Type II endoleaks: diagnosis and treatment algorithm
Introduction
Elective abdominal aortic aneurysm (AAA) repair is recommended for aneurysms greater than 5.5 cm, symptomatic, and/or rapidly expanding more than 0.5 cm in 6 months (1). Guidelines recommend repair when an aneurysm exceeds 5.0 cm for women given evidence that AAA rupture at smaller sizes than men and have poorer outcomes. Seventy-five percent of AAA s today are treated with endovascular aneurysm repair (EVAR) rather than open repair, given decreased periprocedural mortality, complications, and length of hospital stay (2-4). However, some studies have demonstrated EVAR to result in higher reintervention rates, largely secondary to endoleak, a complication not present in the surgical counterpart (5-8). An endoleak results from continued perfusion of the aneurysm sac despite endograft deployment. It occurs in 20–25% of patients, and are categorized from type I to V (9,10). Type II is the most common, making up 10–25% of all endoleaks (10). They occur from retrograde collateral blood flow into the aneurysm sac, typically from a lumbar artery or the inferior mesenteric artery (IMA). Other less common sources include retrograde flow from accessory renal, gonadal, and median sacral arteries, and the internal iliac artery if not embolized when covered by a limb extending into the external iliac artery (11).
Type II endoleaks, can potentially enlarge and pressurize the aneurysm sac with a risk of rupture. However, many type II endoleaks spontaneously resolve or never lead to sac enlargement. This is different than a type I and III endoleak which significantly pressurizes the aneurysm sac with significant risk of rupture. In a study involving 474 patients with type II endoleaks, there were no late AAA ruptures attributable to a type II endoleak. All-cause mortality and aneurysm-related mortality did not differ between patients with and without a type II endoleak. In addition, there was no difference in all-cause mortality or aneurysm-related mortality in patients who had a type II endoleak-associated sac growth who underwent reintervention and those in whom the type II endoleak was not treated (12). Similar findings were seen in an older study by Silverberg, et al. with 154 patients with type II endoleaks. Seventy-five percent of type II endoleaks resolved spontaneously and no pure type II endoleak was associated with rupture (13). Some studies have shown different results. In a study by El Batti et al., patients with a type II endoleak had more complications, including death, rupture, reintervention or conversion (14). In a meta-analysis of outcome data of 10 EVAR trials, analysis showed that in the absence of a type I or III endoleak, intervention on a type II endoleak should be reserved if a type II endoleak occurs after 6 months, persisted more than 12 months, or aneurysm sac pressure was >20% of systolic blood pressure (15). Conversely, a more recent meta-analysis also analyzing data from 10 EVAR trials failed to demonstrate adequate information to support a threshold for intervention due to the rarity of rupture and sac expansion associated with pure type II endoleak (16).
It should be noted that a few have advocated preemptive embolization of the IMA to mitigate type II endoleaks. A meta-analysis demonstrated that the rate of type II endoleaks after IMA embolization was 19.9%, compared to 41.4% without IMA embolization. However, the authors surmised that given the treatment of type II endoleaks is needed in less than 20% of cases, that this complication can be treated successfully in 60–70% of cases, and that the aneurysm rupture risk is <1% with an isolated type II endoleak, data did not support preemptive IMA embolization (17).
Type II endoleaks can be divided into type IIa where there is a single causative vessel involved with “to-and-fro” flow in the aneurysm sac, and type IIb, where multiple vessels are involved, behaving similar to arteriovenous malformations (AVM). Type IIa endoleaks have greater propensity to spontaneously resolve than type IIb, which are more complex and difficult to treat (18). Predictors of persistent type II endoleaks include numerous collaterals, large central nidus (>15 mm), high blood flow (velocity >100 cm/s), and anticoagulation. Factors associated with aneurysm growth include a type IIb endoleak, IMA >2.5 mm, a lumbar artery >1.9 mm, and more than 2 lumbar arteries that extend from the aneurysm sac (19). A type II endoleak may be early, occurring within 30 days of EVAR, persistent, lasting longer than 6 months, or late, occurring after 1 year (18).
Imaging of type 2 endoleak
After EVAR has been completed, our follow-up protocol is a CT angiography (CTA) at 1, 6, and 12 months, and annually thereafter. In the setting of aneurysm sac shrinkage and absence of an endoleak, some patients may be followed every 2 years. In patients with renal insufficiency, follow up may be performed with duplex ultrasound and non-contrast CT. Contrast enhanced ultrasound has emerged as an alternative strategy and has a high sensitivity and specificity for the detection of endoleaks. Time-resolved magnetic resonance angiography is used selectively at our institutions, sometimes to better determine the flow dynamics of an endoleak seen on CTA as well as to optimize a treatment strategy in complex cases (20).
A proper imaging protocol is necessary to ensure endoleaks are identified. A three-phase scan consisting of a non-contrast scan, an arterial phase, and delayed imaging is considered the standard of care, and review of previous studies is mandatory. There is newer data that suggests dual-source dual-energy multidetector CT may be as accurate as the standard triphasic protocol with a significant radiation dose reduction (21-23). The latter protocol is especially promising given the significant radiation exposure patients encounter during CT follow-up imaging after EVAR. Once an endoleak is identified, it is important to determine the endoleak type in order to direct urgency and management. Cross-sectional imaging may not always elucidate the type of endoleak present, making angiography the next step in management. Diagnostic angiography should include an aortogram, as well as selective angiography of the superior mesenteric artery (SMA) and bilateral hypogastric arteries. Power injection runs with adequate contrast volume and frame rate are required. Super-selective angiography of secondary and tertiary branches of the SMA and hypogastric arteries is often necessary to identify endoleaks which may not be well seen on nonselective angiograms. If a type III endoleak is suspected, angiography performed with a pigtail catheter tip within the endograft may be useful. Placement of an occlusion balloon above the pigtail catheter in the graft may increase the sensitivity for type III endoleak assessment. A type Ib endoleak can be uncovered in a similar fashion, wherein a compliant balloon can be deployed in the iliac limb as an angiogram is performed with the pigtail tip at the distal seal site. This may force contrast into the aneurysm sac from the distal seal site if a type Ib endoleak is present. Furthermore, filling of the IMA or lumbar arteries on cross-sectional imaging does not always represent a type II endoleak as we have seen many patients who have a subtle type Ia endoleak creating an inflow into the aneurysm sac, with resultant outflow in the IMA or lumbar vessels. This is best appreciated on aortography.
It is important to remember that the presence of one type of endoleak does not exclude the presence of another. Moreover, treatment of one endoleak does not preclude the subsequent development of another. Many times, endoleaks are complex and must be managed expectantly. Therefore, longitudinal follow-up of these patients is mandatory (20).
Treatment strategies
There are multiple approaches to the management of type II endoleaks, including transarterial, translumbar, transcaval, and surgical approaches. Transarterial and translumbar approaches are the most commonly used. There are conflicting data in the literature regarding the best approach. An early study published in 2002 looked at transarterial embolization vs. translumbar embolization, where 20 patients underwent IMA embolization and 13 underwent translumbar embolization. There was an 80% failure rate with transarterial embolization with recanalization of the original endoleak cavity compared to 8% failure rate with translumbar embolization (24). A more recent study published in 2017 reported 23 patients undergoing 35 embolizations. There was no significant difference in aneurysm sac growth, persistent type 2 endoleak, or complications. However, direct sac puncture did carry a shorter fluoroscopy time and total procedure time (25). A larger retrospective study with 84 patients with a type 2 endoleak compared 62 patients undergoing translumbar embolization with coils and n-butyl cyanoacrylate and 23 patients undergoing transarterial embolization with embolization of the inferior mesenteric or lumbar artery. Success was 72% in patient undergoing translumbar embolization, and 78% with transarterial embolization, which was not statistically different (26).
Transarterial embolization
The transarterial approach is the first line of approach at our institutions. When addressing the IMA, a microcatheter is advanced in a retrograde fashion from the SMA via the arc of Riolan or the marginal artery of Drummond to the IMA. When embolizing a lumbar artery, the microcatheter is advanced from the internal iliac artery to the iliolumbar artery to the culprit lumbar artery (Figure 1). In all cases, it is important to advance the microcatheter to the aneurysm sac; however, collateral pathways can be long and tortuous and potentially very difficult or impossible to maneuver. The goal is to completely obliterate the endoleak nidus and eliminate all inflow and outflow vessels. Proximal embolization is not recommended as a type II endoleak will recur by recruiting additional aortic branch vessels.
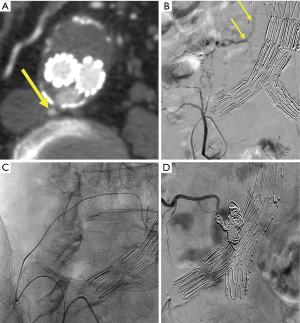
The procedure is performed with stable access in the SMA or the internal iliac artery with a 5-Fr Cobra catheter or reverse-curve catheter, such as a Sos or Simmons. If there is significant tortuosity and inability to achieve stable access, a steerable guiding sheath such as a Destino (Oscor Inc., Palm Harbor, USA) or Morph (BioCardia, Inc., San Carlos, USA) can be utilized. A 150-cm long microcatheter with a 0.021-inch inner diameter or smaller, such as Echelon or Rebar (Medtronic, Minneapolis, USA) is recommended for Onyx [ethylene-vinyl alcohol copolymer (EVOH)] liquid embolic system (Medtronic).
Possible embolization materials include coils, EVOH, n-butyl-cyanoacrylate glue, and coils. Coils are the most widely used. The advantage of EVOH is that it can fill the endoleak nidus and the inflow and outflow vessels. It is also radiopaque, therefore, monitoring the injection and avoiding nontargeted embolization can be performed. Cyanoacrylate glue can be utilized in a similar manner such as after coiling when blood flow has slowed. Its viscosity can be adjusted by adjusting the quantity of ethiodol (27). A less typical approach is to utilize an MVP microvascular plug (Medtronic). The advantage of such a plug is that it leads to minimal artifact on imaging after intervention. Whereas EVOH, glue and coils make assessing for endoleak on post intervention CT scans difficult due to beam hardening artifact, the MVP plug has little associated artifact. Alternatively, MRI is an alternative to image for endoleak which minimizes artifact from the above embolic agents.
Translumbar embolization
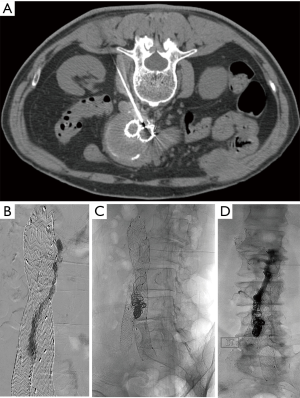
Some advocate translumbar embolization as the first line of therapy. Ideally, translumbar embolization is performed with combination of CT and fluoroscopy. If CT cannot be utilized, landmarks or cone beam CT may be utilized. The aneurysm sac is accessed at the level the endoleak as demonstrated on CTA. While fusion imaging can be helpful in accessing the proper level, fluoroscopy alone may be used when these more sophisticated technologies are not available. The operator will observe pulsatile blood once the aneurysm sac is successfully accessed. A baseline pressure should be recorded. A diagnostic angiogram or a “saccogram” is performed via a sheath needle to delineate the endoleak cavity and inflow and outflow vessels. A microcatheter is typically advanced to the nidus and attempt to embolize all inflow and outflow vessels as well as the nidus is performed. If there is difficulty addressing all inflow and outflow vessels, EVOH or cyanoacrylate glue may be utilized to embolize the nidus and vessels (Figure 2). A final intrasac pressure should be obtained at the conclusion of embolization.
Transcaval approach
This is rarely performed and is reserved if the endoleak is visualized on the right side or in close proximity to the inferior vena cava (IVC). In this technique, the internal jugular or common femoral vein is accessed and a 10 Fr 40 cm transjugular intrahepatic portosystemic shunt sheath is pressed up against the wall of the IVC and a Colapinto needle (Cook Medical, Winston-Salem, USA) is used to accessed the aneurysm sac. Once arterial flow is identified, a 5-F cannula with catheter is navigated into the endoleak nidus. Embolization is then performed similarly to translumbar embolization. A cavagram is obtained at the conclusion of embolization. Potential risks include retroperitoneal bleed, pulmonary embolus from nontargeted embolization, and aortocaval fistula. In a retrospective study of 26 patients who underwent 29 embolization procedures, none of these complications were seen. There was an 83% technical success rate in achieving transcaval access to the aorta. One-year freedom from intervention was 95% and at a mean of 16.5 months, 70% of patients experienced no further endoleak and had stable or decreasing aneurysm sac diameters (28).
Surgical approach
Endovascular approaches are generally preferred over surgical technique. However, sometimes endovascular approaches have suboptimal results and there is continued growth of the aneurysm sac despite multiple endovascular procedures. Surgical approaches include laparoscopic, robotic, and open surgical ligation of mesenteric, lumbar, and other offending arteries, as well as plication of the aneurysm, and graft explantation.
Future considerations
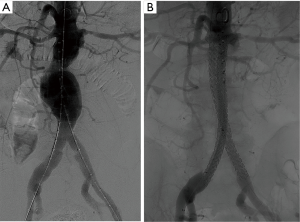
The Nellix endograft (Endologix Inc., Irvine, USA), currently has an FDA Investigational Device Exemption, undergoing efficacy trials, and a European CE mark approval. The device is unique. The sac anchoring endovascular aneurysm sealing system is comprised of two balloon expandable stents that extend in parallel from the non-aneurysmal aorta proximally into the iliac arteries distally. Each balloon expandable stent is surrounded by a polymer filled endobag. The endobags obliterate the aneurysm flow lumen to achieve a seal to resist both lateral and longitudinal displacement forces. Given the filling of the aneurysm sac by the polymer-filled endobag, the device may decrease the incidence of type 2 endoleaks and reintervention rates (Figure 3). In a multicenter study with 171 patients treated with the Nellix device and observed for a median of 5 months (range, 0–14 months), technical success was 99% and type II endoleak rate was 2% (29). There were no aneurysm ruptures or need for open surgical conversion.
Conclusions
EVAR continues to be the favored option for AAA repair given its decreased periprocedural morbidity and mortality risk compared to that of surgical repair. However, endoleaks, especially type II endoleak, continue to be a plague for interventionalists following EVAR. Further investigation is needed to determine the most effective management for optimal durable results.
Acknowledgements
None.
Footnote
Conflicts of Interest: RT Gandhi is a consultant to Medtronic. The other authors have no conflicts of interest to declare.
References
- Brewster DC, Cronenwett JL, Hallett JW Jr, et al. Joint Council of the American Association for Vascular Surgery and Society for Vascular Surgery Guidelines for the treatment of abdominal aortic aneurysms. Report of a subcommittee of the Joint Council of the American Association for Vascular Surgery and Society for Vascular Surgery. J Vasc Surg 2003;37:1106. [Crossref] [PubMed]
- Malas M, Arhuidese I, Qazi U, et al. Perioperative mortality following repair of abdominal aortic aneurysms: application of a randomized clinical trial to real-world practice using a validated nationwide data set. JAMA Surg 2014;149:1260-5. [Crossref] [PubMed]
- Jackson RS, Chang DC, Freischlag JA. Comparison of long-term survival after open vs endovascular repair of intact abdominal aortic aneurysm among Medicare beneficiaries. JAMA 2012;307:1621-8. [Crossref] [PubMed]
- Speicher PJ, Barbas AS, Mureebe L. Open versus endovascular repair of ruptured abdominal aortic aneurysms. Ann Vasc Surg 2014;28:1249-57. [Crossref] [PubMed]
- Al-Jubouri M, Comerota AJ, Thakur S, et al. Reintervention after EVAR and open surgical repair of AAA: a 15-year experience. Ann Surg 2013;258:652-7; discussion 657-8. [PubMed]
- Becquemin JP, Pillet JC, Lescalie F, et al. A randomized controlled trial of endovascular aneurysm repair versus open surgery for abdominal aortic aneurysms in low- to moderate-risk patients. J Vasc Surg 2011;53:1167-73.e1. [Crossref] [PubMed]
- Greenhalgh RM, Brown LC, Powell JT, et al. United Kingdom EVAR Trial Investigators. Endovascular versus open repair of abdominal aortic aneurysm. N Engl J Med 2010;362:1863-71. [Crossref] [PubMed]
- Stather PW, Sidloff DA, Dattani N, et al. Systematic review and meta-analysis of the early and late outcomes of open and endovascular repair of abdominal aortic aneurysm. Br J Surg 2013;100:863-72. [Crossref] [PubMed]
- Hellinger JC. Endovascular repair of thoracic and abdominal aortic aneurysms: pre- and postprocedural imaging. Tech Vasc Interv Radiol 2005;8:2-15. [Crossref] [PubMed]
- Veith FJ, Baum RA, Ohki T, et al. Nature and significance of endoleaks and endotension: summary of opinions expressed at international conference. J Vasc Surg 2002;35:1029-35. [Crossref] [PubMed]
- Sidloff DA, Stather PW, Choke E, et al. Type II endoleak after endovascular aneurysm repair. Br J Surg 2013;100:1262-70. [Crossref] [PubMed]
- Walker J, Tucker LY, Goodney P, et al. Type II endoleak with or without intervention after endovascular aortic aneurysm repair does not change aneurysm-related outcomes despite sac growth. J Vasc Surg 2015;62:551-61. [Crossref] [PubMed]
- Silverberg D, Baril DT, Ellozy SH, et al. An 8-year experience with type II endoleaks: natural history suggests selective intervention is a safe approach. J Vasc Surg 2006;44:453-9. [Crossref] [PubMed]
- El Batti S, Cochennec F, Roudot-Thoraval F, et al. Type II endoleaks after endovascular repair of abdominal aortic aneurysm are not always a benign condition. J Vasc Surg 2013;57:1291-7. [Crossref] [PubMed]
- Gelfand DV, White GH, Wilson SE. Clinical significance of type II endoleak after endovascular repair of abdominal aortic aneurysm. Ann Vasc Surg 2006;20:69-74. [Crossref] [PubMed]
- Karthikesalingam A, Thrumurthy SG, Jackson D, et al. Current evidence is insufficient to define an optimal threshold for intervention in isolated type II endoleak after endovas-cular aneurysm repair. J Endovasc Ther 2012;19:200-8. [Crossref] [PubMed]
- Biancari F, Makela J, Venermo M. Is inferior mesenteric artery embolization indicated prior to endovascular repair of abdominal aortic aneurysm? Eur J Vasc Endovasc Surg 2015;50:671-4. [Crossref] [PubMed]
- Brown A, Saggu G, Brown M, et al. Type II endoleaks: challenges and solutions. Vasc Health Risk Manag 2016;12:53-63. [PubMed]
- Otsu M, Ishizaka T, Watanabe M, et al. Analysis of anatomical risk factors for persistent type II endoleaks following endovascular abdominal aortic aneurysm repair using CT angiography. Surg Today 2016;46:48-55. [Crossref] [PubMed]
- Uthoff H, Peña C, Katzen B, et al. Current clinical practice in postoperative endovascular aneurysm repair imaging surveillance. J Vasc Interv Radiol 2012;23:1152-9.e6. [Crossref] [PubMed]
- Chandarana H, Godoy MC, Vlahos I, et al. Abdominal aorta: evaluation with dual-source dual-energy multidetector CT after endovascular repair of aneurysms—initial observations. Radiology 2008;249:692-700. [Crossref] [PubMed]
- Ascenti G, Mazziotti S, Lamberto S, et al. Dual-energy CT for detection of endoleaks after endovascular abdominal aneurysm repair: usefulness of colored iodine overlay. AJR Am J Roentgenol 2011;196:1408-14. [Crossref] [PubMed]
- Flors L, Leiva-Salinas C, Norton PT, et al. Endoleak detection after endovascular repair of thoracic aortic aneurysm using dual-source dual-entery CT: suitable scanning protocols and potential radiation dose reduction. AJR Am J Roentgenol 2013;200:451-60. [Crossref] [PubMed]
- Baum RA, Carpenter JP, Golden MA, et al. Treatment of type 2 endoleaks after endovascular repair of abdominal aortic aneurysms: comparison of transarterial and translumbar techniques. J Vasc Surg 2002;35:23-9. [Crossref] [PubMed]
- Yang RY, Tan KT, Beecroft JR, et al. Direct sac puncture versus transarterial embolization of type II endoleaks: an evaluation and comparison of outcomes. Vascular 2017;25:227-33. [Crossref] [PubMed]
- Stavropoulos SW, Park J, Fairman R, et al. Type 2 endoleak embolization comparison: translumbar embolization versus modified transarterial embolization. J Vasc Interv Radiol 2009;20:1299-302. [Crossref] [PubMed]
- Wilmot A, Stavropoulos SW. Embolization of recurrent type 2 endoleak using the liquid embolic n-Butyl cyanoacrylate. Semin Intervent Radiol 2007;24:38-42. [Crossref] [PubMed]
- Giles KA, Fillinger MF, De Martino RR, et al. Results of transcaval embolization for sac expansion from endoleaks after endovascular aneurysm repair. J Vasc Surg 2015;61:1129-36. [Crossref] [PubMed]
- Böckler D, Holden A, Thompson M, et al. Multicenter Nellix EndoVascular Sealing system experience in aneurysm sealing. J Vasc Surg 2015;62:290-8. [Crossref] [PubMed]


