Requiem for routine thrombus aspiration
Interventional cardiologists share a feeling of accomplishment having just aspirated a particularly large thrombus from a coronary artery in a patient with myocardial infarction. Narcissistic personalities even call for a colleague to inspect and worship the feat. However, the satisfying visual feedback of thrombus aspiration might lead to flawed conclusions.
Indeed—much to the regret of the interventional community—recent large randomized studies have shown disappointing results for routine thrombus aspiration in patients with ST-elevation myocardial infarction (STEMI).
Jolly et al. now published a collaborative meta-analysis of the three largest randomized controlled trials (TAPAS, TASTE and TOTAL) comparing percutaneous coronary intervention (PCI) with routine manual thrombus aspiration versus PCI alone in patients with STEMI (1-4). The authors found a statistical trend (P=0.06) towards reduced cardiovascular and all-cause death favoring additional thrombus aspiration at 30 days (P=0.06 for both), which was attenuated at 1 year (cardiovascular death: P=0.15; all-cause death: P=0.18). However, there was also a signal of a possible higher rate of stroke or transient ischemic attack (TIA) in patients undergoing thrombectomy, although not statistically significant (30 days: P=0.06; 1 year: P=0.11). All other clinical endpoints studied such as recurrent myocardial infarction, stent thrombosis, congestive heart failure or target revascularization were clearly not significantly different between the two treatment strategies. Exploratory subgroup analyses revealed that patients with high thrombus burden had a numerically lower rate of cardiovascular death, however, at the expense of a possibly higher risk of stroke/TIA.
The work is particularly valuable for distinct reasons. First, the field of manual thrombus aspiration lends itself for summarizing evidence by means of meta-analysis. Unlike other areas of cardiovascular research, there are several large-scale randomized trials which form the backbone of the analysis and provide a high degree of scientific certainty (1,2,4). Specifically, the meta-analysis comprises datasets from over 18,000 STEMI patients. Second, its conclusions are drawn from individual patient data rather than aggregate summary data. This established gold standard is the most powerful method of meta-analysis.
Although the analysis is of the highest quality, some aspects deserve a second look. The conclusion that patients with high thrombus burden may benefit from thrombus aspiration calls for careful consideration of statistical details. The simple comparison of nominal event rates in this high-risk subgroup showed indeed a significant difference for cardiovascular death (favoring thrombus aspiration) and stroke/TIA (to the disadvantage of thrombus aspiration). However, the more refined analysis by interaction terms revealed a significant difference between the groups only for the risk of stroke/TIA. In other words, the subgroup of patients with high thrombus burden does not seem to benefit in terms of cardiovascular death, but is still at risk of stroke or TIA. This does not seem to be a desirable deal. Furthermore, as acknowledged by the authors, analyses of subgroups are in general vulnerable to overinterpretation of positive results, especially if there is no adjustment for multiple comparisons.
Surprisingly, the authors favored a fixed-effects over a random-effects model. It is unlikely that independent clinical studies can be considered equivalent to a degree that using a fixed-effects model is appropriate (in fact, study-level interaction was found to be significant for the primary safety endpoint). Of note, the choice of model should not be based on statistical tests for heterogeneity, since these suffer from low power in detecting true clinical heterogeneity (5). It is rather a matter of clinical judgment.
Why has thrombus aspiration not met its high expectations?
The most straightforward explanation for the lack of efficacy is that the effect of routine upfront thrombus aspiration is simply not of sufficient size to change hard clinical endpoints and/or the outcome is dominated by additional factors other than removal of thrombus.
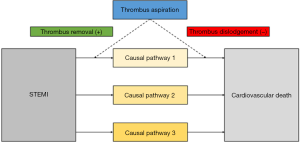
Second, while aspiration will remove thrombotic material in many patients (as verified by analysis of the aspirate), manipulation with the catheter might also dislodge thrombotic material with subsequent embolization into the microcirculation and expansion of the necrotic zone. These opposing effects might balance each other resulting in neutral outcome for efficacy. One might hypothesize that creation of only a small channel within the thrombus might allow for sufficient blood flow and oxygen delivery to keep the myocardial cells alive, though not fully functional (contracting). Any additional manipulation other than stent deployment (such as thrombus aspiration) might incur the risk of adverse effects such as thrombus embolization or vasospasm. In addition, possible embolization of thrombus from the coronary into the systemic vasculature raises safety concerns. This is further illustrated in Figure 1.
Are there any niche indications left for manual thrombus aspiration?
The current meta-analysis provides little argument for routine manual thrombus aspiration. It is, however, worthwhile to look at the evidence of thrombus aspiration in specific groups of patients.
The trials of the present meta-analysis explored the unselected routine use of thrombus aspiration in patients with acute STEMI. To date, there are no randomized data in patients with large residual thrombus, slow or absent flow after unsuccessful conventional PCI. Thrombus aspiration remains an option in these bailout scenarios after careful balancing of possible risk and benefit. However, evidence in favor of thrombus aspiration in this clinical setting is anecdotal.
In the only randomized trial which studied routine thrombus aspiration in patients with non-STEMI, patients did not benefit with regard to a reduction in microvascular obstruction in cardiac magnetic resonance imaging or clinical endpoints (6). The same holds true for STEMI patients who present late (≥12 hours) after symptom onset. Although, these patients display particularly high thrombus burden due to long dwelling times, routine manual thrombus aspiration before PCI did not reduce the extent of microvascular obstruction on cardiac magnetic resonance imaging compared with conventional PCI without thrombectomy in a randomized trial (7).
Based on subgroup analyses of the current meta-analysis, the authors bring into play a randomized controlled trial specifically in patients with high thrombus burden. However, as outlined above, this conclusion seems to be a far stretch. Furthermore, as calculated by the authors, such a study would require approximately 26,000 patients. Notwithstanding the question of practical execution, the number needed to treat would likely be exceedingly high if such a trial were to be positive for thrombus aspiration. It is questionable if the cardiology community should invest resources into such an endeavor.
Conclusions
For the foreseeable future, the meta-analysis by Jolly et al. can be regarded as the final evidence for routine thrombus aspiration. There are no studies in sight that will significantly alter the conclusion of the work. It is unlikely that thrombus aspiration will be successfully resuscitated by new trials or devices. Unfortunately, the interventional community’s confidence in the method was greater than objective evidence that followed.
Acknowledgements
The first author has received an unrestricted research grant from Medtronic.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Frobert O, Lagerqvist B, Olivecrona GK, et al. Thrombus aspiration during ST-segment elevation myocardial infarction. N Engl J Med 2013;369:1587-97. [Crossref] [PubMed]
- Jolly SS, Cairns JA, Yusuf S, et al. Randomized trial of primary PCI with or without routine manual thrombectomy. N Engl J Med 2015;372:1389-98. [Crossref] [PubMed]
- Jolly SS, James S, Dzavik V, et al. Thrombus aspiration in ST-segment-elevation myocardial infarction: an individual patient meta-analysis: Thrombectomy Trialists Collaboration. Circulation 2017;135:143-52. [Crossref] [PubMed]
- Vlaar PJ, Svilaas T, van der Horst IC, et al. Cardiac death and reinfarction after 1 year in the Thrombus Aspiration during Percutaneous coronary intervention in Acute myocardial infarction Study (TAPAS): a 1-year follow-up study. Lancet 2008;371:1915-20. [Crossref] [PubMed]
- Borenstein M, Hedges H, Higgins JP, et al. editors. Introduction to meta-analysis. Cornwall, UK: John Wiley & Sons Ltd., 2009.
- Thiele H, de Waha S, Zeymer U, et al. Effect of aspiration thrombectomy on microvascular obstruction in NSTEMI patients: the TATORT-NSTEMI trial. J Am Coll Cardiol 2014;64:1117-24. [Crossref] [PubMed]
- Desch S, Stiermaier T, de Waha S, et al. Thrombus aspiration in patients with ST-segment elevation myocardial infarction presenting late after symptom onset. JACC Cardiovascular interventions 2016;9:113-22. [Crossref] [PubMed]


