PET flow tracer is performed. Sympathetic activation by CPT induces noradrenaline release. The direct effect of noradrenaline on smooth muscle cells is vasoconstriction, which is mediated by activation of α 1 and 2-adrenoceptors (33). When endothelial function is normal, this vasoconstrictor effect is counteracted by endothelial α 2-adrenoceptor-mediated stimulation of nitric oxide release with a net increase in coronary flow (34). In contrast, when endothelial cell function is abnormal, the vasoconstrictive response to noradrenaline predominates, and therefore an attenuated MBF response to CPT is observed (30). Typically, an increase of more than 50% of MBF respect to the resting flow is considered a preserved endothelial function. The accuracy and reproducibility of PET MBF measurements during CPT have been validated in several studies (32,35-37).
CT angiography can be performed in conjunction with myocardial perfusion PET imaging. This noninvasive approach allows integrating epicardial anatomy with the extent and characteristics of nonobstructive disease, which has been related to microvascular coronary flow (38). The prognostic impact of nonobstructive CAD has been demonstrated with cardiac CT, especially using atherosclerotic burden indexes like the segment involvement score (SIS) (39) or the CT-Leaman score (40). Both indexes were able to identify that a subset of patients that had “only” nonobstructive CAD but by having a high (SIS >5 or CT-Leaman score >5) coronary atherosclerotic burden were at an increased risk for coronary events, in the range of what is expected for the traditionally considered higher risk patients of the obstructive CAD subset.
In parallel, CT coronary angiography has evolved to evaluate the hemodynamic significance of coronary lesions by several approaches, including stress myocardial perfusion, fractional flow reserve (FFR)-CT, transluminal attenuation gradients, atherosclerotic plaque burden and characteristics, and more recently, CFR by means of dynamic CT perfusion acquisition techniques (41-43) (see Figure 1).

Diagnostic accuracy and prognostic value of PET in women
Current literature supports a high diagnostic accuracy for cardiac PET perfusion imaging. The reasons for this are multiple including routine attenuation correction, image quality, as well as the properties of PET tracers that follow MBF in a more linear fashion than SPECT tracers (44). A meta-analysis of the cardiac PET literature demonstrated high sensitivity (92%) and specificity (85%) for the detection of CAD (45). Two meta-analyses comparing Rubidium-82 and N-13 Ammonia PET data to SPECT confirmed the diagnostic accuracy. Both demonstrated a higher diagnostic accuracy for PET with a 3-5% increase in specificity and 4–5% increase in sensitivity (46,47) (see Table 1).

Full table
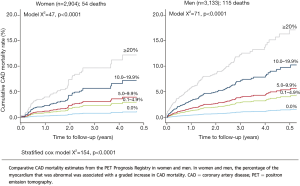
Myocardial perfusion imaging with PET has a high diagnostic accuracy for both women and men, which is higher than conventional SPECT (88% vs. 67%, P=0.009) (48). In particular, in the PET Prognosis Multicenter Registry, Kay et al. (49) described in a total of 6,307 women and men that stress myocardial perfusion with Rb-82 provided significant and clinically meaningful risk stratification in both genders. There was a proportional relationship between CAD mortality and stress Rb-82 PET myocardial perfusion defect size and extent, as assessed by percentage of myocardium involved (see Figure 2). The unadjusted 5-year CAD mortality ranged from 0.9% to 12.9% for women (P<0.0001) and from 1.5% to 17.4% for men (P<0.0001) for 0% to ≥15% abnormal myocardium at stress. Importantly, in this study the percentage of abnormal stress myocardium was independently predictive of CAD mortality in both men and women. The finding of sex equity in risk stratification with Rb-82 PET supports comparability with evidence from SPECT and other stress imaging modalities (49).
Identification and prognostic value of microvascular dysfunction in women: role of quantification of MBF by PET
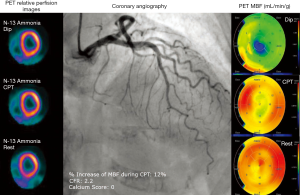
As previously discussed, the accurate diagnosis of IHD in women is often difficult because complex interactions between focal stenosis, diffuse epicardial coronary narrowing, impaired endothelial shear stress and microvascular dysfunction are responsible for their symptoms and the disease (14). Moreover, symptomatic women with chest pain undergoing coronary angiography may exhibit normal or insignificant CAD in up to 50% of the cases (5). In this scenario, establishing the diagnosis based on the relative distribution of a flow tracer in the myocardium may underestimate their risk. Instead, absolute quantification of MBF can identify more accurately women at risk in absence of obstructive CAD (see Figure 3).
In fact, impaired CFR and abnormal coronary vascular responsiveness to CPT have been reported in women with risk factors for CAD and without stress-regional myocardial perfusion defects (50-53). These functional alterations at microcirculation level in women at risk for IHD are in agreement with observations using dynamic PET imaging in patients with traditional coronary risk factors (33,51,54,55). For example, Di Carli et al. (56) using PET imaging have compared MBF responses to CPT and during maximal hyperemia in three groups of asymptomatic women: healthy premenopausal women, premenopausal women with diabetes and healthy postmenopausal women. All women exhibited normal relative myocardial perfusion PET scans at rest and during stress. However, absolute measurements of MBF showed a diminished CFR and an impaired response of MBF to CPT in diabetic premenopausal women, similar to those observed in postmenopausal women. Thus, the presence of diabetes removes the protective vascular effects observed in premenopausal women which have prognostic implications that can be detected by dynamic PET imaging (57).
The WISE study reported a subgroup of symptomatic women for chest pain with non-obstructive CAD that showed a microvascular dysfunction (defined as CFR <2.5 in response to adenosine, as evaluated by PET) distributed heterogeneously in the myocardium (58). The investigators found that resting absolute MBF was significantly higher in the left anterior descending coronary artery territory than in the left circumflex coronary artery and right coronary artery territories. Accordingly, a comparison of the CFR in the three coronary arteries demonstrated that the left circumflex coronary artery distribution had lower values than the other two vascular territories. This finding suggests that microvascular dysfunction manifested by impaired CFR may be a localized process, which is distributed heterogeneously in the myocardium in symptomatic women.
Abnormal MBF responses to CPT and an impaired CFR are associated with future cardiac events in both men and women even in absence of epicardial obstructive CAD (36,59-62). Importantly, noninvasive assessment of coronary microcirculation function by PET provides incremental risk stratification beyond measures of clinical risk, including estimations of left ventricular systolic function and the extent and severity of myocardial ischemia (by semiquantitative analysis), resulting in an incremental risk reclassification of patients with known or suspected CAD (59-62).
Naya et al. (63) have shown that a preserved CFR has a high negative predictive-value for excluding high-risk CAD on coronary angiography. On the other hand, although an abnormal CFR increases the likelihood of significant obstructive CAD, it cannot differentiate significant epicardial stenosis from nonobstructive disease, diffuse atherosclerosis or microvascular dysfunction.
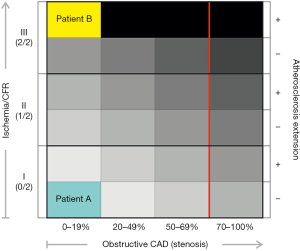
Interestingly, it has been described the association between CFR, angiographic CAD and cardiovascular outcomes (64). The authors included 329 patients referred for coronary angiography after stress PET and followed by a median of 3.1 years. They found a modest correlation between CFR and CAD prognostic index and both parameters were independently associated with cardiac events. Patients with low CFR experienced rates of events similar to those of subjects with high angiographic scores, and those with low CFR who underwent coronary revascularization by CABG experienced event rates comparable to those with preserved CAD (independently of revascularization). Thus, based on this observational study global CFR confers risk independently of luminal angiographic severity. This is an attractive concept which has recently been explored in symptomatic women and men without CAD. Murthy et al. (65) investigated 405 men and 813 women referred for evaluation of suspected CAD and without myocardial perfusion defects at rest and during stress by PET. Major cardiac events (MACE) were assessed over a median follow-up of 1.3 years. Coronary microvascular dysfunction (CFR <2) was a powerful predictor of MACE and resulted in favorable net reclassification improvement after adjustment for clinical risk and left ventricular function. Importantly, these findings were regardless of gender. Thus, coronary vasomotor dysfunction as assessed by PET reveals men and women at increased clinical risk. More recently, it has been reported that excess cardiovascular risk in women was independently associated with severely impaired CFR (<1.6) in absence of obstructive CAD by invasive coronary angiography (66) (see Table 2). This finding is of clinical relevance because, as mentioned before, women have significantly lower burden of epicardial CAD as compared to men and absolute quantification of MBF may allow reclassifying their risk (see Figure 4).

Full table
Conclusions and future directions
Taken all together, symptomatic women with nonobstructive CAD are at risk when coronary vascular dysfunction is present. Observational studies have demonstrated that PET imaging can identify coronary microvascular dysfunction and predict outcomes in women with IHD. Randomized and multicenter studies are needed to standardize the routine clinical application of quantification of PET-MBF and CFR in such patients. Of note, in the current multimodality imaging era we can evaluate the whole spectrum of the atherosclerosis process and predict risk. Further studies are warranted to explore the complementary role of cardiac imaging techniques to identify women at risk.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Writing Group Members. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation 2016;133:e38-360. [Crossref] [PubMed]
- Gupta A, Wang Y, Spertus JA, et al. Trends in acute myocardial infarction in young patients and differences by sex and race, 2001 to 2010. J Am Coll Cardiol 2014;64:337-45. [Crossref] [PubMed]
- Townsend N, Nichols M, Scarborough P, et al. Cardiovascular disease in Europe 2015: epidemiological update. Eur Heart J 2015;36:2673-4. [Crossref] [PubMed]
- Pepine CJ, Ferdinand KC, Shaw LJ, et al. Emergence of Nonobstructive Coronary Artery Disease: A Woman's Problem and Need for Change in Definition on Angiography. J Am Coll Cardiol 2015;66:1918-33. [Crossref] [PubMed]
- Dean J, Cruz SD, Mehta PK, et al. Coronary microvascular dysfunction: sex-specific risk, diagnosis, and therapy. Nat Rev Cardiol 2015;12:406-14. [Crossref] [PubMed]
- Campisi R. Noninvasive assessment of coronary microvascular function in women at risk for ischaemic heart disease. Int J Clin Pract 2008;62:300-7. [Crossref] [PubMed]
- Eastwood JA, Johnson BD, Rutledge T, et al. Anginal symptoms, coronary artery disease, and adverse outcomes in Black and White women: the NHLBI-sponsored Women's Ischemia Syndrome Evaluation (WISE) study. J Womens Health (Larchmt) 2013;22:724-32. [Crossref] [PubMed]
- Reis SE, Holubkov R, Conrad Smith AJ, et al. Coronary microvascular dysfunction is highly prevalent in women with chest pain in the absence of coronary artery disease: results from the NHLBI WISE study. Am Heart J 2001;141:735-41. [Crossref] [PubMed]
- Pepine CJ. Multiple causes for ischemia without obstructive coronary artery disease: not a short list. Circulation 2015;131:1044-6. [Crossref] [PubMed]
- Lin FY, Shaw LJ, Dunning AM, et al. Mortality risk in symptomatic patients with nonobstructive coronary artery disease: a prospective 2-center study of 2,583 patients undergoing 64-detector row coronary computed tomographic angiography. J Am Coll Cardiol 2011;58:510-9. [Crossref] [PubMed]
- Sharaf B, Wood T, Shaw L, et al. Adverse outcomes among women presenting with signs and symptoms of ischemia and no obstructive coronary artery disease: findings from the National Heart, Lung, and Blood Institute-sponsored Women's Ischemia Syndrome Evaluation (WISE) angiographic core laboratory. Am Heart J 2013;166:134-41. [Crossref] [PubMed]
- Johnson BD, Shaw LJ, Pepine CJ, et al. Persistent chest pain predicts cardiovascular events in women without obstructive coronary artery disease: results from the NIH-NHLBI-sponsored Women's Ischaemia Syndrome Evaluation (WISE) study. Eur Heart J 2006;27:1408-15. [Crossref] [PubMed]
- Gould KL, Johnson NP, Bateman TM, et al. Anatomic versus physiologic assessment of coronary artery disease. Role of coronary flow reserve, fractional flow reserve, and positron emission tomography imaging in revascularization decision-making. J Am Coll Cardiol 2013;62:1639-53. [Crossref] [PubMed]
- Patel MB, Bui LP, Kirkeeide RL, et al. Imaging Microvascular Dysfunction and Mechanisms for Female-Male Differences in CAD. JACC Cardiovasc Imaging 2016;9:465-82. [Crossref] [PubMed]
- Campisi R, Di Carli MF. Assessment of coronary flow reserve and microcirculation: a clinical perspective. J Nucl Cardiol 2004;11:3-11. [Crossref] [PubMed]
- Camici PG, Rimoldi OE. The clinical value of myocardial blood flow measurement. J Nucl Med 2009;50:1076-87. [Crossref] [PubMed]
- Schindler TH. Myocardial blood flow: Putting it into clinical perspective. J Nucl Cardiol 2016;23:1056-71. [Crossref] [PubMed]
- Bateman TM, Lance Gould K, Di Carli MF. Proceedings of the Cardiac PET Summit, 12 May 2014, Baltimore, MD: 3: Quantitation of myocardial blood flow. J Nucl Cardiol 2015;22:571-8. [Crossref] [PubMed]
- Dorbala S, Di Carli MF, Delbeke D, et al. SNMMI/ASNC/SCCT guideline for cardiac SPECT/CT and PET/CT 1.0. J Nucl Med 2013;54:1485-507. [Crossref] [PubMed]
- Taqueti VR, Dorbala S. The role of positron emission tomography in the evaluation of myocardial ischemia in women. J Nucl Cardiol 2016;23:1008-15. [Crossref] [PubMed]
- Udelson JE, Heller GV, Wackers FJ, et al. Randomized, controlled dose-ranging study of the selective adenosine A2A receptor agonist binodenoson for pharmacological stress as an adjunct to myocardial perfusion imaging. Circulation 2004;109:457-64. [Crossref] [PubMed]
- Cerqueira MD. The future of pharmacologic stress: selective A2A adenosine receptor agonists. Am J Cardiol 2004;94:33D-40D; discussion 40D-42D.
- Schindler TH, Zhang XL, Vincenti G, et al. Role of PET in the evaluation and understanding of coronary physiology. J Nucl Cardiol 2007;14:589-603. [Crossref] [PubMed]
- Schindler TH, Schelbert HR, Quercioli A, et al. Cardiac PET imaging for the detection and monitoring of coronary artery disease and microvascular health. JACC Cardiovasc Imaging 2010;3:623-40. [Crossref] [PubMed]
- Di Carli M, Czernin J, Hoh CK, et al. Relation among stenosis severity, myocardial blood flow, and flow reserve in patients with coronary artery disease. Circulation 1995;91:1944-51. [Crossref] [PubMed]
- Buus NH, Bottcher M, Hermansen F, et al. Influence of nitric oxide synthase and adrenergic inhibition on adenosine-induced myocardial hyperemia. Circulation 2001;104:2305-10. [Crossref] [PubMed]
- Kaufmann PA, Gnecchi-Ruscone T, Yap JT, et al. Assessment of the reproducibility of baseline and hyperemic myocardial blood flow measurements with 15O-labeled water and PET. J Nucl Med 1999;40:1848-56. [PubMed]
- Nagamachi S, Czernin J, Kim AS, et al. Reproducibility of measurements of regional resting and hyperemic myocardial blood flow assessed with PET. J Nucl Med 1996;37:1626-31. [PubMed]
- Manabe O, Yoshinaga K, Katoh C, et al. Repeatability of rest and hyperemic myocardial blood flow measurements with 82Rb dynamic PET. J Nucl Med 2009;50:68-71. [Crossref] [PubMed]
- Nabel EG, Ganz P, Gordon JB, et al. Dilation of normal and constriction of atherosclerotic coronary arteries caused by the cold pressor test. Circulation 1988;77:43-52. [Crossref] [PubMed]
- Zeiher AM, Drexler H, Wollschlaeger H, et al. Coronary vasomotion in response to sympathetic stimulation in humans: importance of the functional integrity of the endothelium. J Am Coll Cardiol 1989;14:1181-90. [Crossref] [PubMed]
- Siegrist PT, Gaemperli O, Koepfli P, et al. Repeatability of cold pressor test-induced flow increase assessed with H(2)(15)O and PET. J Nucl Med 2006;47:1420-6. [PubMed]
- Campisi R, Czernin J, Schoder H, et al. L-Arginine normalizes coronary vasomotion in long-term smokers. Circulation 1999;99:491-7. [Crossref] [PubMed]
- Kichuk MR, Seyedi N, Zhang X, et al. Regulation of nitric oxide production in human coronary microvessels and the contribution of local kinin formation. Circulation 1996;94:44-51. [Crossref] [PubMed]
- Schindler TH, Zhang XL, Vincenti G, et al. Diagnostic value of PET-measured heterogeneity in myocardial blood flows during cold pressor testing for the identification of coronary vasomotor dysfunction. J Nucl Cardiol 2007;14:688-97. [Crossref] [PubMed]
- Schindler TH, Nitzsche EU, Schelbert HR, et al. Positron emission tomography-measured abnormal responses of myocardial blood flow to sympathetic stimulation are associated with the risk of developing cardiovascular events. J Am Coll Cardiol 2005;45:1505-12. [Crossref] [PubMed]
- Schindler TH, Zhang XL, Prior JO, et al. Assessment of intra- and interobserver reproducibility of rest and cold pressor test-stimulated myocardial blood flow with (13)N-ammonia and PET. Eur J Nucl Med Mol Imaging 2007;34:1178-88. [Crossref] [PubMed]
- Rodriguez-Granillo GA, Campisi R, Carrascosa P. Noninvasive Cardiac Imaging in Patients with Known and Suspected Coronary Artery Disease: What is in it for the Interventional Cardiologist? Curr Cardiol Rep 2016;18:3. [Crossref] [PubMed]
- Bittencourt MS, Hulten E, Ghoshhajra B, et al. Prognostic value of nonobstructive and obstructive coronary artery disease detected by coronary computed tomography angiography to identify cardiovascular events. Circ Cardiovasc Imaging 2014;7:282-91. [Crossref] [PubMed]
- Mushtaq S, De Araujo Goncalves P, Garcia-Garcia HM, et al. Long-term prognostic effect of coronary atherosclerotic burden: validation of the computed tomography-Leaman score. Circ Cardiovasc Imaging 2015;8:e002332. [Crossref] [PubMed]
- Gonçalves PA, Rodriguez-Granillo GA, Spitzer E, et al. Functional Evaluation of Coronary Disease by CT Angiography. JACC Cardiovasc Imaging 2015;8:1322-35. [Crossref] [PubMed]
- Koo BK, Erglis A, Doh JH, et al. Diagnosis of ischemia-causing coronary stenoses by noninvasive fractional flow reserve computed from coronary computed tomographic angiograms. Results from the prospective multicenter DISCOVER-FLOW (Diagnosis of Ischemia-Causing Stenoses Obtained Via Noninvasive Fractional Flow Reserve) study. J Am Coll Cardiol 2011;58:1989-97. [Crossref] [PubMed]
- Min JK, Koo BK, Erglis A, et al. Usefulness of noninvasive fractional flow reserve computed from coronary computed tomographic angiograms for intermediate stenoses confirmed by quantitative coronary angiography. Am J Cardiol 2012;110:971-6. [Crossref] [PubMed]
- Beanlands R, Heller GV. Proceedings of the ASNC Cardiac PET Summit, 12 May 2014, Baltimore, MD: 1: The value of PET: Integrating cardiovascular PET into the care continuum. J Nucl Cardiol 2015;22:557-62. [Crossref] [PubMed]
- Nandalur KR, Dwamena BA, Choudhri AF, et al. Diagnostic performance of positron emission tomography in the detection of coronary artery disease: a meta-analysis. Acad Radiol 2008;15:444-51. [Crossref] [PubMed]
- Mc Ardle BA, Dowsley TF, deKemp RA, et al. Does rubidium-82 PET have superior accuracy to SPECT perfusion imaging for the diagnosis of obstructive coronary disease?: A systematic review and meta-analysis. J Am Coll Cardiol 2012;60:1828-37. [Crossref] [PubMed]
- Parker MW, Iskandar A, Limone B, et al. Diagnostic accuracy of cardiac positron emission tomography versus single photon emission computed tomography for coronary artery disease: a bivariate meta-analysis. Circ Cardiovasc Imaging 2012;5:700-7. [Crossref] [PubMed]
- Bateman TM, Heller GV, McGhie AI, et al. Diagnostic accuracy of rest/stress ECG-gated Rb-82 myocardial perfusion PET: comparison with ECG-gated Tc-99m sestamibi SPECT. J Nucl Cardiol 2006;13:24-33. [Crossref] [PubMed]
- Kay J, Dorbala S, Goyal A, et al. Influence of sex on risk stratification with stress myocardial perfusion Rb-82 positron emission tomography: Results from the PET (Positron Emission Tomography) Prognosis Multicenter Registry. J Am Coll Cardiol 2013;62:1866-76. [Crossref] [PubMed]
- Peterson LR, Eyster D, Davila-Roman VG, et al. Short-term oral estrogen replacement therapy does not augment endothelium-independent myocardial perfusion in postmenopausal women. Am Heart J 2001;142:641-7. [Crossref] [PubMed]
- Campisi R, Nathan L, Pampaloni MH, et al. Noninvasive assessment of coronary microcirculatory function in postmenopausal women and effects of short-term and long-term estrogen administration. Circulation 2002;105:425-30. [Crossref] [PubMed]
- Duvernoy C, Martin J, Briesmiester K, et al. Myocardial blood flow and flow reserve in response to hormone therapy in postmenopausal women with risk factors for coronary disease. J Clin Endocrinol Metab 2004;89:2783-8. [Crossref] [PubMed]
- Schindler TH, Campisi R, Dorsey D, et al. Effect of hormone replacement therapy on vasomotor function of the coronary microcirculation in post-menopausal women with medically treated cardiovascular risk factors. Eur Heart J 2009;30:978-86. [Crossref] [PubMed]
- Di Carli MF, Janisse J, Grunberger G, et al. Role of chronic hyperglycemia in the pathogenesis of coronary microvascular dysfunction in diabetes. J Am Coll Cardiol 2003;41:1387-93. [Crossref] [PubMed]
- Guethlin M, Kasel AM, Coppenrath K, et al. Delayed response of myocardial flow reserve to lipid-lowering therapy with fluvastatin. Circulation 1999;99:475-81. [Crossref] [PubMed]
- Di Carli MF, Afonso L, Campisi R, et al. Coronary vascular dysfunction in premenopausal women with diabetes mellitus. Am Heart J 2002;144:711-8. [Crossref] [PubMed]
- Murthy VL, Naya M, Foster CR, et al. Association between coronary vascular dysfunction and cardiac mortality in patients with and without diabetes mellitus. Circulation 2012;126:1858-68. [Crossref] [PubMed]
- Marroquin OC, Holubkov R, Edmundowicz D, et al. Heterogeneity of microvascular dysfunction in women with chest pain not attributable to coronary artery disease: implications for clinical practice. Am Heart J 2003;145:628-35. [Crossref] [PubMed]
- Murthy VL, Naya M, Foster CR, et al. Improved cardiac risk assessment with noninvasive measures of coronary flow reserve. Circulation 2011;124:2215-24. [Crossref] [PubMed]
- Ziadi MC, Dekemp RA, Williams KA, et al. Impaired myocardial flow reserve on rubidium-82 positron emission tomography imaging predicts adverse outcomes in patients assessed for myocardial ischemia. J Am Coll Cardiol 2011;58:740-8. [Crossref] [PubMed]
- Fukushima K, Javadi MS, Higuchi T, et al. Prediction of short-term cardiovascular events using quantification of global myocardial flow reserve in patients referred for clinical 82Rb PET perfusion imaging. J Nucl Med 2011;52:726-32. [Crossref] [PubMed]
- Herzog BA, Husmann L, Valenta I, et al. Long-term prognostic value of 13N-ammonia myocardial perfusion positron emission tomography added value of coronary flow reserve. J Am Coll Cardiol 2009;54:150-6. [Crossref] [PubMed]
- Naya M, Murthy VL, Taqueti VR, et al. Preserved coronary flow reserve effectively excludes high-risk coronary artery disease on angiography. J Nucl Med 2014;55:248-55. [Crossref] [PubMed]
- Taqueti VR, Hachamovitch R, Murthy VL, et al. Global coronary flow reserve is associated with adverse cardiovascular events independently of luminal angiographic severity and modifies the effect of early revascularization. Circulation 2015;131:19-27. [Crossref] [PubMed]
- Murthy VL, Naya M, Taqueti VR, et al. Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation 2014;129:2518-27. [Crossref] [PubMed]
- Taqueti VR, Shaw LJ, Cook NR, et al. Excess Cardiovascular Risk in Women Relative to Men Referred for Coronary Angiography is Associated with Severely Impaired Coronary Flow Reserve, not Obstructive Disease. Circulation 2017;135:566-77. [Crossref] [PubMed]


