The relationship between total ischemic time and mortality in patients with STEMI: every second counts
We read with great interest the recently published article in the October 2016 issue of JACC Cardiovasc Interv by Rashid et al. (1). Briefly, it was a retrospective, single center study that analyzed ST-elevation myocardial infarction (STEMI) patients with symptom onset <12 hours. Patients with primary percutaneous intervention (PCI) were compared with those who received fibrinolytic therapy prior to arrival at the hospital due to non-availability of primary PCI. The authors concluded that the pharmaco-invasive strategy was associated with similar rates of the composite endpoint of mortality, reinfarction, or stroke as compared with a primary PCI strategy. There was propensity for increased bleeding in the pharmaco-invasive group, with a significant fraction (approximately 1 in every 21 patients) having major bleeding, including intracranial bleeding.
Non-inferiority of pharmaco-invasive strategy compared to primary PCI strategy in this study emphasizes the importance of total ischemic time. These are patients who received fibrinolytic therapy within few minutes of presentation at the initial hospital (with no primary PCI capability) which could have led to dissolution of the thrombus and re-canalization of the culprit artery, and hence reduction of the total ischemic time. The similar outcomes between the groups despite a big difference in the door-to-balloon times are most likely due to the shortening of the total ischemic time by the administration of fibrinolysis. However, we must not ignore the limitations of the study. There was a disparity between the control group vs. test group, i.e., 236 pharmaco-invasive strategy patients vs. 980 primary PCI patients (approximately 4:1 ratio), which may have influenced the statistical model. As the patients were not randomized, unknown confounders may have affected the results owing to the retrospective nature of the study. For example, the median time from symptom onset to arrival at the first hospital was significantly shorter in the pharmacoinvasive group, which may have favored these patients. The study only studied the in-hospital results and the long-term outcomes are not known. The infarct size as measured by peak creatine-kinase MB levels, was significantly higher in pharmaco-invasive group, and we already know that the peak infarct size is related to worse long-term outcomes from several large trials in the past (2). However, this difference may not be clinically significant as the composite of mortality, reinfarction, or stroke was similar between the two groups. Nevertheless, the long-term outcomes are unknown in these patients. Also, the infarct zone at risk was not defined so that the selection bias for patients who were destined to have larger infarcts was not eliminated. To better define the zone at risk an MRI study like the one done by Eitel et al. would have been very useful (3). Furthermore, the pharmacologic regimen used for the groups were different. The pharmaco-invasive group patients received a lower dose of clopidogrel and an infusion of heparin. Whereas, the primary PCI patients received only a bolus of heparin and 600 mg of clopidogrel. This might be important since the bleeding rates between the groups were different. We know from the ASSENT-4 trial that when fibrinolysis is used in combination with suboptimal antiplatelet use the outcome might not be so favorable (4). However, there is no head to head comparison of different anti-platelet regimens in this group of patients, and an “optimal regimen” remains unclear, regarding both the choice and dosing of P2Y12 inhibitor. Nevertheless, we know that 600-mg loading dose of clopidogrel has better angiographic end points and 1-year clinical outcomes compared to a 300-mg dose in patients who undergo primary PCI for STEMI (5). The relatively short duration between fibrinolytic administration and first balloon inflation (260 min) might also have increased the bleeding risk. Besides, the infusion of heparin might also be the reason for increased bleeding seen in these patients. In the AMICO and PATCAR studies the bleeding rates were exceedingly low when only a single bolus heparin was used together with 600 mg of clopidogrel and fibrinolysis. The PATCAR pilot trial also used half-dose fibrinolysis (6). While the limitations of the study are clearly present, it is often hard to achieve an ideal design. The results have to be interpreted while acknowledging both the strengths and weaknesses to practically apply the results in real life settings.
Several studies in the past have shown the importance of total ischemic time, and how it correlates better with overall short term and long term cardiovascular prognosis than FMC-device time (6). Figure 1 depicts all the components of total ischemic time, the delay of any of which could increase the total ischemic time and lead to poor outcomes. In 1977, transient left circumflex coronary artery ligation was performed in dogs for different durations (7). Longer durations of blood flow interruption were directly associated with the degree of transmural myocardial injury. The percentage transmural necrosis increased from 38% at 40 minutes duration of circumflex artery ligation to 85% at 24 hours duration (7). Therefore, one may conclude that the benefit of reperfusion significantly depends on the ischemic time. Similar results have been reported when evaluating cardiovascular outcomes in STEMI patients receiving fibrinolysis. For every 1,000 patients, 15 more lives were saved at one month follow up if the patients received an hour earlier treatment in the European Myocardial Infarction Project Group (8). A meta-analysis of 22 randomized controlled trials studied over 50,000 STEMI patients who received fibrinolysis. If fibrinolysis was achieved within one hour of symptom onset, there were 65 fewer deaths for every 1,000 patients when compared to patients with longer delay from symptom onset to fibrinolysis (9). These data indicate that FMC-device time is only a component of the total ischemic time, and the latter is more accurately correlated with overall prognosis.
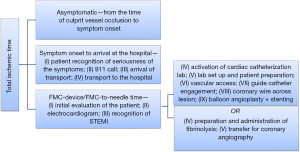
The relationship between total ischemic time and cardiovascular outcomes has also been studied in STEMI patients undergoing primary PCI. In a study of 1,791 STEMI patients who underwent primary angioplasty for STEMI, every 30-minute delay in reperfusion resulted in a 7.5% increased mortality at one year follow up (10). This suggests that the total ischemic time determines both the short-term and long-term prognosis. Most studies evaluating the link between total ischemic time and cardiovascular outcomes being retrospective studies have inherent survivor bias, as all STEMI patients who died before reaching the hospital were not included. However, had they been included, the data to support lessening total ischemic time would have been even more compelling. The importance of total ischemic time has also been highlighted in cardiac magnetic resonance (CMR) imaging studies. Transmural necrosis, as identified by CMR, was directly related to the duration of ischemic time in a study of 77 STEMI patients. For every 30 minutes of treatment delay, there was a 37% increased risk of transmural necrosis (11).
The most recent American College of Cardiology/American Heart Association guidelines on STEMI care in 2015 (12) focus on targeting the period after FMC. The STEMI guidelines from 2013 acknowledge the importance of total ischemic time, and that broader initiatives at a system level are required to improve cardiovascular outcomes, however the performance measures still not give emphasis on the pre-hospital phase (13). In the current study, it is interesting to note that in the pharmacoinvasive group, once the patients received fibrinolytics, the median time to first balloon inflation was 260 min (IQR: 201 to 385 min). Although these results suggest that earlier intervention within the 3–24 hours’ period may confer benefit over later intervention, reducing the total ischemic time and influencing outcomes. They also show that the major goal of this treatment strategy was not to reduce total ischemic time since it took 260 minutes to deliver definitive therapy. A significant percentage of patients in the pharmaco-invasive group (25.5%) ended up having TIMI 0 flow at the time of coronary angiography. The 85% of the patients in the pharmaco-invasive group ended up getting percutaneous coronary intervention. These results strongly suggest that after receiving fibrinolytic therapy, these patients should still have been rushed for urgent coronary angiography to further reduce the total ischemic time and positively influence the outcomes. This specific question has been studied by several trials. There is no penalty paid in the contemporary era by the increased use of radial artery interventions for immediately following the fibrinolysis by coronary intervention.
There have been six major published trials that have studied fibrinolysis followed by urgent PCI in STEMI patients (4,14-19). The comparison group of three of these trials was primary PCI, while for rest it was conservative or conventional strategy i.e., STEMI patients transferred for coronary angiogram within 3–24 hours. The results of these trials are summarized in Table 1. The ASCENT-4 trial (4) was the only study to show increased incidence of primary efficacy endpoint in the fibrinolysis-urgent PCI arm. However, the primary endpoint was driven largely by the recurrent MI, and patients in fibrinolysis-urgent PCI group had a significantly lower use of the P2Y12 inhibitor (clopidogrel) compared to the primary PCI group. The FINESSE trial (18) had a unique design to compare fibrinolysis-urgent PCI with primary PCI, as all the patients in the fibrinolysis-urgent PCI group received half-dose reteplase followed by abciximab. There was no difference in the primary efficacy endpoint between the two groups 9.8% vs. 10.7% (P=0.55). It should also be noted that in the FINESSE trial patients who are >75 years of age received half dose fibrinolysis and the number of patients with intracranial bleeds among those patients were 0. The design and results of the following three trials, namely, CARESS-in-MI (17), TRANSFER-AMI (15) and NORDISTEMI (16) were similar. All three studies compared STEMI patients who had presented to non-PCI capable hospitals with fibrinolysis-urgent PCI vs. conservative strategy. The CARESS-in-MI trial used half-dose reteplase along with abciximab, while the other two trials used full dose tenecteplase. In both CARESS-in-MI and TRANSFER-AMI trials, there was a significant reduction in primary outcome in the fibrinolytic-urgent PCI group vs. conservative strategy. The third one, NORDISTEMI trial, did not have a difference in the primary efficacy endpoint (Death, MI, stroke or ischemia at 12 months) between the two groups; however, there was a trend towards benefit in the fibrinolysis-early PCI group (21% vs. 27%, P=0.19). Moreover, one of the secondary outcomes, composite of death, reinfarction, or stroke at 12 months was significantly reduced in the fibrinolysis-early PCI group compared with the conservative group (6% vs. 16%, hazard ratio: 0.36, 95% confidence interval: 0.16 to 0.81, P=0.01).

Full table
The safety endpoints were also evaluated in all these trials (4,14-19). In all the three trials that compared fibrinolytic-early PCI with conservative strategy, there was no significant difference in major bleeding or intracranial bleeding between the two groups. One of the two trials that compared fibrinolytic-early PCI strategy vs. primary PCI showed increased major bleeding while the other one demonstrated increased intracranial bleeding, in the fibrinolysis-early PCI groups. AMICO trial’s design was unique, as this is the only trial to study half-dose fibrinolytic agent without IIB/IIIA inhibitor (14). The comparison group was primary PCI in these patients. One-month mortality was significantly lower with FAST-PCI strategy compared to primary PCI strategy (3.8% vs. 6.4%, P=0.002). All secondary outcomes including combined triple end-point of death, reinfarction, or stroke, incidence of Killip class IV symptoms, infarct-related artery TIMI flow grades were comparatively superior in FAST-PCI strategy. The STREAM trial (19), published in 2013, evaluated all patients with STEMI who had a delay in primary PCI. They were randomized to receive either full dose fibrinolysis or PCI. The primary efficacy outcome (death, shock, CHF or MI at 30 days) was unchanged between the fibrinolysis vs. PCI groups (12.4% vs. 14.3%, P=0.21). Emergent coronary angiography was needed in 36% of the patients in fibrinolysis arm.
Taken together, a comprehensive review of these trials suggest that administration of fibrinolytic therapy should be followed by immediate coronary angiography with percutaneous coronary intervention to improve ischemic outcomes. Given the increased bleeding tendency with some trials that used either full dose fibrinolysis or half dose fibrinolysis coupled with IIB/IIIA inhibitor, one may suggest using half-dose fibrinolysis without concomitant IIb/IIIa inhibition, but with 600 mg of clopidogrel, as the that strategy has shown improved ischemic outcomes without increased bleeding (14). A meta-analysis showed similar results with fibrinolysis and early PCI having significantly reduced re-infarction and recurrent ischemia at 1 month, with no significant increase in adverse bleeding events compared to standard therapy (20).
In summary, total ischemic time, rather than FMC-device time, should be the real target for improving mortality in STEMI patients. Several factors can influence the total ischemic time, as depicted in Figure 1. Any delay to reperfusion may adversely influence patient outcomes and should therefore be minimized. Clinical trials should focus on diminishing the total ischemic time and its impact on the incidence of shock and other adverse cardiovascular events upon arrival to the hospital (21). In our opinion, if there is delay in transfer, most STEMI patients should receive fibrinolysis followed by early coronary angiography (and PCI, when appropriate). The ischemic time can thus be reduced with fibrinolysis, and possible re-occlusion can be detected and treated with coronary angiography/PCI. It is also not uncommon to have non-anticipated delays during transfer (e.g., weather, traffic). The protocol that needs retesting is one in which all patients not showing up to a PCI capable hospital receive half-dose fibrinolysis with adequate antiplatelet agents and then transferred for coronary angiography. Such a protocol will likely help shorten the total ischemic time for these patients. It doesn’t matter for the myocardium whether the patient presents to a non-PCI capable or a PCI capable hospital. The myocardium only keeps on dying with every second of delay.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Rashid MK, Guron N, Bernick J, et al. Safety and Efficacy of a Pharmacoinvasive Strategy in ST-Segment Elevation Myocardial Infarction: A Patient Population Study Comparing a Pharmacoinvasive Strategy With a Primary Percutaneous Coronary Intervention Strategy Within a Regional System. JACC Cardiovasc Interv 2016;9:2014-20. [Crossref] [PubMed]
- Stone GW, Selker HP, Thiele H, et al. Relationship Between Infarct Size and Outcomes Following Primary PCI: Patient-Level Analysis From 10 Randomized Trials. J Am Coll Cardiol 2016;67:1674-83. [Crossref] [PubMed]
- Eitel I, Desch S, Fuernau G, et al. Prognostic significance and determinants of myocardial salvage assessed by cardiovascular magnetic resonance in acute reperfused myocardial infarction. J Am Coll Cardiol 2010;55:2470-9. [Crossref] [PubMed]
- Assessment of the Safety and Efficacy of a New Treatment Strategy with Percutaneous Coronary Intervention (ASSENT-4 PCI) investigators. Primary versus tenecteplase-facilitated percutaneous coronary intervention in patients with ST-segment elevation acute myocardial infarction (ASSENT-4 PCI): randomised trial. Lancet 2006;367:569-78. [Crossref] [PubMed]
- Mangiacapra F, Muller O, Ntalianis A, et al. Comparison of 600 versus 300-mg Clopidogrel loading dose in patients with ST-segment elevation myocardial infarction undergoing primary coronary angioplasty. Am J Cardiol 2010;106:1208-11. [Crossref] [PubMed]
- Weaver WD. Time to thrombolytic treatment: factors affecting delay and their influence on outcome. J Am Coll Cardiol 1995;25:3S-9S. [Crossref] [PubMed]
- Reimer KA, Lowe JE, Rasmussen MM, et al. The wave front phenomenon of ischemic cell death: 1. Myocardial infarct size vs. duration of coronary occlusion in dogs. Circulation 1977;56:786-94. [Crossref] [PubMed]
- The European Myocardial Infarction Project Group. Prehospital thrombolytic therapy in patients with suspected acute myocardial infarction. N Engl J Med 1993;329:383-9. [Crossref] [PubMed]
- Boersma E, Maas AC, Deckers JW, et al. Early thrombolytic treatment in acute myocardial infarction: reappraisal of the golden hour. Lancet 1996;348:771-5. [Crossref] [PubMed]
- De Luca G, Suryapranata H, Ottervanger JP, et al. Time delay to treatment and mortality in primary angioplasty for acute myocardial infarction: every minute of delay counts. Circulation 2004;109:1223-5. [Crossref] [PubMed]
- Tarantini G, Cacciavillani L, Corbetti F, et al. Duration of ischemia is a major determinant of transmurality and severe microvascular obstruction after primary angioplasty: a study performed with contrast-enhanced magnetic resonance. J Am Coll Cardiol 2005;46:1229-35. [Crossref] [PubMed]
- Levine GN, Bates ER, Blankenship JC, et al. 2015 ACC/AHA/SCAI Focused Update on Primary Percutaneous Coronary Intervention for Patients With ST-Elevation Myocardial Infarction: An Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention and the 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction. J Am Coll Cardiol 2016;67:1235-50. [Crossref] [PubMed]
- American College of Emergency Physicians. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;61:e78-140. [Crossref] [PubMed]
- Denktas AE, Athar H, Henry TD, et al. Reduced-dose fibrinolytic acceleration of ST-segment elevation myocardial infarction treatment coupled with urgent percutaneous coronary intervention compared to primary percutaneous coronary intervention alone results of the AMICO (Alliance for Myocardial Infarction Care Optimization) Registry. JACC Cardiovasc Interv 2008;1:504-10. [Crossref] [PubMed]
- Cantor WJ, Fitchett D, Borgundvaag B, et al. Routine early angioplasty after fibrinolysis for acute myocardial infarction. N Engl J Med 2009;360:2705-18. [Crossref] [PubMed]
- Bøhmer E, Hoffmann P, Abdelnoor M, et al. Efficacy and safety of immediate angioplasty versus ischemia-guided management after thrombolysis in acute myocardial infarction in areas with very long transfer distances results of the NORDISTEMI (NORwegian study on DIstrict treatment of ST-elevation myocardial infarction). J Am Coll Cardiol 2010;55:102-10. [Crossref] [PubMed]
- Di Mario C, Dudek D, Piscione F, et al. Immediate angioplasty versus standard therapy with rescue angioplasty after thrombolysis in the Combined Abciximab REteplase Stent Study in Acute Myocardial Infarction (CARESS-in-AMI): an open, prospective, randomised, multicentre trial. Lancet 2008;371:559-68. [Crossref] [PubMed]
- Ellis SG, Tendera M, de Belder MA, et al. Facilitated PCI in patients with ST-elevation myocardial infarction. N Engl J Med 2008;358:2205-17. [Crossref] [PubMed]
- Armstrong PW, Gershlick AH, Goldstein P, et al. Fibrinolysis or primary PCI in ST-segment elevation myocardial infarction. N Engl J Med 2013;368:1379-87. [Crossref] [PubMed]
- Borgia F, Goodman SG, Halvorsen S, et al. Early routine percutaneous coronary intervention after fibrinolysis vs. standard therapy in ST-segment elevation myocardial infarction: a meta-analysis. Eur Heart J 2010;31:2156-69. [Crossref] [PubMed]
- Bhatt NS, Solhpour A, Balan P, et al. Comparison of in-hospital outcomes with low-dose fibrinolytic therapy followed by urgent percutaneous coronary intervention versus percutaneous coronary intervention alone for treatment of ST-elevation myocardial infarction. Am J Cardiol 2013;111:1576-9. [Crossref] [PubMed]


