Paget-Schroetter syndrome: treatment of venous thrombosis and outcomes
Introduction
Thoracic outlet syndrome (TOS) describes compression of the structures of the thoracic outlet as they exit the thoracic cavity. Any of the neurovascular structures in this area can be affected, accounting for variations in its presentations: neurogenic (95%), venous (4%), and arterial (1%) (1-3). This review will focus on venous thoracic outlet syndrome (VTOS).
Epidemiology, pathophysiology, anatomy
There are three types of VTOS: intermittent/positional obstruction, secondary subclavian vein thrombosis, and primary “effort thrombosis”. Secondary thrombosis most commonly results from iatrogenic causes such as catheter or pacemaker insertion and is not due to compression (2). Paget-Schroetter syndrome (PSS), also called primary “effort thrombosis”, is associated with both compression and thrombosis of the subclavian vein.
PSS is a rare condition with a yearly incidence of 1–2 per 100,000 people and accounts for 1–4% of all venous thrombosis episodes (2). Young, otherwise healthy, males in the early 30s is the most commonly affected group. At particular risk are athletes, such as baseball players, swimmers, and weight lifters, or workers with repetitive overhead arm motion, such as mechanics or electricians (3-5). It is controversial whether coagulopathy increases the risk of VTOS, but likely thrombotic disorders play a role in idiopathic, unprovoked VTOS (6-9).
PSS is believed to be incited by repeated abnormal and strenuous interaction between the venous system and the structures within the thoracic outlet. The thoracic outlet is bordered by the clavicle superiorly, the first rib inferiorly, the costoclavicular ligament medially, and the anterior scalene muscle laterally. As the axillary vein passes over the first rib and under the clavicle, it becomes the subclavian vein. To reach the internal jugular vein, the subclavian vein passes through the thoracic outlet (10). Chronic compression of the vein between the angle of the clavicle and first rib with arm abduction leads to venous endothelial cell damage, inflammation, scaring, and potential thrombosis (11). Most patients with VTOS have a costoclavicular ligament which inserts more laterally, contributing to subclavian vein compression (12). Additionally, hypertrophy of muscles in the area, including the subclavius and anterior scalene muscles can also contribute to venous compression and injury (2,3).
Clinical presentation and diagnosis
Patients commonly present within 24 h of an inciting event, with history of excessive activity of the upper extremity and/or dehydration (4,13,14). The upper extremity and chest is painful, congested, and cyanotic appearing. Superficial veins can appear engorged and occasionally thrombosed veins can be palpated in the axilla (15). Pain involving the affected arm is characteristically sudden, involving the dominant extremity. Physical exam provocative tests have a high false positive rate and are more likely to detect neurogenic TOS rather than VTOS (16). PSS can also present with or be complicated by pulmonary embolism (PE). Studies report rates of PE and PSS ranging from 20–30% (17-19). Among upper extremity deep vein thromboses (DVT), PE occurs more often with secondary upper extremity DVT. When considering all DVT, PE is more associated with lower extremity DVT than upper extremity DVT (20,21). Although the risk of PE is smaller in PSS than other DVT states, it is important to be aware of the phenomenon.
Diagnosis is usually achieved with history and physical examination, and can be confirmed with imaging. Initial evaluation includes duplex ultrasonography revealing partial or complete thrombosis of the axillary and/or subclavian veins (Figure 1). Duplex imaging is the gold standard in diagnosis with reports of 80–100% sensitivity and specificity (22).
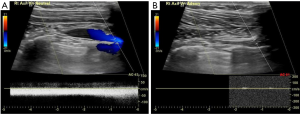
If duplex ultrasound is inconclusive, other imaging techniques may be used to confirm the diagnosis. Catheter-directed venography was historically the gold-standard for diagnostic imaging, but due to its invasive nature, higher costs, and accuracy of non-invasive ultrasound, it is now reserved for cases with high clinical suspicion and equivocal non-invasive images (2). Venography via computed tomographic (CT) or magnetic resonance (MR) can also be performed in the setting of atypical symptoms or equivocal ultrasound to interrogate the surrounding anatomy. However, both have their drawbacks. CT venography is complicated by risk of contrast and radiation exposure, while MR carries high costs and limited availability. When faced with an inconclusive non-invasive ultrasound and high clinical suspicion for upper extremity DVT, providers have the option of choosing among the above venography techniques. Alla et al. propose an algorithm to follow equivocal Doppler ultrasound with MR venogram if a high index of suspicion and no alternative explanation exists. A positive MR venogram is then followed by catheter-directed thrombolysis and early thoracic outlet decompression in patients with symptoms present for less than 2–6 weeks (8).
Plasma D-dimer levels may be elevated with upper extremity DVT, however specificity ranges from 14–60%. As PSS is a rare disorder, no guidelines exist on routine D-dimer testing. It may be useful as an adjunct, but is not recommended as a confirmatory test (23).
Patients complaining of neuropathic symptoms warrant nerve conduction studies, although the venous swelling can sometimes produce paresthesias unrelated to neurogenic TOS (16,24). Routine hypercoagulable workup for upper extremity thrombosis is not recommended. However, if a patient presents with an unexplained thrombosis and/or family history, hypercoagulable work-up should be performed (25,26). Hypercoagulable investigations include mutations in factor V Leiden and prothrombin G20210A, and deficiencies in antithrombin, protein C, and protein S. Lupus anticoagulant screening and anticardiolipin/anti-β2-glycoprotein antibodies may also help direct clinical management (25,27,28).
Cancer screening following unexplained upper extremity thromboses follows similar recommendations for those in the lower extremities. Routine screening is not recommended, however may be considered in unexplained thromboses (29). In these cases, a thorough history and physical examination, routine laboratory studies, abdominal ultrasound, chest X-ray as well as any other age or gender specific screenings should be performed (25).
Treatment
Treatment of PSS consists of relieving symptoms due to obstruction, preventing complications from DVT, and preventing recurrence. This can be achieved through anticoagulation, thrombolysis, and/or surgical decompression. Initiation of systemic anticoagulation immediately after diagnosis is the first step in treatment. Although not specific for PSS, the 2016 CHEST Guideline and Expert Panel Report on antithrombotic therapy for VTE disease recommends dabigatran, rivaroxaban, apixaban or edoxaban for patients with VTE and no cancer over vitamin K antagonists. Vitamin K antagonists are recommended over low-molecular-weight-heparin (30).
Anticoagulation alone for treatment of PSS is generally not recommended. A more aggressive approach involving thrombolysis and surgery is superior to anticoagulation alone in patient-reported outcomes, such as resolution of symptoms and return to work (31,32). If there are no contraindications, therapeutic anticoagulation for at least 5 days, followed by venography and catheter-directed thrombolysis is optimal if performed within 2 weeks after the onset of symptoms (Figure 2) (3,33). Early catheter-directed thrombolysis success rates are reported between 75–84% (34,35). Treatment of a clot older than 2 weeks is less successful as the thrombus is considered chronic and less susceptible to thrombolytic therapy (36,37). One study reported a 29% success rate of thrombolysis 2–12 weeks following onset of symptoms (33).
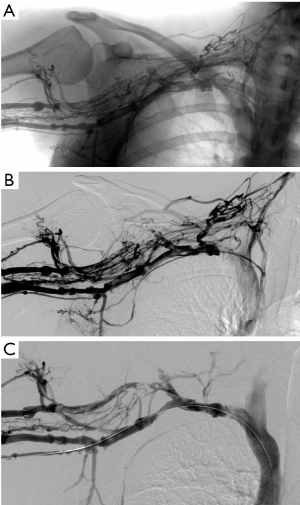
Despite initial relief of symptoms following anticoagulation and thrombolysis, re-thrombosis can occur in up to one-third of patients (38). Therefore, more definitive treatment with thoracic outlet decompression is recommended for patients who are good surgical candidates (39,40). In the absence of specific anatomic abnormalities, decompression with first rib resection is performed via a transaxillary, supraclavicular or infraclavicular approach. No clinical trials exist to support the superiority of one particular approach. If a cervical rib is present, it should also be removed. If indicated, partial resection of the subclavius and anterior scalene muscle helps to de-bulk the thoracic outlet and prevent recurrence of symptoms (39).
There is no consensus on duration of anticoagulation for PSS. The 2016 CHEST Guideline and Expert Panel Report on antithrombotic therapy for VTE disease recommends a 3-month course of therapy following any upper extremity DVT, regardless of thrombolytic interventions (30). Others suggest a more tailored approach using post-operative venography. If vein patency is demonstrated, no further intervention is required and anticoagulation can be stopped. However, if persistent stenosis or re-thrombosis is identified, anticoagulation is continued and repeat provocative duplex ultrasound examination is performed monthly for 6 months (3,41,42). Additional measures such as surgical thrombectomy, balloon venoplasty, and stenting have been used in the past for persistent stenosis or re-thrombosis, but have fallen out of favor secondary to poor success rates and high morbidity and are generally not recommended as initial treatment (12,43,44).
Outcomes
Following PSS treatment, significant morbidity can occur due to post-thrombotic syndrome (PTS). It is characterized by pain, heaviness and swelling of the affected extremity and can be a chronic, debilitating condition (21,45). PTS occurs in 7–46% of patients with upper extremity DVT and it is more common in primary rather than secondary upper extremity DVT (8,45). It is difficult to prevent all cases of PTS, but data has shown that early treatment correlates with improved patient-reported outcomes.
Success rates of 90–95% have been noted if PSS is diagnosed and intervened quickly with immediate decompression (2). Urschel et al. reported 95% of patients indicating “excellent/good” results with early thrombolysis and first rib resection, in regards to pain relief, employment and recreation (12). Other studies have reported similar findings, supporting the use of early surgical decompression (46,47). Patients with VTOS who undergo first rib resection and scalenectomy report improved quality of life, with near full recovery 1 year after surgery (39). Recurrence rates are low, with only 18% of VTOS postoperative patients requiring physical therapy to further reduce symptoms with no further operative interventions required (39). A recent meta-analysis on surgical outcomes of TOS demonstrated surgical intervention to be safe and beneficial with 90% of VTOS patients reporting “excellent/good” outcomes. However, heterogeneity among studies proved difficult to draw many further conclusions (48).
Conclusions
Rapid diagnosis and treatment for PSS is essential for good outcomes. Treatment involves immediate anticoagulation. Venography with catheter-directed thrombolysis can confirm the diagnosis and is potentially therapeutic. Surgical decompression should follow soon after, both for completion of treatment and prevention of recurrent thromboses. Managed in this fashion, the vast majority of patients with VTOS report beneficial outcomes with near full recovery at 1 year.
Acknowledgements
Funding: R Oklu gratefully acknowledges funding from the National Institutes of Health (EB021148, CA172738, EB024403, HL137193) and the Mayo Clinic.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Fugate MW, Rotellini-Coltvet L, Freischlag JA. Current management of thoracic outlet syndrome. Curr Treat Options Cardiovasc Med 2009;11:176-83. [Crossref] [PubMed]
- Illig KA, Doyle AJ. A comprehensive review of Paget-Schroetter syndrome. J Vasc Surg 2010;51:1538-47. [Crossref] [PubMed]
- Moore R, Wei Lum Y. Venous thoracic outlet syndrome. Vasc Med 2015;20:182-9. [Crossref] [PubMed]
- DeLisa LC, Hensley CP, Jackson S. Diagnosis of Paget-Schroetter Syndrome/Primary Effort Thrombosis in a Recreational Weight Lifter. Phys Ther 2017;97:13-9. [PubMed]
- Chandra V, Little C, Lee JT. Thoracic outlet syndrome in high-performance athletes. J Vasc Surg 2014;60:1012-7; discussion 1017-8. [Crossref] [PubMed]
- Cassada DC, Lipscomb AL, Stevens SL, et al. The importance of thrombophilia in the treatment of Paget-Schroetter syndrome. Ann Vasc Surg 2006;20:596-601. [Crossref] [PubMed]
- Martinelli I, Battaglioli T, Bucciarelli P, et al. Risk factors and recurrence rate of primary deep vein thrombosis of the upper extremities. Circulation 2004;110:566-70. [Crossref] [PubMed]
- Alla VM, Natarajan N, Kaushik M, et al. Paget-Schroetter Syndrome: Review of Pathogenesis and Treatment of Effort Thrombosis. West J Emerg Med 2010;11:358-62. [PubMed]
- Héron E, Lozinguez O, Alhenc-Gelas M, et al. Hypercoagulable states in primary upper-extremity deep vein thrombosis. Arch Intern Med 2000;160:382-6. [Crossref] [PubMed]
- Urschel HC Jr. Anatomy of the thoracic outlet. Thorac Surg Clin 2007;17:511-20. [Crossref] [PubMed]
- Aziz S, Straehley CJ, Whelan TJ Jr. Effort-related axillosubclavian vein thrombosis. A new theory of pathogenesis and a plea for direct surgical intervention. Am J Surg 1986;152:57-61. [Crossref] [PubMed]
- Urschel HC Jr, Patel AN. Surgery remains the most effective treatment for Paget-Schroetter syndrome: 50 years' experience. Ann Thorac Surg 2008;86:254-60; discussion 260. [Crossref] [PubMed]
- Klitfod L, Broholm R, Baekgaard N. Deep venous thrombosis of the upper extremity. A review. Int Angiol 2013;32:447-52. [PubMed]
- Farrar TA, Rankin G, Chatfield M. Venous thoracic outlet syndrome: approach to diagnosis and treatment with focus on affected athletes. Curr Sports Med Rep 2014;13:81-5. [Crossref] [PubMed]
- Mall NA, Van Thiel GS, Heard WM, et al. Paget-Schroetter Syndrome: A Review of Effort Thrombosis of the Upper Extremity From a Sports Medicine Perspective. Sports Health 2013;5:353-6. [Crossref] [PubMed]
- Hooper TL, Denton J, McGalliard MK, et al. Thoracic outlet syndrome: a controversial clinical condition. Part 1: anatomy, and clinical examination/diagnosis. J Man Manip Ther 2010;18:74-83. [Crossref] [PubMed]
- Bushnell BD, Anz AW, Dugger K, et al. Effort Thrombosis Presenting as Pulmonary Embolism in a Professional Baseball Pitcher. Sports Health 2009;1:493-9. [Crossref] [PubMed]
- Shimada T, Tounai T, Syoji T, et al. Acute Pulmonary Embolism due to Paget-Schroetter Syndrome. Intern Med 2015;54:1875-9. [Crossref] [PubMed]
- Kaczynski J, Sathiananthan J. Paget-Schroetter syndrome complicated by an incidental pulmonary embolism. BMJ Case Rep 2017;2017. pii: bcr-2017-219982.
- Joffe HV, Kucher N, Tapson VF, et al. Upper-extremity deep vein thrombosis: a prospective registry of 592 patients. Circulation 2004;110:1605-11. [Crossref] [PubMed]
- Mai C, Hunt D. Upper-extremity Deep Venous Thrombosis: A Review. Am J Med 2011;124:402-7. [Crossref] [PubMed]
- Chin EE, Zimmerman PT, Grant EG. Sonographic evaluation of upper extremity deep venous thrombosis. J Ultrasound Med 2005;24:829-38. [Crossref] [PubMed]
- Kraaijpoel N, van Es N, Porreca E, et al. The diagnostic management of upper extremity deep vein thrombosis: A review of the literature. Thromb Res 2017;156:54-9. [Crossref] [PubMed]
- Sanders RJ, Hammond SL, Rao NM. Diagnosis of thoracic outlet syndrome. J Vasc Surg 2007;46:601-4. [Crossref] [PubMed]
- Czihal M, Hoffmann U. Upper extremity deep venous thrombosis. Vasc Med 2011;16:191-202. [Crossref] [PubMed]
- Linnemann B, Meister F, Schwonberg J, et al. Hereditary and acquired thrombophilia in patients with upper extremity deep-vein thrombosis. Results from the MAISTHRO registry. Thromb Haemost 2008;100:440-6. [PubMed]
- Hoffmann U, Spannagl M. Deep venous thrombosis of the upper extremity: is thrombophilia a relevant clinical issue? Thromb Haemost 2008;100:369-70. [PubMed]
- Bernardi E, Pesavento R, Prandoni P. Upper extremity deep venous thrombosis. Semin Thromb Hemost 2006;32:729-36. [Crossref] [PubMed]
- Girolami A, Prandoni P, Zanon E, et al. Venous thromboses of upper limbs are more frequently associated with occult cancer as compared with those of lower limbs. Blood Coagul Fibrinolysis 1999;10:455-7. [Crossref] [PubMed]
- Kearon C, Akl EA, Ornelas J, et al. Antithrombotic Therapy for VTE Disease. Chest 2016;149:315-52. [Crossref] [PubMed]
- AbuRahma AF, Sadler D, Stuart P, et al. Conventional versus thrombolytic therapy in spontaneous (effort) axillary-subclavian vein thrombosis. Am J Surg 1991;161:459-65. [Crossref] [PubMed]
- Urschel HC Jr, Razzuk MA. Paget-Schroetter syndrome: what is the best management? Ann Thorac Surg 2000;69:1663-8; discussion 1668-9.
- Molina JE, Hunter DW, Dietz CA. Paget-Schroetter syndrome treated with thrombolytics and immediate surgery. J Vasc Surg 2007;45:328-34. [Crossref] [PubMed]
- Doyle A, Wolford HY, Davies MG, et al. Management of effort thrombosis of the subclavian vein: today's treatment. Ann Vasc Surg 2007;21:723-9. [Crossref] [PubMed]
- Taylor JM, Telford RJ, Kinsella DC, et al. Long-term clinical and functional outcome following treatment for Paget-Schroetter syndrome. Br J Surg 2013;100:1459-64. [Crossref] [PubMed]
- Oklu R, Wicky S. Catheter-directed thrombolysis of deep venous thrombosis. Semin Thromb Hemost 2013;39:446-51. [Crossref] [PubMed]
- Wicky S, Pinto EG, Oklu R. Catheter-directed thrombolysis of arterial thrombosis. Semin Thromb Hemost 2013;39:441-5. [Crossref] [PubMed]
- Kunkel JM, Machleder HI. Spontaneous subclavain vein thrombosis: a successful combined approach of local thrombolytic therapy followed by first rib resection. Surgery 1989;106:114. [PubMed]
- Chang DC, Rotellini-Coltvet LA, Mukherjee D, et al. Surgical intervention for thoracic outlet syndrome improves patient's quality of life. J Vasc Surg 2009;49:630-5; discussion 635-7. [Crossref] [PubMed]
- Caparrelli DJ, Freischlag J. A unified approach to axillosubclavian venous thrombosis in a single hospital admission. Semin Vasc Surg 2005;18:153-7. [Crossref] [PubMed]
- Freischlag J, Orion K. Understanding Thoracic Outlet Syndrome. Scientifica 2014;2014:248163. [PubMed]
- Bamford RF, Holt PJ, Hinchliffe RJ, et al. Modernizing the treatment of venous thoracic outlet syndrome. Vascular 2012;20:138-44. [Crossref] [PubMed]
- Urschel HC Jr, Patel AN. Paget-Schroetter syndrome therapy: failure of intravenous stents. Ann Thorac Surg 2003;75:1693-6; discussion 1696.
- Lee JT, Karwowski JK, Harris EJ, et al. Long-term thrombotic recurrence after nonoperative management of Paget-Schroetter syndrome. J Vasc Surg 2006;43:1236-43. [Crossref] [PubMed]
- Elman EE, Kahn SR. The post-thrombotic syndrome after upper extremity deep venous thrombosis in adults: a systematic review. Thromb Res 2006;117:609-14. [Crossref] [PubMed]
- Lee MC, Grassi CJ, Belkin M, et al. Early operative intervention after thrombolytic therapy for primary subclavian vein thrombosis: an effective treatment approach. J Vasc Surg 1998;27:1101-7; discussion 1107-8. [Crossref] [PubMed]
- Azakie A, McElhinney DB, Thompson RW, et al. Surgical management of subclavian-vein effort thrombosis as a result of thoracic outlet compression. J Vasc Surg 1998;28:777-86. [Crossref] [PubMed]
- Peek J, Vos CG, Ünlü Ç, et al. Outcome of Surgical Treatment for Thoracic Outlet Syndrome: Systematic Review and Meta-Analysis. Ann Vasc Surg 2017;40:303-26. [Crossref] [PubMed]


