Impact of plaque characteristics on the degree of functional stenosis
Coronary atherosclerosis assessment by CCTA: detailed plaque features and atherosclerotic burden scores
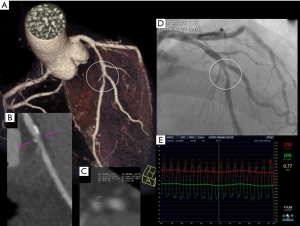
Coronary CT angiography (CCTA) is now widely recognized as a useful tool to noninvasively rule out significant coronary stenosis, and is increasingly used as a gatekeeper for invasive coronary angiography, which is becoming more and more reserved for patients at high probability of coronary artery disease in need of a revascularization procedure (1). Although still not in the clinical arena, one of the most appealing features of CCTA is the ability to look be-yond the luminal stenosis and provide a detailed evaluation of the coronary wall depicting several features like plaque composition (calcified, noncalcified or mixed), plaque burden, remodeling index and degree of attenuation measured in Hounsfield units (HU), and several of these features have been associated with the future development of events (2-8) (Figure 1). In fact, the potential prognostic value of these detailed plaque features provided by CCTA has opened a new field of research in coronary artery disease, which in the last 2–3 decades has been struggling in the search of the vulnerable plaque (10,11). In addition, when looking for the same disease using a different imaging modality, is seems wise to follow the steps of previous research and therefore the excitement in this field has to be balanced by the difficulties that other imaging modalities, some of which of very high spatial resolution, have faced in the search of these vulnerable plaques. Some landmark studies that used invasive intracoronary imaging have prospectively linked several plaque features to the development of future coronary events, although with a modest positive predictive value (12-14). Studies with CCTA have also demonstrated the prognostic value of some of these detailed plaque features like positive remodeling, the presence of low attenuation plaques and the presence of a napkin ring sign. Since several coronary plaques are frequently found in the coronary tree even in low to intermediate CAD probability patients undergoing CCTA to rule out coronary stenosis (15,16), and since detailed plaque evaluation is time consuming, requires very good image quality and can be associated with low interobserver variability, another possible approach is to provide the total coronary atherosclerotic burden as a score, and some have been developed and validated as prognostic tools (17-19), providing imagers and researcher with additional tools and a change of focus from just the vulnerable plaque to the vulnerable coronary tree (20).
The search of functional information with CT angiography
Recently, CTA has been increasingly looked as a possible provider of functional information, moving from the traditional view of a purely anatomical exam. The rational for this is two-sided: on one hand, current clinical decision making on the need for myocardial revascularization is based on the presence of ischemia, since it has been extensively documented that there is no expected benefit of intervention for coronary stenosis that are not functionally significant (21,22). On the other hand, although several diagnostic tools are already available, some of them have limited diagnostic performance (like exercise ECG), several patient related potential limitations (like stress Echocardiography and cardiac magnetic resonance imaging), depend on a significant amount of radiation exposure (like single photon emission computed tomography) and have availability issues apart from some selected centers (like cardiac MRI) (23). In what concerns CCTA, the impressive reductions in radiation exposure of last generation scanners (24-26), coupled with their increasingly availability makes CTA a very attractive 2 in 1 modality to evaluate both the presence and the impact of CAD. Therefore, cardiac CT is becoming more and more a modality that is able not only to rule out CAD with a very high accuracy but also to provide functional information, moving beyond the usual classification of obstructive vs. nonobstructive to a more functional-based interpretation of signipara-doxicallyficant vs. nonsignificant CAD, a feature that is more in line with current clinical decision algorithm (9).
In line with this rational, the following research lines have been explored like myocardial perfusion imaging, fractional flow reserve computed from CTA (FFRCT), transluminal attenuation gradients and corrected coronary opacification indexes, which have been recently reviewed elsewhere (9), and some of them are revisited in the same issue of this journal.
One additional line of research aimed at extracting functional significance out of CTA datasets, the more recent and least explored of these, is the link between certain atherosclerotic plaque characteristics and the functional impact of coronary lesions, which will be developed further in the following chapter.
Atherosclerotic plaque characteristics and functional information: beyond stenosis severity
The concept that there is a modest correlation between the degree of anatomical stenosis and functional significance is not new and has been pointed out in several studies using ICA and the gold standard invasive FFR (21,27) and also with CCTA and noninvasive FFR (FFRCT) (28,29). Also important is the fact that these mismatches can also be observed on their counterparts, meaning that not only some of the apparently significant lesions (>50% and even >70% stenosis) are not causing ischemia (27), but also that nonobstructive (<50% stenosis) lesions can paradoxically be associated with ischemia, both on invasive (ICA/FFR) (30) and noninvasive (CCTA/FFRCT) (31) coronary evaluation. In a study including 1,000 patients evaluated by invasive coronary angiography and FFR, 16% of those with nonobstructive (<50% stenosis) were reversed mismatches, having a significant (<0.80) FFR (30). Although the limitations in quantifying stenosis severity using ICA have to be acknowledged, this study reinforces the concept that nonobstructive lesions can be associated with ischemia. Interestingly in this study was the fact that these reverse mismatches were more often found in the left main and independently associated with a left anterior descending artery location and the presence of a larger plaque burden and plaque rupture, features that link atherosclerotic plaque features to functional significance of coronary lesions. Recently this relation was also depicted using CCTA. In a study that included 252 stable patients undergoing both CCTA and ICA with FFR, Park et al. were able to document an association between certain CCTA atherosclerotic plaque characteristics (APCs) and the presence of ischemia by invasive FFR (31). In their study, lesion length, positive remodelling (index ≥1.1), and the presence of low attenuation plaque (<30 HU) were independent predictors of ischemia by invasive FFR. Besides the expected finding that a significant percentage of CCTA obstructive (≥50% stenosis) lesions were not functionally significant by FFR, the most but striking result was the fact that even among nonobstructive CAD lesions, 17% were associatathero-scleroticed with ischemia, a result that was in line with the previously discussed studies that documented the visual functional mismatches between FFR and invasive coronary angiography. In another small study, including 58 patients with intermediate stenosis on CCTA undergoing ICA with FFR, the extent of coronary atherosclerotic burden quantified as aggregate plaque volume (APV), has been found to be incremental to several luminal narrowing measurements (diameter stenosis, minimum luminal diameter, area stenosis and minimum lumen area) to predict functional significance (FFR <0.80) (32). Taken together these studies underline the fact that some nonobstructive lesions but with high CTA risk features can be associated with the presence of ischemia. The reason for this might be related to the fact that these lesions with high plaque burden can be associated with endothelial dysfunction, since it has been demonstrated in small studies using virtual histology (IVUS-VH) that plaques with larger necrotic core are more prevalent in patients with endothelial dysfunction (33). The pathophysiological mechanism linking plaque features and ischemia deserves further evaluation but in any case the prognostic impact of coronary plaque burden is not a new concept. In a large study including 4,137 patients pooled from 6 IVUS studies, Nicholls et al. were able to demonstrate that percent atheroma volume (PAV) was an independent predictor of events (34). Also interesting in that study was the fact that not only PAV but also disease burden progression was associated with outcomes, a result that is in line with the recent study of Motoyama et al. relating coronary events to plaque progression documented on patients undergoing serial scans (6). Similar observations have been made using oresultptical coherence tomography and simultaneous functional assessment of the lesions. Yonetsu et al. showed a direct association of a larger lipidic arc and lipid length with microcirculatory disturbances (35). Usui et al. reported that physiological severe stenosis were twice as prevalent in thin cap fibroatheroma lesions. These reports suggest that these high risk lesions may affect the microcirculation downstream (36). Taqueti et al. recently presented data comparing CAD lesions in women and men assessed by angiography and coronary flow reserve (CFR) in which women had lower plaque burden. The adjusted cardiovascular events were higher in the women’s group, two times higher when compared to men’s, despite the lower CFR values in women’s group (37).
Recently a new terminology was proposed regarding those mismatches between anatomy and function: The NIPSS (no ischemia in the presence of significant stenosis) and the PINSS (presence of ischemia with no significant steno-sis), recognizing this interesting subset of patients in which certain plaque features might be responsible for the apparent contradiction between anatomy and physiology (38). In fact, lesions in this last subgroup can be considered “false negative” of CCTA and might explain the worse than expected prognosis of patients that don’t have obstructive lesions but have high disease burden, as it has been recently demonstrated with the use of CCTA coronary atherosclerotic burden scores (17-19) that take in consideration all lesions independent of stenosis severity. As the knowledge of coronary disease pathophysiology advances, the traditional anatomic-based dichotomic concept of obstructive vs. nonobstructive is becoming more and more outdated and becoming replaced by a concept of high vs. low risk plaque features/burden, more in line with the recent evidence provided by both invasive and noninvasive atherosclerosis imaging. Further, the field of functional assessment will be revisited in light of the recent reports. In the FUTURE trial (American Heart Association 2016), Rioufol et al., showed an increase in mortality from any cause in FFR guided management group of patients when compared to the optimal medical treatment alone, interrupting prematurely the study (39).
Intravascular coronary imaging and its relation with functional lesion assessments
The 3-dimensional and dynamic nature of the coronary vasculature cannot be fully appreciated by planar angiography. Frequently, defining the proper angiographic angulation that provides a straight, nonforeshortened view of the target coronary segment without overlapping of other vessels may be a challenge in the catheter laboratory. In addition, determination of disease severity by angiography is hampered by the diffuse nature of atherosclerosis and its most common eccentric growth in the vessel wall. Hence, lesions can appear more stenotic in one orthogonal view than in the other, making clinical decisions difficult. The so-called intermediate lesion is the more prevalent phenotype in the coronary tree. The American Heart Association/American College of Cardiology/Society for angiography and Interventions (AHA/ACC/ SCAI) guidelines define an intermediate coronary lesion as a plaque producing a 50–70% angiographic stenosis (40). These plaques represent a heterogeneous group of coronary lesions, which may or may not be hemodynamically flow limiting. Intravascular imaging, particularly IVUS, was granted a class IIb indication (level of evidence B) for the “IVUS may be reasonable for the assessment of non–left main coronary arteries with angiographically intermediate coronary stenoses” and a class IIa indication (level of evidence B) for the “IVUS is reasonable for the assessment of angiographically indeterminant left main coronary artery disease” in the American Guidelines. In the European guidelines is also a class IIa indication (level of evidence B) for the “IVUS to assess severity and optimize treatment of unprotected left main lesions”. For other intermediate lesions IVUS is not endorsed by the European Guidelines in the absence of prospective randomised evidence (41).
IVUS minimum luminal cross-sectional area (MLA) has proved to be a good morphometric surrogate of coronary physiology. IVUS MLA showed a direct correlation with coronary flow reserve determined by Doppler flow-wire (r=0.831, P<0.001) (42). FIRST trial, demonstrated, in a cohort of 350 patients with intermediate lesions and FFR ≤0.8, a MLA <3.07 mm2 (64.0% sensitivity, 64.9% specificity and area under curve of 0.65) and a FFR correlation with plaque burden size (r=−0.220, P<0.001) (43). Han et al. reported similar results after assessing 881 lesions with a MLA <3.0 mm2 (and MLA<2.75 mm2 for Asians) (44). In 2014, a meta-analysis pooled 15 studies enrolling a total of 3,428 patients (3,775 lesions), showing a mean MLA of 2.59 mm2 with 73% sensitivity, 66% of specificity and AUC =0.778 (45). The optical coherence tomography (OCT) was also used to evaluate lesions with FFR ≤0.75 and demonstrated a correlation with MLA <1.91 mm2, minimum lumen diameter (MLD) <1.35 mm, plaque burden >70% (46). Thus, accessing only FFR ≤0.8 lesions, Gonzalo et al., compared OCT and IVUS measurements presenting an IVUS MLA of 2.36 mm2 and 1.95 mm2 for OCT, with a relative difference of 32.3±26% and absolute difference of 0.65±0.62 mm2 (limits of agreement of –0.57 to 1.87 mm2) (47). The MLD value for IVUS was 1.59 mm2, 1.34 mm2 for OCT with a relative difference of 19.5±16%, absolute difference of 0.27±0.23 mm2 (limits of agreement of –0.20 to 0.72 mm2) and area of stenosis of 61% for IVUS and 70% for OCT (46). When assessed using virtual histology (IVUS-VH) inter-mediate lesions showed a prone to have less necrotic core (14.2±8% vs. 19.2±10.2%, P=0.08) and greater plaque burden (54.6±0.7% vs. 51.7±0.7% P=0.1) (Table S1) (48). In 73 patients studied pre-intervention, an MLA of ≥ 4.0 mm2 had a diagnostic accuracy of 89% in predicting a coronary flow reserve >2.0. Likewise, IVUS has been correlated with noninvasive single-photon emission computed tomography (SPECT) (49). A 4 mm2 MLA by IVUS had 88% sensitivity and 90% specificity to discriminate the SPECT (+) group from the SPECT (–) group. The relatively simple cut-off of 4.0 mm2 MLA can be used as a criterion in the clinical decision making process. This cut-off value has also been identified using fractional flow reserve (FFR) as the gold standard in the assessment of lesion severity (50). The association of MLA and ischaemia has been revisited more recently using an existing IVUS imaging database: 170 coronary lesions (150 patients) which were imaged with IVUS and underwent stress myocardial single-photon emission computed tomography (SPECT) were analysed. By receiver operator characteristic curve analysis, the best cut-off value of MLA was ≤2.1 mm2 (38.6% positive predictive value, and a 91.3% negative predictive value versus lesions with a positive SPECT) area under the curve: 0.690, 95% CI: 0.615 to 0.759, P<0.01 (51).
Full table
There have been previous comparative studies showing good to excellent correlation between CCTA and IVUS geometrical parameters (52). This has led to the use of CCTA to prospectively evaluate changes in IVUS-like parameters such as MLA (53).
It is worth noting that this value does not apply to small vessels (54) or to large segments such as the left main (LM) or venous bypass grafts.
Conclusions
In this fast moving field of advanced coronary imaging, CTA has moved from just a “rule-out CAD” modality and has been explored in several different lines of research, due to its unique ability to non-invasively depict several atherosclerotic plaque features and quantify the extent of plaque burden in the coronary tree. One of the most interesting research lines is exploring the relation between these plaque features and coronary burden of disease, not only with adverse outcomes but also recently with the development of ischemia, which might help to understand the pathophysiological link between anatomy and function in ischemic heart disease and a better identification of patients at risk of future coronary events.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- de Araujo Goncalves P, Campos CA, Serruys PW, et al. Computed tomography angiography for the in-terventional cardiologist. Eur Heart J Cardiovasc Imaging 2014;15:842-54. [Crossref] [PubMed]
- Motoyama S, Kondo T, Sarai M, et al. Multislice computed tomographic characteristics of coronary lesions in acute coronary syndromes. J Am Coll Cardiol 2007;50:319-26. [Crossref] [PubMed]
- Motoyama S, Sarai M, Harigaya H, et al. Computed tomographic angiography characteristics of ath-erosclerotic plaques subsequently resulting in acute coronary syndrome. J Am Coll Cardiol 2009;54:49-57. [Crossref] [PubMed]
- Maurovich-Horvat P, Hoffmann U, Vorpahl M, et al. The napkin-ring sign: CT signature of high-risk coronary plaques? JACC Cardiovasc Imaging 2010;3:440-4. [Crossref] [PubMed]
- Otsuka K, Fukuda S, Tanaka A, et al. Napkin-ring sign on coronary CT angiography for the predic-tion of acute coronary syndrome. JACC Cardiovasc Imaging 2013;6:448-57. [Crossref] [PubMed]
- Motoyama S, Ito H, Sarai M, et al. Plaque Characterization by Coronary Computed Tomography Angiography and the Likelihood of Acute Coronary Events in Mid-Term Follow-Up. J Am Coll Cardiol 2015;66:337-46. [Crossref] [PubMed]
- Min JK, Shaw LJ, Devereux RB, et al. Prognostic value of multidetector coronary computed tomo-graphic angiography for prediction of all-cause mortality. J Am Coll Cardiol 2007;50:1161-70. [Crossref] [PubMed]
- Rodriguez-Granillo GA, Carrascosa P, Bruining N, et al. Defining the non-vulnerable and vulnerable patients with computed tomography coronary angiography: evaluation of atherosclerotic plaque burden and composition. Eur Heart J Cardiovasc Imaging 2016;17:481-91. [Crossref] [PubMed]
- Goncalves Pde A, Rodriguez-Granillo GA, Spitzer E, et al. Functional Evaluation of Coronary Dis-ease by CT Angiography. JACC Cardiovasc Imaging 2015;8:1322-35. [Crossref] [PubMed]
- Falk E. Morphologic features of unstable atherothrombotic plaques underlying acute coronary syn-dromes. Am J Cardiol 1989;63:114E-20E. [Crossref] [PubMed]
- Arbab-Zadeh A, Nakano M, Virmani R, et al. Acute coronary events. Circulation 2012;125:1147-56. [Crossref] [PubMed]
- Stone GW, Maehara A, Lansky AJ, et al. A prospective natural-history study of coronary athero-sclerosis. N Engl J Med 2011;364:226-35. [Crossref] [PubMed]
- Calvert PA, Obaid DR, O'Sullivan M, et al. Association between IVUS findings and adverse out-comes in patients with coronary artery disease: the VIVA (VH-IVUS in Vulnerable Atherosclerosis) Study. JACC Cardiovasc Imaging 2011;4:894-901. [Crossref] [PubMed]
- Cheng JM, Garcia-Garcia HM, de Boer SP, et al. In vivo detection of high-risk coronary plaques by radiofrequency intravascular ultrasound and cardiovascular outcome: results of the ATHEROREMO-IVUS study. Eur Heart J 2014;35:639-47. [Crossref] [PubMed]
- Min JK, Dunning A, Lin FY, et al. Age- and sex-related differences in all-cause mortality risk based on coronary computed tomography angiography findings results from the International Multicen-ter CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multi-center Registry) of 23,854 patients without known coronary artery disease. J Am Coll Cardiol 2011;58:849-60. [Crossref] [PubMed]
- de Araujo Goncalves P, Garcia-Garcia HM, Carvalho MS, et al. Diabetes as an independent predic-tor of high atherosclerotic burden assessed by coronary computed tomography angiography: the coro-nary artery disease equivalent revisited. Int J Cardiovasc Imaging 2013;29:1105-14. [Crossref] [PubMed]
- Bittencourt MS, Hulten E, Ghoshhajra B, et al. Prognostic value of nonobstructive and obstructive coronary artery disease detected by coronary computed tomography angiography to identify cardiovas-cular events. Circ Cardiovasc Imaging 2014;7:282-91. [Crossref] [PubMed]
- Mushtaq S, De Araujo Goncalves P, Garcia-Garcia HM, et al. Long-term prognostic effect of coro-nary atherosclerotic burden: validation of the computed tomography-leaman score. Circ Cardiovasc Imaging 2015;8:e002332. [Crossref] [PubMed]
- Hadamitzky M, Achenbach S, Al-Mallah M, et al. Optimized prognostic score for coronary com-puted tomographic angiography: results from the CONFIRM registry (COronary CT Angiography EvaluatioN For Clinical Outcomes: An InteRnational Multicenter Registry). J Am Coll Cardiol 2013;62:468-76. [Crossref] [PubMed]
- de Araujo Goncalves P, Garcia-Garcia HM, Dores H, et al. Coronary computed tomography angi-ography-adapted Leaman score as a tool to noninvasively quantify total coronary atherosclerotic burden. Int J Cardiovasc Imaging 2013;29:1575-84. [Crossref] [PubMed]
- Tonino PA, De Bruyne B, Pijls NH, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med 2009;360:213-24. [Crossref] [PubMed]
- De Bruyne B, Pijls NH, Kalesan B, et al. Fractional flow reserve-guided PCI versus medical thera-py in stable coronary disease. N Engl J Med 2012;367:991-1001. [Crossref] [PubMed]
- Task Force M. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the Eu-ropean Society of Cardiology. Eur Heart J 2013;34:2949-3003. [Crossref] [PubMed]
- Achenbach S, Goroll T, Seltmann M, et al. Detection of coronary artery stenoses by low-dose, pro-spectively ECG-triggered, high-pitch spiral coronary CT angiography. JACC Cardiovasc Imaging 2011;4:328-37. [Crossref] [PubMed]
- de Graaf FR, Schuijf JD, van Velzen JE, et al. Diagnostic accuracy of 320-row multidetector com-puted tomography coronary angiography in the non-invasive evaluation of significant coronary artery disease. Eur Heart J 2010;31:1908-15. [Crossref] [PubMed]
- Schuhbaeck A, Achenbach S, Layritz C, et al. Image quality of ultra-low radiation exposure coro-nary CT angiography with an effective dose <0.1 mSv using high-pitch spiral acquisition and raw data-based iterative reconstruction. Eur Radiol 2013;23:597-606. [Crossref] [PubMed]
- Tonino PA, Fearon WF, De Bruyne B, et al. Angiographic versus functional severity of coronary artery stenoses in the FAME study fractional flow reserve versus angiography in multivessel evaluation. J Am Coll Cardiol 2010;55:2816-21. [Crossref] [PubMed]
- Min JK, Leipsic J, Pencina MJ, et al. Diagnostic accuracy of fractional flow reserve from anatomic CT angiography. JAMA 2012;308:1237-45. [Crossref] [PubMed]
- Norgaard BL, Leipsic J, Gaur S, et al. Diagnostic performance of noninvasive fractional flow re-serve derived from coronary computed tomography angiography in suspected coronary artery disease: the NXT trial (Analysis of Coronary Blood Flow Using CT Angiography: Next Steps). J Am Coll Cardiol 2014;63:1145-55. [Crossref] [PubMed]
- Park SJ, Kang SJ, Ahn JM, et al. Visual-functional mismatch between coronary angiography and fractional flow reserve. JACC Cardiovasc Interv 2012;5:1029-36. [Crossref] [PubMed]
- Park HB, Heo R. Atherosclerotic Plaque Characteristics by CT Angiography Identify Coronary Lesions That Cause Ischemia: A Direct Comparison to Fractional Flow Reserve. JACC Cardiovasc Imaging 2015;8:1-10. [Crossref] [PubMed]
- Nakazato R, Shalev A, Doh JH, et al. Aggregate plaque volume by coronary computed tomography angiography is superior and incremental to luminal narrowing for diagnosis of ischemic lesions of inter-mediate stenosis severity. J Am Coll Cardiol 2013;62:460-7. [Crossref] [PubMed]
- Lavi S, Bae JH, Rihal CS, et al. Segmental coronary endothelial dysfunction in patients with minimal atherosclerosis is associated with necrotic core plaques. Heart 2009;95:1525-30. [Crossref] [PubMed]
- Nicholls SJ, Hsu A, Wolski K, et al. Intravascular ultrasound-derived measures of coronary athero-sclerotic plaque burden and clinical outcome. J Am Coll Cardiol 2010;55:2399-407. [Crossref] [PubMed]
- Yonetsu T, Murai T, Kanaji Y et al. Association between plaque instability assessed by optical coherence tomography and microvascular resistance. Eur Heart J 2016;37(abstract supplement):600.
- Usui E, Kakuta T, Yonetsu T et al. Relevance of optical coherence tomography derived un-stable plaque features and physiological stenosis severity determined by fractional flow reserve. Eur Heart J 2016;37(abstract supplement):600.
- Taqueti VR, Shaw LJ, Cook NR, et al. Excess Cardiovascular Risk in Women Relative to Men Re-ferred for Coronary Angiography Is Associated With Severely Impaired Coronary Flow Reserve, Not Obstructive Disease. Circulation 2017;135:566-577. [Crossref] [PubMed]
- Ahmadi A, Kini A, Narula J. Discordance between ischemia and stenosis, or PINSS and NIPSS: are we ready for new vocabulary? JACC Cardiovasc Imaging 2015;8:111-4. [Crossref] [PubMed]
- Riufol G, Mewton N, Rabilloud M et al. Functional Testing Underlying Revascularization (FUTURE trial). ahead of print, presented AHA 2016
- Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and In-terventions. J Am Coll Cardiol 2011;58:e44-122. [Crossref] [PubMed]
- Kolh P, Windecker S, Alfonso F, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur J Cardiothorac Surg 2014;46:517-92. [Crossref] [PubMed]
- Abizaid A, Mintz GS, Pichard AD, et al. Clinical, intravascular ultrasound, and quantitative angio-graphic determinants of the coronary flow reserve before and after percutaneous transluminal coronary angioplasty. Am J Cardiol 1998;82:423-8. [Crossref] [PubMed]
- Waksman R, Legutko J, Singh J, et al. FIRST: Fractional Flow Reserve and Intravascular Ultra-sound Relationship Study. J Am Coll Cardiol 2013;61:917-23. [Crossref] [PubMed]
- Han JK, Koo BK, Park KW, et al. Optimal intravascular ultrasound criteria for defining the func-tional significance of intermediate coronary stenosis: an international multicenter study. Cardiology 2014;127:256-62. [Crossref] [PubMed]
- Jang JK, Song JY, Jin HY, Seo JS, Yang TH, Kim DK, Kim DS. TCT347 Assessment of Intravascular Ultrasound-derived Minimal Lumen Area and Fractional Flow Reserve to Evaluate Functionally Significant Coronary Artery Disease: A Meta-Analysis. JACC. 2014; Vol 64/11/Suppl B:B101
- Shiono Y, Kitabata H, Kubo T, et al. Optical coherence tomography-derived anatomical criteria for functionally significant coronary stenosis assessed by fractional flow reserve. Circ J 2012;76:2218-25. [Crossref] [PubMed]
- Gonzalo N, Escaned J, Alfonso F, et al. Morphometric assessment of coronary stenosis relevance with optical coherence tomography: a comparison with fractional flow reserve and intravascular ultra-sound. J Am Coll Cardiol 2012;59:1080-9. [Crossref] [PubMed]
- Brugaletta S, Garcia-Garcia HM, Shen ZJ, et al. Morphology of coronary artery lesions assessed by virtual histology intravascular ultrasound tissue characterization and fractional flow reserve. Int J Cardiovasc Imaging 2012;28:221-8. [Crossref] [PubMed]
- Nishioka T, Amanullah AM, Luo H, et al. Clinical validation of intravascular ultrasound imaging for assessment of coronary stenosis severity: comparison with stress myocardial perfusion imaging. J Am Coll Cardiol 1999;33:1870-8. [Crossref] [PubMed]
- Briguori C, Anzuini A, Airoldi F, et al. Intravascular ultrasound criteria for the assessment of the functional significance of intermediate coronary artery stenoses and comparison with fractional flow re-serve. Am J Cardiol 2001;87:136-41. [Crossref] [PubMed]
- Ahn JM, Kang SJ, Mintz GS, et al. Validation of minimal luminal area measured by intravascular ultrasound for assessment of functionally significant coronary stenosis comparison with myocardial per-fusion imaging. JACC Cardiovasc Interv 2011;4:665-71. [Crossref] [PubMed]
- Costa MA, Sabate M, Staico R, et al. Anatomical and physiologic assessments in patients with small coronary artery disease: final results of the Physiologic and Anatomical Evaluation Prior to and After Stent Implantation in Small Coronary Vessels (PHANTOM) trial. Am Heart J 2007;153:296.e1-7. [Crossref] [PubMed]
- Ben-Dor I, Torguson R, Gaglia MA Jr, et al. Correlation between fractional flow reserve and intra-vascular ultrasound lumen area in intermediate coronary artery stenosis. EuroIntervention 2011;7:225-33. [Crossref] [PubMed]
- Lee CH, Tai BC, Soon CY, et al. New set of intravascular ultrasound-derived anatomic criteria for defining functionally significant stenoses in small coronary arteries (results from Intravascular Ultra-sound Diagnostic Evaluation of Atherosclerosis in Singapore [IDEAS] study). Am J Cardiol 2010;105:1378-84. [Crossref] [PubMed]
- Takagi A, Tsurumi Y, Ishii Y, et al. Clinical potential of intravascular ultrasound for physiological assessment of coronary stenosis: relationship between quantitative ultrasound tomography and pressure-derived fractional flow reserve. Circulation 1999;100:250-5. [Crossref] [PubMed]
- Kang SJ, Lee JY, Ahn JM, et al. Validation of intravascular ultrasound-derived parameters with fractional flow reserve for assessment of coronary stenosis severity. Circ Cardiovasc Interv 2011;4:65-71. [Crossref] [PubMed]
- Koo BK, Yang HM, Doh JH, et al. Optimal intravascular ultrasound criteria and their accuracy for defining the functional significance of intermediate coronary stenoses of different locations. JACC Cardiovasc Interv 2011;4:803-11. [Crossref] [PubMed]
- Kang SJ, Ahn JM, Song H, et al. Usefulness of minimal luminal coronary area determined by intra-vascular ultrasound to predict functional significance in stable and unstable angina pectoris. Am J Cardiol 2012;109:947-53. [Crossref] [PubMed]
- Ben-Dor I, Torguson R, Deksissa T, et al. Intravascular ultrasound lumen area parameters for as-sessment of physiological ischemia by fractional flow reserve in intermediate coronary artery stenosis. Cardiovasc Revasc Med 2012;13:177-82. [Crossref] [PubMed]
- Kwan TW, Yang S, Xu B, et al. Optimized quantitative angiographic and intravascular ultrasound parameters predicting the functional significance of single de novo lesions in the left anterior descend-ing artery. Chin Med J (Engl) 2012;125:4249-53. [PubMed]
- Chen SL, Xu B, Chen JB, et al. Diagnostic accuracy of quantitative angiographic and intravascular ultrasound parameters predicting the functional significance of single de novo lesions. Int J Cardiol 2013;168:1364-9. [Crossref] [PubMed]
- Cui M, Zhu D, Guo LJ, et al. Usefulness of lumen area parameters determined by intravascular ul-trasound to predict functional significance of intermediate coronary artery stenosis. Chin Med J (Engl) 2013;126:1606-11. [PubMed]
- Naganuma T, Latib A, Costopoulos C, et al. The role of intravascular ultrasound and quantitative angiography in the functional assessment of intermediate coronary lesions: correlation with fractional flow reserve. Cardiovasc Revasc Med 2014;15:3-7. [Crossref] [PubMed]
- Yang HM, Tahk SJ, Lim HS, et al. Relationship between intravascular ultrasound parameters and fractional flow reserve in intermediate coronary artery stenosis of left anterior descending artery: intra-vascular ultrasound volumetric analysis. Catheter Cardiovasc Interv 2014;83:386-94. [Crossref] [PubMed]
- Doh JH, Koo BK, Nam CW, et al. Diagnostic value of coronary CT angiography in comparison with invasive coronary angiography and intravascular ultrasound in patients with intermediate coronary artery stenosis: results from the prospective multicentre FIGURE-OUT (Functional Imaging criteria for GUiding REview of invasive coronary angiOgraphy, intravascular Ultrasound, and coronary computed Tomographic angiography) study. Eur Heart J Cardiovasc Imaging 2014;15:870-7. [Crossref] [PubMed]


