Valve-sparing aortic root replacement—midterm outcomes and quality of life
Introduction
Surgery of aortic root aneurysm has consistently changed over the last three decades. The gold standard treatment for aortic root pathology with or without aortic valve disease has traditionally been the use of composite valve and root replacement (Bentall and De Bono procedure) along with various modifications (1). The composite valved-graft could include either mechanical or bioprosthetic valve. The Bentall operation has demonstrated excellent mid-term to long-term survival (2,3) including few cardiac deaths. On 5, 10, and 15 years actuarial composite valve graft-related event-free survival was 97%, 82% and 54%, respectively (4-7). There was no difference in long-term survival between bioprosthetic and mechanical valves (8). All-cause mortality and valve-related complications were not significantly different between these two types of prostheses (9). However, a potential shortcoming to this procedure is the risk of valve-related events: reoperation, particularly structural deterioration for biologic valves, bleeding (9–11%) and thromboembolism (4–11%) for mechanical aortic valve (5,8). The linearized rate for valve/ascending aorta reoperation was 0.86% after Bentall procedure with mechanical valves and 2.5% after Bentall with the bioprosthetic valve (8). A good quality of life described by health-related quality-of-life (HRQOL), was highlighted after Bentall procedure, also independent of the type of prosthesis (9).
Valve sparing root replacement is an attractive treatment option compared with composite valve and root replacement for patients with aortic root aneurysm. This procedure can be performed by either remodeling (Yacoub) or reimplantation (David) technique (10,11). The 10-year survival is excellent reaching that of the general population of the same age. On year 10 during the follow-up, the risk of reoperation appears to be moderate (5–15%). Aortic valve preservation reduces the risk of valve-related complications (2).
In this study, we examined the midterm outcomes, the valve related events and quality of life of patients treated by valve-sparing aortic root replacement (VSRR).
Methods
From January 2003 to December 2014, 88 consecutive patients with an aortic root aneurysm or ascending aortic aneurysms with or without aortic regurgitation (AR) underwent VSRR surgery.
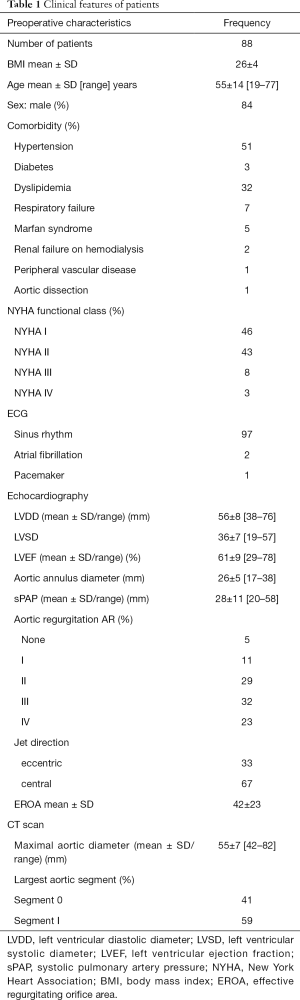
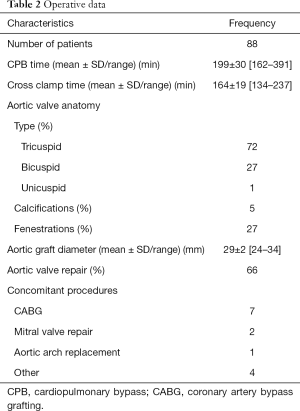
Clinical features of all patients are listed in Table 1. Eighty-six patients were living in France and 2 came from abroad (1 Canadian and 1 Senegalese). All operations were performed through a median sternotomy, with standard cardiopulmonary bypass (CPB), in normothermia, with cannulation of aortic arch, right atrium, and superior pulmonary vein venting. Myocardial protection was accomplished with a combination of antegrade and retrograde cold blood cardioplegia. The ascending aorta was transversely opened, above the tops of the commissures; cutting of the coronary ostia, dissection from the aortic root to the aortic annulus plane, resection of the sinuses. Proximal sub annular fixation of the vascular prosthesis using eleven U-shaped stitches. After aortic valve reimplantation and co-optation assessment, repair of the aortic valve was done if necessary by plicature of central free marginal of the cusps. Reimplantation of the coronary ostia is performed by the button technique. Operative data of the patients are listed in Table 2. We found 24 (27.3%) patients with bicuspid valve, 55% had AR grade III or IV. We used a straight aortic graft in 5 cases (HEMAGUARD® InterVascular, La Ciotat, France) and a Valsalva aortic graft in 83 cases (Vascutek Gelweave® Vascutek Terumo, Glasgow, Scotland; or CARDIOROOT MAQUET®, InterVascular, La Ciotat, France).
Full table
Full table
Patients were followed every year by family doctors and referent cardiologists. Doppler echocardiographic examinations were obtained annually in most patients. Valve-related morbidity and EuroQoL (EQ) scores were obtained by mail, self-administered questionnaires. From July 1 to September 1, 2015, questionnaires were sent to patients, referent cardiologists and practitioners. This mailing, in case of “missing response” was completed by direct phone call (patients, practitioners, and cardiologists). All patients had an echocardiographic study during the last year of follow-up, which was closed on September 1, 2015. The mean follow-up was 5.3±3 (range 1–12) years.
No patient was missing during the follow-up. Outcome events were registered according to the 2008 American Association for Thoracic Surgery/Society of Thoracic Surgeons/European Association for Cardiothoracic Surgery guidelines for reporting mortality and morbidity after cardiac valve interventions (12). The EQ instrument is a 5-item general questionnaire assessing five health domains, mobility, self-care, usual activity, pain and discomfort, and anxiety and depression, from which the “EQ index” is then calculated, producing a score from 0 to 1.0, with a higher score indicating best quality of life (13). The EQ visual analogue scale (EQ VAS) records the respondent’s self-rated health on a vertical from 0 to 100, visual analogue scale where the endpoints are labelled ‘best imaginable health state’ and ‘worst imaginable health state’. This information can be used as a quantitative measure of health as judged by the individual respondents (13).
Statistical analysis
All analyses were conducted with Stata 13 (StataCorp, College Station, TX, USA) under a bilateral hypothesis with a type-I error set at 5%. For descriptive analyses, the categorical variables were expressed as number and percentage; the quantitative variables were expressed as mean ± standard deviation in case of Gaussian distribution, or by quartiles and range otherwise. Normality and homoscedasticity were respectively checked with the Shapiro-Wilk’s and the Fisher-Snedecor’s test. Between-group comparisons for categorical variables were conducted by the chi-square or Fisher’s exact test where appropriate. Comparisons quantitative variables were conducted by the Student’s t or the Mann-Whitney’s, where appropriate. Censored data were estimated by the Kaplan-Meier’s method and compared between groups by the Cox regression proportional hazard model. In multivariable analyses, these models were implemented by including covariates selected in accordance to the results of univariate analysis and to clinical relevance or current knowledge. Schoenfeld’s assumptions were checked, and results were expressed in terms of chance ratio and its 95% confidence interval. Most of the secondary analyses were exploratory and therefore lacked statistical power. As discussed by Feise in 2002 (14), particular attention was given to the magnitude of the differences, and not only to significance. Then, no correction of the type-I error was done.
Results
Early postoperative outcomes
Hospital mortality was 1%; one patient died as the result of acute respiratory failure in intensive care on day 7. The median length of intensive care unit stay was 2 days ranging from 1 to 64 days. The median length of hospital was 10 days ranging from 6 to 156 days. The mean aortic valve area was 2.3±0.5 cm2 ranging from 1.5 to 3.7 cm2. The mean left ventricular ejection fraction (LVEF) was 58±11% ranging from 25% to 79%. The mean aortic gradient was 7±4 mmHg ranging from 0 to 26 mmHg.
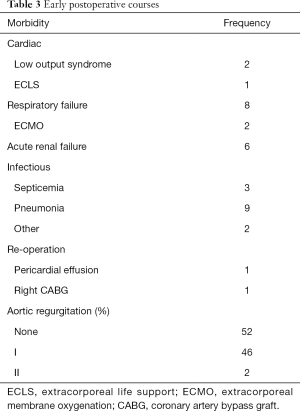
Hospital morbidity and postoperative echocardiography examinations are listed in Table 3.
Full table
Medium-term outcomes
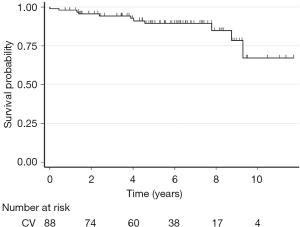
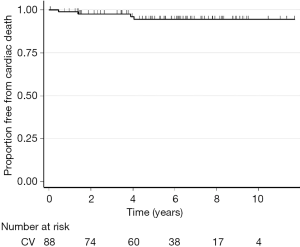
During the follow-up, there were 10 late deaths (11%). Causes of death were cardiac in 5 cases (post heart transplant cardiogenic shock, myocardial infarction, acute left ventricular dysfunction, sudden death and ischemic stroke) and non-cardiac in 5 cases (2 lung cancer, 1 drowning, 1 acute myeloid leukemia, 1 head trauma). Figures 1 and 2 show the Kaplan-Meier global and free from cardiac death survival curves.
Three patients required a successful reoperation. Two for a root surgery complication on postoperative years 2 and 6: one for an acute vascular graft infection with a false aneurysm and an aortic insufficiency repaired by mechanical Bentall procedure; and a second one for sterilized endocarditis with a severe aortic insufficiency treated by transcatheter aortic valve implantation (TAVI) due to age over 83 years. One patient underwent mitral valve replacement 4 years after aortic root surgery.
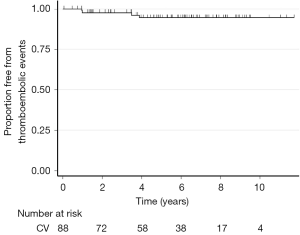
Aortic valve endocarditis and prosthetic graft infection occurred in two patients, medically treated for the first one and surgically managed for the second one. Three patients suffered from ischemic stroke, one of the patients had a pace maker, the two others were in sinus rhythm. None of the following morbidity has been observed: structural valve deterioration, nonstructural dysfunction, valve thrombosis, bleeding event. The Figure 3 shows the Kaplan-Meier survival curve free from thromboembolic events.
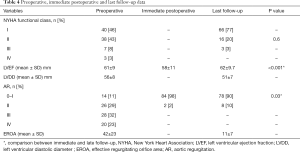
At the last check of the follow-up, 90% of patients had sinus rhythm, 6% atrial fibrillation and 4% pacemaker. Table 4 reported univariate analysis between pre- and postoperative functional class and echocardiographic data.
Full table
Quality of life after VSRR
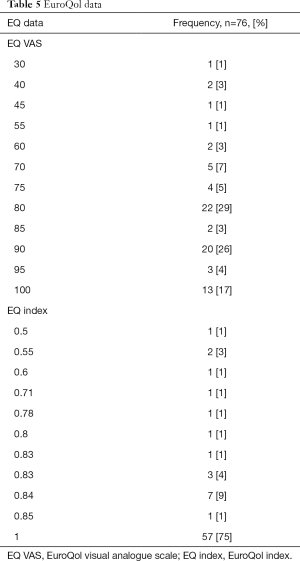
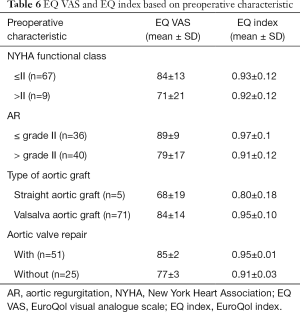
Mean EQ VAS was 83±15 ranging from 30 to 100, with a median of 82.5. Mean EQ index was 0.94±0.12 ranging from 0.5 to 1, with a median of 1 (Tables 5 and 6).
Full table
Full table
Risk factor analysis of medium-term outcome and quality of life
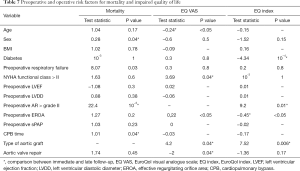
On 8 years, most relevant factors influencing mortality and quality of life were age, sex, BMI, preoperative respiratory failure, diabetes, preoperative AR, preoperative EROA, preoperative aortic annulus diameter, type of aortic graft CPB time (Table 7).
Full table
Discussion
Mid-term results and quality of life after VSRR are excellent in our experience as shown in this study. VSRR can offer an important alternative to Bentall technique for many reasons: optimal haemodynamic conditions, no need for anticoagulation, more physiological virtual basal ring growth in young patients and lower valve-related complication rate (15,16).
Our hospital mortality of 1% is comparable to other reports of aortic valve sparing root replacement (0.9–1%) (17,18); and less or equivalent to Bentall surgery (between 3% and 11%) (4-7).
In our population, the few valve complications encountered were infection in 2% and embolic event in 3%. There were no bleeding events in our series. Valve-related complications after VSRR are fewer compared with Bentall procedure which, in the literature, arose with a frequency of 13% for reoperation, 7% for nonstructural dysfunction, 4–18% for thromboembolic events, 12% for endocarditis, 9–13% for bleeding events (5,8,9,19).
The risk factors that influenced the overall mortality were sex, preoperative respiratory failure, preoperative AR > grade II, CPB time. Beside classical risk factors that influence mortality such as sex, respiratory failure and higher CPB time we found in this study that preoperative factor such as higher AR > grade II, has detrimental effect. However, an effect of preoperative LVEF could not be evidenced. Therefore, a possible explanation could be that the severity of AR is more pertinent and earlier parameter influencing mortality than ejection fraction. Similar risk factors of early mortality were found in the literature like cross clamp time, bypass time, the New York Heart Association (NYHA) functional class >II (20).
In this midterm study, valve function appears to be quite stable over time as shown by the absence of reoperation for AR and the absence of AR > grade II. In our study, while comparing early postoperative and late echography only 6 patients with immediate postoperative echocardiography grade I increase to grade II on late examination, 78 patients didn’t modify their AR grade. Concerning follow up AR quantification, reported results seems to be slightly worst with 35%, of mild AR, 2% of moderate and 3% of severe 3% (21). In the literature, recurrence risk factors of AR are: preoperative AR grade and specifically grade IV AR, performing of sub-commissural annuloplasty, aortic valve repair, aortic annular dilatation (aortic annulus >25 mm), mild AR prior to discharge (21,22).
Quality of life perceived by our patients is similar to healthy patients in 79%. EQ index in our study was 0.94±0.12, slightly superior to those reported after Bentall procedure EQ index =0.90±0.04 (9). Regarding both clinical outcome and postoperative quality of life, the superiority of the aortic valve reimplantation compared with the aortic composite replacement is demonstrated (23).
Beside classical risk factors that influence quality of life such as age and diabetes we found in this study that preoperative factors such as higher NYHA class, AR > grade II, higher EROA have detrimental effect. We could hypothesize that delayed operative decision at higher functional class or severe aortic insufficiency, and despite preserved LVEF, had a pejorative impact on midterm quality of life.
Study limitations
This manuscript reports single-centre retrospective observational analysis with a limited number of patients. But clinical characteristic and echocardiographic examinations were collected prospectively. However early and late echocardiography was done by different cardiologists.
Conclusions
Although the technical difficulties, we believe that the VSRR surgery can be performed with the same and even lower rate of postoperative morbidity and mortality and the same or better quality of life. Moreover, the outcomes of this procedure in term of aortic valve competency remain stable in time. Therefore, valve sparing aortic root replacement seems to be a promising method, and offer an interesting alternative treatment to traditional Bentall procedure. In properly selected patients, we consider it as the gold standard.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical statement: The study was approved by institutional ethics committee of Gabriel Montpied University Hospital of Clermont-Ferrand (ETSH1200330A) and informed consent was taken from all the patients.
References
- Bentall H, De Bono A. A technique for complete replacement of the ascending aorta. Thorax 1968;23:338-9. [Crossref] [PubMed]
- Toeg H, Chan V, Rao RV, et al. Contemporary midterm echocardiographic outcomes of bentall procedure and aortic valve sparing root replacement. Annals of Thoracic Surgery 2014;98:590-6. [Crossref] [PubMed]
- Gaudino M, Lau C, Munjal M, et al. Contemporary outcomes of surgery for aortic root aneurysms: A propensity-matched comparison of valve-sparing and composite valve graft replacement. J Thorac Cardiovasc Surg 2015;150:1120-9.e1. [Crossref] [PubMed]
- Varrica A, Satriano A, De Vincentiis C, et al. Bentall operation in 375 patients: long-term results and predictors of death. J Heart Valve Dis 2014;23:127-34. [PubMed]
- Kim TS, Na CY, Oh SS, et al. Long-term mortality and morbidity after button Bentall operation. J Card Surg 2013;28:280-4. [Crossref] [PubMed]
- Verbakel KM, van Straten AH, Hamad MA, et al. Results of one-hundred and seventy patients after elective Bentall operation. Asian Cardiovasc Thorac Ann 2012;20:418-25. [Crossref] [PubMed]
- Etz CD, Homann TM, Silovitz D, et al. Long-term survival after the Bentall procedure in 206 patients with bicuspid aortic valve. Ann Thorac Surg 2007;84:1186-93. [Crossref] [PubMed]
- Etz CD, Bischoff MS, Bodian C, et al. The Bentall procedure: is it the gold standard? A series of 597 consecutive cases. J Thorac Cardiovasc Surg 2010;140:S64-70. [Crossref] [PubMed]
- Lehr EJ, Wang PZ, Oreopoulos A, et al. Midterm outcomes and quality of life of aortic root replacement: mechanical vs biological conduits. Can J Cardiol 2011;27:262.e15-20. [Crossref] [PubMed]
- Yacoub M, Fagan A, Tassano P, et al. Result of valve conserving operations for aortic regurgitation Circulation 1983;68:321. (abstract). [PubMed]
- David TE, Feindel CM. An aortic valve-sparing operation for patients with aortic incompetence and aneurysm of the ascending aorta. J Thorac Cardiovasc Surg 1992;103:617-21. [PubMed]
- Akins CW, Miller DC, Turia MI, et al. Guidelines for reporting mortality and morbidity after cardiac valve interventions. Ann Thorac Surg 2008;85:1490-5. [Crossref] [PubMed]
- EuroQol Group Association. Euroqol-5D User guide. Available online: https://euroqol.org/publications/user-guides/
- Feise RJ. Do multiple outcome measures require p-value adjustment? BMC Med Res Methodol 2002;2:8. [Crossref] [PubMed]
- Price J, De Kerchove L, Glineur D, et al. Risk of valve-related events after aortic valve repair. Ann Thorac Surg 2013;95:606-12. [Crossref] [PubMed]
- Aicher D, Fries R, Rodionycheva S, et al. Aortic valve repair leads to a low incidence of valve-related complications. Eur J Cardiothorac Surg 2010;37:127-32. [Crossref] [PubMed]
- Coselli JS, Hughes MS, Green SY, et al. Valve-sparing aortic root replacement: early and midterm outcomes in 83 patients. Ann Thorac Surg 2014;97:1267-73. [Crossref] [PubMed]
- Kvitting JP, Kari FA, Fischbein MP, et al. David valve-sparing aortic root replacement: equivalent midterm outcome for different valve types with or without connective tissue dis- order. J Thorac Cardiovasc Surg 2013;145:117-26. [Crossref] [PubMed]
- Sabol F, Kolesar A, Jankajova M, et al. Aortic valve-sparing operation versus Bentall and mechanical aortic valve replacement--midterm results. Bratisl Lek Listy 2014;115:292-9. [PubMed]
- Jasinski MJ, Gocol R, Malinowski M, et al. Predictors of early and medium-term outcome of 200 consecutive aortic valve and root repairs. J Thorac Cardiovasc Surg 2015;149:123-9. [Crossref] [PubMed]
- Koolbergen DR, Manshanden JS, Bouma BJ, et al. Valve-sparing aortic root replacement. Eur J Cardiothorac Surg 2015;47:348-54; discussion 354. [Crossref] [PubMed]
- Stephens EH, Liang DH, Kvitting JP, et al. Incidence and progression of mild aortic regurgitation after Tirone David reimplantation valve-sparing aortic root replacement. J Thorac Cardiovasc Surg 2014;147:169-77. [Crossref] [PubMed]
- Franke UF, Isecke A, Nagib R, et al. Quality of life after aortic root surgery: reimplantation technique versus composite replacement. Ann Thorac Surg 2010;90:1869-75. [Crossref] [PubMed]