Kounis syndrome - an atopic monster for the heart
Introduction
Allergic reactions to chemicals, food products or even insect bites are encountered all over the world with a variety of manifestations. Symptoms range from the development of a minor rash to life threatening anaphylactic reactions. Rarely, such allergic reactions can precipitate acute organ involvement – in our case, acute coronary syndrome. Acute coronary syndrome or ST-Segment elevation myocardial infarction resulting from an allergic reaction, also referred to as Kounis syndrome, is rarely described and not clearly understood (1). We present a case of Kounis syndrome secondary to a bee sting in a patient with previously treated coronary disease.
Case report
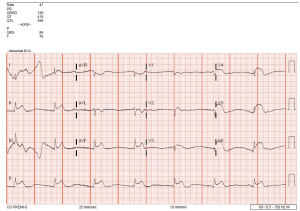
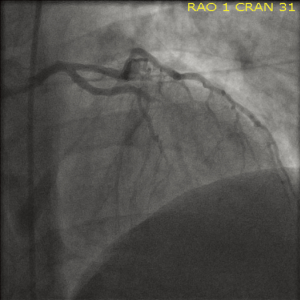
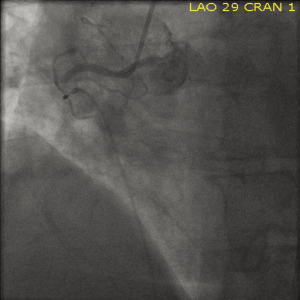
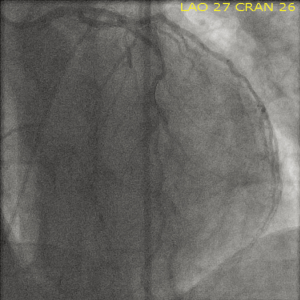
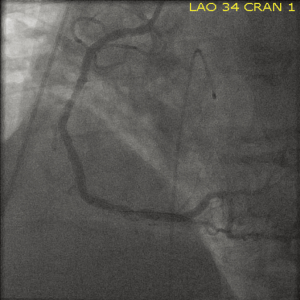
A 51-year old Caucasian male presented to the emergency room with complaints of rash and itching over his back secondary to a bee sting occurring 60 minutes prior to presentation. Past medical history was significant for hypertension, dyslipidemia, diabetes, tobacco abuse as well as established coronary artery disease with a percutaneous coronary intervention and drug eluting stent placement to right coronary artery about 6 months ago. Within 20 minutes of arrival the patient’s clinical status deteriorated with development of shortness of breath, retrosternal chest pressure and hemodynamic instability (Blood pressure 80/60 mmHg and HR 40 bpm). Subsequent ECG demonstrated ST-segment elevation in the antero-septal and inferior distributions suggestive of acute myocardial infarction (Figure 1). Urgent left heart catheterization and angiography revealed acute thrombotic occlusion of the proximal left anterior descending (LAD) and proximal right coronary arteries (RCA) (Figures 2,3). In this setting of cardiogenic shock, an intra-aortic balloon pump was inserted with subsequent successful percutaneous coronary intervention to both culprit vessels with good angiographic results (Figures 4,5). The patient’s cardiac intervention was further complicated by worsening shortness of breath requiring intubation with mechanical ventilation and development of transient complete heart block requiring transvenous pacemaker placement.
The patient’s remaining hospital course was lengthy and fraught with a variety of associated insults. It initially consisted of removal of the intra-aortic balloon pump, weaning off of mechanical ventilation, and stabilization of recurrent ventricular arrhythmias with antiarrhythmic medications (namely amiodarone and betablockers). He also suffered a single episode of gastrointestinal bleeding supported with packed red blood cell (PRBC) transfusion with continuation of dual antiplatelet therapy due to the high risk of acute stent thrombosis. In addition, despite adequate deep vein thrombosis prophylaxis and dual antiplatelet therapy, patient developed a right upper lobe pulmonary embolus which was initially treated with intravenous heparin. However, after being exposed to heparin, he subsequently developed heparin induced thrombocytopenia (HIT) confirmed with positive HIT-antibody test, for which an inferior vena cava filter was introduced and intravenous argatroban started until he was therapeutic on Coumadin. The patient was eventually stabilized on appropriate cardiac, antiplatelet and anti-arrhythmic medications and was discharged safely home to be followed up closely as an outpatient.
Discussion
Kounis syndrome is characterized by a group of symptoms that manifest as unstable vasospastic or non-vasospastic angina secondary to a hypersensitivity reaction (2). This syndrome was first described and therefore named after Dr. Nicholas Kounis in 1991 coining it “allergic angina” (3). Since then this condition has evolved to include a number of mast cell activation disorders which are associated with acute coronary syndrome (4,5). Examples include reactions to multiple medications (NSAIDs, antibiotics, and antineoplastic agents), contrast exposure, poison ivy, bee stings and reaction to shellfish (1,6). In addition to coronary arterial involvement, the entity “Kounis syndrome” today encompasses other arterial systems with similar physiology such as mesenteric and cerebral circulation resulting in ischemia/infarction of the vital organs supplied (7,8). Incidence of this condition is hard to delineate due to the number of potential instigating factors and its relatively infrequent documentation in literature.
Pathophysiologically, Kounis syndrome typically results from mast cell degranulation in the setting of an allergic insult with subsequent release of numerous inflammatory mediators such as histamine, neutral proteases, arachidonic acid products, platelet activating factor and a variety of cytokines and chemokines (1). It is these chemical mediators that have been implicated in coronary vasospasm and atheromatous plaque rupture leading to acute coronary syndrome (9). Another pathway described in literature is the acute release of tryptase which in turn activates interstitial collagenase, gelatinase and stromelysin which erode the atheromatous plaques leading to plaque rupture and initiation of inflammatory cascade leading to acute thrombus formation (10). These mechanisms have been pathologically substantiated by a study in 1995 where Kovanen et al. examined specimens of coronary arteries from patients who had died of acute myocardial infarction and found that the degree of mast cell degranulation was much higher (200 to 1 ratio) at the sites of plaque erosion or rupture than in adjacent areas (11).
Uniquely, in our patient in addition to acute coronary thrombosis his hospital course was also complicated by acute venous thrombosis and heparin induced thrombocytopenia which may suggest a disorder of inherent coagulopathy behind Kounis syndrome. A case report by Lomabardini et al. described a state of massive mast cell degranulation in a human following a wasp sting leading to significant anti-thrombotic laboratory disturbances but no acute bleeding. This case reported that significant mast cell activation can result in release of heparin which can act on anticoagulation by binding to antithrombin while extensive release of “tryptase” enzyme inhibits fibrinogen mediated thrombin initiated clot formation (12). However, it is also postulated that the same tryptase is also involved in acute atheromatous plaque rupture demonstrating a pro-thrombotic and anti-thrombotic action on the coagulation cascade. Further studies are needed to identify which pathway predominates in patients with Kounis syndrome.
Clinically, anaphylactic reactions occur as a systemic phenomenon leading to activation of mast cells dwelling in the cardiac tissue producing tachycardia, ventricular dysfunction and even atrio-ventricular conduction block (3). The constellation of symptoms involved in Kounis syndrome have been described in three different settings of coronary artery disease: TYPE I, chest pain secondary to coronary artery vasospasm in patients with no previous history of coronary artery disease; TYPE II, includes patients with culprit but quiescent pre-existing atheromatous disease in whom the acute release of inflammatory mediators can induce either coronary artery spasm with normal cardiac enzymes and troponins or plaque erosion or rupture manifesting as acute myocardial infarction; and TYPE III, includes patients with coronary thrombosis (including stent thrombosis) in whom aspirated thrombus specimens stained with hematoxylin-eosin and Giemsa demonstrate the presence of eosinophils and mast cells respectively (1,6,8). Vessel involvement is varied; however, most cases reported identify single vessel involvement as opposed to two vessel involvement as seen in our case.
As Kounis syndrome represents a constellation of cardiovascular symptoms as a result of an acute allergic insult, clinical diagnosis is often made retrospectively and without definite markers. However, there are some studies that suggest measurement of inflammatory mediators in the serum soon after disease onset can help better appreciate an allergic etiology to the acute coronary event especially in those with no previous risk factors or known coronary disease. Such markers as prostaglandin D2, carpoxypeptidase, CD63, interleukin 4 and 6, CRP and tryptase have been evaluated to help elucidate a diagnosis of anaphylaxis with routine tests still being developed (13). Histamine released by degranulation of mast cells can also be measured within five to ten minutes but only remains elevated for 30-60 mins and therefore has a very limited value (14). To date, serum tryptase has only been identified as a reliable marker of an anaphylactic reaction. Review of literature has suggested that serum tryptase can be considered as a new marker of the instability of atheromatous plaque in relation to the existence of mastocytes in heart tissue (15). Nevertheless, regardless of documented lab evidence of anaphylaxis a diagnosis can still be achieved based on the clinical presentation and treatment carried out accordingly.
Treatment of Kounis syndrome consists mostly of aborting the allergic/anaphylactic reaction followed by stabilization of the coronary vasculature with known medical/interventional techniques. Abortive therapies include the use of corticosteroids, epinephrine and antihistamines until therapeutic effect is achieved. Such measures are thought to improve vasospasm by reducing transcription of several pro-inflammatory cytokines and their subsequent effect on unstable coronary plaques (16). Typical medical therapy for acute coronary syndrome can then be instituted bearing in mind such medications as beta-blockers may make the use of epinephrine ineffective requiring beta-blocker reversal with glucagon. Analgesia with fentanyl and its derivatives is better suited as morphine may increase mast cell degranulation thereby potentiating the allergic reaction (17). Type I Kounis syndrome characterized by coronary vasospasm responds well to treatment with corticosteroids and H1/H2 receptor blockers in addition to coronary vasodilators such as nitrates/CCBs if tolerated. Similar therapy is employed in patients with Type II/III Kounis syndrome where coronary interventions are often required to manage acute plaque rupture and thrombus formation (18). Once the allergic component is identified, desensitization measures should be employed if possible to prevent such events in the future. Mast cell stabilizers like sodium cromoglycate, ketotifen, sodium nedocromil, lodoxamide which could potentially suppress degranulation of mast cells need more exploration to be used as prevention strategies in such situations (1).
Conclusions
Kounis syndrome is a complicated condition that results in significant cardiovascular manifestations such as an acute myocardial infarction. Increasing reports of this entity in literature warrant paying more attention to prevention rather than cure in known atopic individuals, because sometimes it may be too late before patients reach the hospital for the cure!
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Kounis NG. Kounis syndrome (allergic angina and allergic myocardial infarction): a natural paradigm? Int J Cardiol 2006;110:7-14.
- Kogias JS, Sideris SK, Anifadis SK. Kounis syndrome associated with hypersensitivity to hymenoptera sting. Int J Cardiol 2007;114:252-5.
- Kounis NG, Zavras GM. Histamine-induced coronary artery spasm: the concept of allergic angina. Br J Clin Pract 1991;45:121-8.
- Lopez PR, Peiris AN. Kounis syndrome. South Med J 2010;103:1148-55.
- Waller BF. Non atherosclerotic coronary heart disease. In: Fuster V, Wane Alexander A, O’Rourke RA. eds. Hurst’s The Heart, 13th edn. New York: McGraw-Hill; 2010.
- Taggar JS, Watson T, Musarrat K. Kounis syndrome presenting as ST-segment elevation myocardial infarction following a hymenoptera (bee) sting. Int J Cardiol 2009;136:e29-30.
- Goto M, Matszaki AM, Fuchinoue A, et al. Chronic atherosclerotic mesenteric ischemia that started to develop symptoms just after anaphylaxis. Case Rep Gastroenterol 2012;6:300-8.
- González-de-Olano D, Alvarez-Twose I, Matito A, et al. Mast cell activation disorders presenting with cerebral vasospasm-related symptoms: a “Kounis-like” syndrome? Int J Cardiol 2011;150:210-1.
- Sakata Y, Komamura K, Hirayama A, et al. Elevation of plasma histamine concentration in the coronary circulation in patients with variant angina. Am J Cardiol 1996;77:1121-6.
- Mytas DZ, Stougiannos PN, Zairis MN, et al. Acute anterior myocardial infarction after multiple bee stings. A case of Kounis syndrome. Int J Cardiol 2009;134:e129-31.
- Kovanen PT, Kaartinen M, Paavonen T. Infiltrates of activated mast cells at the site of coronary atheromatous erosion or rupture in myocardial infarction. Circulation 1995;92:1084-8.
- Lombardini C, Helia RE, Boehlen F, et al. “Heparinization” and hyperfibrinogenolysis by wasp sting. Am J Emerg Med 2009;27:1176.e1-3.
- Sainte-Laudy J, Cado S. Comparison of the levels of histamine, tryptase, and interleukin-6 for the investigation of anaphylactoid drug reactions. Allerg Immunol (Paris) 1998;30:209-11.
- Bjornsson HM, Graffeo CS. Improving diagnostic accuracy of anaphylaxis in the acute care setting. West J Emerg Med 2010;11:456-61.
- Kounis NG. Serum tryptase levels and Kounis syndrome. Int J Cardiol 2007;114:407-8.
- Takagi S, GotoY, Hirose E, et al. Successful treatment of refractory vasospastic angina with corticosteroids: coronary arterial hyperactivity caused by local inflammation? Circ J 2004;68:17-22.
- Ridella M, Bagdure S, Nugent K, et al. Kounis syndrome following beta-lactam antibiotic use: review of literature. Inflamm Allergy Drug Targets 2009;8:11-6.
- Sebaldt RJ, Sheller JR, Oates JA, et al. Inhibition of eicosanoid biosynthesis by glucocorticoid in humans. Proc Natl Acad Sci USA 1990;87:6974-8.