Evolving clinical applications of contrast-enhanced ultrasound (CEUS) in the abdominal aorta
Introduction
Aortic diseases account for a significant part of urgent and emergent abdominal pathology. Ultrasound (US) plays a key role in the initial workup and follow-up of a wide range of vascular and specifically aortic abnormalities including dissection, aneurysm, vasculitis and post-endovascular treatment surveillance. B-mode technique but especially flow visualization techniques like color-, power- or non-Doppler techniques and Doppler spectral analysis are the cornerstone of vascular ultrasonographic evaluation. As a result, US has been traditionally used as a first-line imaging modality for prompt evaluation of aortic conditions thanks to its inherent advantages such as its cost-effectiveness, safety profile, repeatability and availability in different settings including at the bedside and as point-of-care US in the emergency department. However, US has its inherent limitations, including operator-dependency, limited field-of view and shadowing from overlying gas-containing bowel loops hindering evaluation of the aorta. When it comes to flow visualization with US, it is not free of limitations either. Low sensitivity to slow flow, blooming artifact and the deep location of aorta are factors limiting the modality’s effectiveness (1,2). But it needs to be emphasized that US with Doppler or Duplex is the only clinically available modality which enables blood flow visualization in real-time. The introduction of multi-detector technology in computed tomography angiography (CTA) and the magnetic resonance angiography (MRA) are valuable modalities for assessment of aortic diseases
The introduction of US contrast agents has augmented US applications in a wide spectrum of organs and clinical conditions, with society guidelines and recommendations being published. The liver has been the primary organ investigated with this modality, although non-hepatic applications are increasingly investigated and recent advances include renal, testicular, lymph nodes, thyroid, prostate and other extra-hepatic applications (1-4). Focal liver lesion characterization with contrast-enhanced ultrasound (CEUS) is already widely performed, showing high concordance rates with other cross-sectional imaging modalities (5). Moreover, detection of liver metastases from colorectal cancer is greatly enhanced by the intravenous administration of ultrasonographic contrast agents, increasing the modality’s accuracy (6). Although CEUS is mainly performed in adult patients, it has also been evaluated in the paediatric population with official recommendations being published (7). Regarding the evaluation of vascular systems including the aorta, US contrast agents enabled physicians to acquire virtually angiographic images. This technique is characterized by increased sensitivity to flow visualization, even in the case of slowly-flowing blood and superior accuracy for lumen delineation. This enhanced accuracy for flow visualization stems from the inherent property of US contrast agents to strictly remain within the vascular lumen (1,2,8,9). CEUS can thus overcome some of the conventional US technique’s limitations and can be performed as a complementary ultrasonographic technique in order to enhance the role of US for the evaluation of vascular pathology. CEUS has been tested in many vascular systems, either arterial or venous, although with varying success. So far, recent research has shown promising results for the use of CEUS in the carotid arteries, where the use of microbubbles increases the accuracy of grading of stenosis and detection of superficial plaque abnormalities like ulcerations. Moreover, CEUS has been found to be an excellent modality for the evaluation of intraplaque neovascularization; a major factor of carotid plaque vulnerability (9-12). CEUS has also been found valuable for the evaluation of superficial arteries like the femoral (2) but the added value compared to the unenhanced technique was rather limited in deep vascular structures like the mesenteric arteries or the renal arteries (13,14). When it comes to the evaluation of the aorta, CEUS has shown promising results, having become already well-established in indications like the detection of endoleaks (Figure 1). US contrast agents consist of microbubbles and have been used in liver, cardiac, breast and vascular applications. In the United States though, the Food and Drug Administration has recently licensed an ultrasonographic contrast agent for use in characterization of focal liver lesions (1,8). This is expected to widen the availability and use of CEUS.
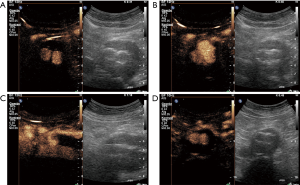
The purpose of this paper is to introduce and discuss aortic applications of CEUS. These applications will be illustrated with representative cases, demonstrating the added value of US contrast-agents, compared to the conventional unenhanced US technique. All patients gave informed consent for the CEUS imaging study.
Technical and safety aspects of CEUS
Once a conventional US examination is complete, having incorporated B-mode, color-Doppler images and spectral analysis, the region of interest is identified, in order to be carefully observed after the administration of US contrast agents. US contrast agents consist of microbubbles made of a phospholipid shell containing a gas. Various contrast agents are currently on the market. SonoVue® (Bracco Spa, Milan, Italy) is the most recently manufactured and widely used contrast agent, approved for cardiac, macrovascular, liver and breast applications in Europe (1,3). In the United States, the counterpart to this agent is Lumason® (Bracco Spa). The mean diameter of contrast US microbubbles is about 2.5 µm while more than 90% of the microbubbles measure less than 8 µm in diameter (8,15). As a result, these microbubbles are smaller than red blood cells in diameter but are large enough so that they cannot pass through the capillary endothelium (8,16). Consequently, and unlike CT and MRI contrast agents, microbubbles remain within the vascular lumen, representing strict intravascular tracers; an inherent property particularly valuable for vascular applications (8,16). The only case in which microbubbles are visualized outside the vascular lumen is in active extravasation or hemorrhage. The dose of microbubbles depends on application and available equipment. In general, 2.4 mL of SonoVue® US contrast agent is typically administered for liver exam indications but vascular CEUS examinations can be successfully performed with 1.0 to 2.4 mL of the microbubbles (1,10,17). Microbubbles are administered as a bolus followed by saline, although continuous injection with a slow rate using a perfusor device has been described as well. If administered in bolus, microbubbles arrive in arteries approximately after 10 to 30 s. Lumen enhancement gradually increases and lasts for up to approximately 2 to 5 min, depending on the mechanical index (MI) used and the duration of scanning. When the enhancement of vascular lumen vanishes, a second dose of microbubbles can be administered if clinically deemed necessary (1,10,17,18).
After the unenhanced US examination, for the CEUS portion a peripheral intravenous line needs to be established. Based on the contrast agent characteristics, several types of catheters or needles can be used, ranging in size from 18 to 21 gauge, resulting in no significant difference in the quality and duration of enhancement (19). After the intravenous access has been established, the ultrasonographic device needs to be set into its contrast-specific mode. In the early stages of CEUS, microbubbles have been used as a means of increasing blood flow signals while using the conventional color or power Doppler techniques; a mode termed “Doppler rescue” (20,21). While this technique did increase US sensitivity to blood flow, it is significantly inferior to modern technologies using harmonic frequencies. It is known that when the US beam hits the microbubbles, the latter start to oscillate in a non-linear patter, meaning that they expand more than they contract. This oscillation results in the reflection of not only the fundamental frequency of emitted US waves but also higher or lower subharmonic frequencies. Contrary to what happens with microbubbles, static tissue mainly shows a linear response to the US beam, reflecting the same frequency. It thus becomes obvious that harmonic ultrasonographic imaging offers improved visualization of flowing microbubbles. Pulse-inversion harmonic imaging is one of the latest advances in CEUS technology, offering the best contrast between microbubbles and static tissues. This is achieved with the simultaneous use of two US pulses being 180° out of phase. The waves reflected linearly by static tissues tend to cancel one another while the harmonic waves produced by the oscillating microbubbles are visualized by the ultrasonographic device. In simple other words, pulse-inversion harmonic imaging achieves visualization of microbubbles with excellent contrast, spatial and temporal resolution while at the same time suppressing static tissues. If combined with a low-MI grey-scale image in a dual-display mode, the physician can simultaneously appreciate the examined area structure and vascularization. An exception to the rule of linear response of static tissues lies in their non-linear response when exposed to high MI US waves. When static tissues produce harmonic frequencies, these are less successfully suppressed and the image quality can be degraded. This combined with the fact that microbubbles rupture quickly when exposed to high-MI explains why it is crucial to use low-MI US pulses when performing CEUS examinations. In general, a MI is considered low when it is less than 0.3, although most US devices achieve values lower than 0.1, providing excellent image quality (1,8,22,23).
As with every contrast agent the safety profile is of utmost importance. It has been concluded that microbubbles are characterized by an excellent safety profile based on the reported incidence of life-threatening allergic reactions occurring in less than 0.002% of cases. The frequency of allergic reactions caused by US contrast agents is lower than that of CT contrast agents and comparable to that reported for MRI agents (1,24,25). The administration of microbubbles needs no prior laboratory testing and has limited contraindications including history of allergic reaction to the active substance or any of its excipients, known right-to-left shunt, severe pulmonary hypertension, uncontrolled systemic hypertension and unclear pregnancy status. However, the lack of substantial evidence has led some to debate to the contraindication of known right-to-left shunt. Microbubbles are not excreted via the urinary tract but are metabolized in the liver and the gas is exhaled. Therefore, these can be safely administered in patients with impaired renal function, contrary to CT and MRI contrast agents (1,8,26).
Aortic aneurysm and post treatment surveillance
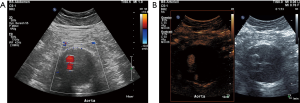
An aneurysm of the abdominal aorta (AAA) can be diagnosed if a segmental, full-thickness enlargement of more than 3 cm in vessel diameter is identified. Alternatively, the abdominal aorta can be characterized as aneurysmal if its diameter is 50% (or 2 standard deviations) greater than its normal diameter (2,27,28). Aortic aneurysmal disease’s natural history is gradual expansion of the aneurysm sac, with a rate varying from 0.1 to 0.3 cm per year and up to a point that the aneurysm may rupture which is life threatening. It is well-established that the aneurysm’s diameter and annual expansion rate of maximum transverse diameter are the best predictors of aortic aneurysm rupture, with the consecutive risk increasing with increasing sac size (27-29). AAA rupture represents a lethal surgical emergency characterized by an overall mortality of up to 90% (2,30-32). Based on this high mortality rate, increased caution is needed for the diagnosis of a ruptured AAA not to be missed. The diagnosis of AAA complications including rupture in patients with acute abdominal pain is primarily performed using cross-sectional imaging techniques like CTA. US represents the first line modality for screening and follow-up of unruptured AAA, showing excellent sensitivity and specificity and strong intra- and inter-observer agreement. US can accurately measure an aneurysm’s diameter, provided that the probe is properly angled so that the measurements are made on a level perpendicular to the longitudinal axis of the aorta. US can be used as a screening tool for men with a smoking history aged 65 to 74 years in order to exclude the presence of an aneurysm and thus reduce aneurysm-related mortality, even in a community setting (28,33-35). US is limited for the evaluation of supra- and infra-renal borders of an AAA and the detection of additional aneurysms affecting the iliac arteries (28). As far as the AAA rupture is concerned, the diagnostic accuracy of US is rather poor with only limited findings being described (36). While the use of microbubbles adds little to the investigation of an unruptured AAA and is not recommended, it was found to significantly increase the modality’s diagnostic accuracy post rupture and/or leaking. CEUS findings of AAA rupture include active extravasation and depending pooling of microbubbles within the abdominal cavity; all findings correlating with CTA. Other CEUS findings of AAA rupture include a focal area of the aneurysmal wall showing no enhancement due to necrosis (28,37,38). Decreasing of the enhancement within the aneurysmal wall may be a finding of impending rupture justifying the use of CEUS when performing surveillance of larger aneurysms. At this point, it should be highlighted that CTA remains the imaging modality of choice for the diagnosis of a ruptured AAA (28,39,40). However, point of care US with CEUS could be considered as a modality in the emergency room for unstable patients who cannot be transported safely to the CT scanner.
The extravasated blood from a ruptured AAA can leak into the lumen of the inferior vena cava, leading to the formation of an aortocaval fistula; a condition requiring specific treatment. This type of vascular communication is best investigated and visualized with CTA. Nevertheless, the use of microbubbles increases the ultrasonographic technique’s accuracy for the diagnosis of this entity. It was reported that CEUS accurately delineates aortocaval communications with high spatial and temporal resolution in a real-time dynamic investigation (40,41).
Based on its unacceptable mortality rates in case of rupture, an AAA with a diameter of more than 55 mm, an annual aneurysm growth rate of >10 mm or a symptomatic AAA should be appropriately treated either with an endovascular repair [endovascular aneurysm repair (EVAR)] or with an open repair (28). EVAR treatment has certain advantages over open repair but lifelong surveillance is needed to assess for endoleaks. Other post EVAR complications include graft migration and fractures. It is important that these complications are early recognized and subsequently managed. Long-term imaging follow-up of post-EVAR aneurysms is routinely performed with CTA and CEUS has been used increasingly for this application. Currently lifelong surveillance is recommended although the risk for rupture has been found most significant within the first two to three years post intervention (28,42-46). When selecting the post EVAR surveillance modality, several factors should be considered. US can be readily performed and is widely available but is less sensitive than CTA for detecting and visualizing flow outside the stent. On the other hand, CTA shows excellent accuracy but has limitations including use of ionizing radiation, costs and iodinated contrast agent; a potential contraindication for patients with chronic renal failure. CTA can acquire a maximum of three phases acting as “snapshots” of a dynamic phenomenon like endoleaks. This static acquisition of images in CTA though may prevent detection of slow-flowing endoleak or its accurate characterization and classification. CEUS could be potentially introduced to the workup algorithms with a purpose to increase US accuracy for detection and characterization of endoleaks (Figures 2 and 3). Potentially CEUS may replace certain CTA exams during the post EVAR surveillance protocol. CEUS advantages for the detection of endoleaks include increased sensitivity for slow flow, superior spatial and temporal resolution, potential of prolonged and continuous scanning and a dynamic and real-time pattern of flow visualization. Additionally, replenishment techniques previously described for CEUS offers the possibility to re-observe the flow characteristics of microbubbles within the aneurysm sac. As a result, both fast- and slow-flowing endoleaks can be readily detected and accurately classified (2,47). Accurate classification of endoleaks is essential as different types require different management; with types 1 and 3 needing intervention while in patients with type 2 endoleak, occlusion is only needed if the aneurysm’s diameter increases, justifying the need for serial follow-up aneurysm diameter measurements.
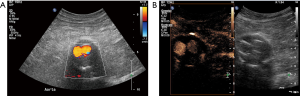
The concept of endoleaks was introduced in 1996 by White et al. in order to describe the presence of persistent blood flow inside the aneurysm sac but outside the stent wall. Five types of endoleaks have been described based on the blood flow’s origin and direction and are presented in Table 1 (28,42,45,46,48,49). Endoleaks can be adequately visualized with US with limitations appreciated due to patient’s body habitus and overlying bowel gas. A wide variety of diagnostic accuracy have been reported with a sensitivity ranging from 33% to 90% and a specificity ranging from 63% to 81% (50-53). The role of CEUS in endoleaks detection has been extensively investigated, showing promising results. In an early study using an older contrast-agent, it was concluded that CEUS can accurately classify type 1 and 2 endoleaks while identifying more cases compared to delayed phase CTA (54). The same type of contrast agent was found to be almost 100% sensitive and 65% specific for diagnosing endoleaks (55). The use of newer contrast agents has shown excellent results with 80% up to 100% sensitivity and 82% to 100% specificity for diagnosing endoleaks. A recent study comparing CEUS and CTA has concluded that CEUS is 97.6% sensitive and 100% specific while CTA is 90.5% sensitive and 100% specific for diagnosing an endoleak. Moreover, CEUS outperformed CTA in terms of endoleak classification (56). Consequently, CEUS is regarded superior to color Doppler technique and equivalent to the gold standard of CTA for endoleak assessment. It is advocated by some that CEUS may even better characterize endoleaks compared to CTA due to its dynamic and real-time pattern of scanning (52,53,57-59). A recently published meta-analysis has concluded that CEUS is characterized by a pooled sensitivity and specificity of 91.4% and 78.2% respectively. Specificity estimation though is considered limited by significant heterogeneity among included studies (60). In keeping with these results, a different systematic review concluded that CEUS and MRA are better than CTA in the detection of endoleaks but equivalent to CTA for classification of endoleaks type 1 and 3 (61). The use of time-intensity curves has demonstrated a 99% sensitivity and 93% specificity for the detection of endoleaks, based on comparison with CTA and therefore these time-intensity may be of particular value when assessing patients for endoleaks. It was also concluded that the enhancement of an aneurysmal sac containing an endoleak is significantly higher than that of a sac without endoleak (62). Patients with fenestrated endografts have been recently included in a study evaluating four-dimensional CEUS for the detection of endoleaks. This study showed that four-dimensional CEUS may be equivalent to CTA in terms of accuracy for the evaluation of aneurysm diameter, volume and endoleak detection (63). In the light of these promising results, CEUS could be incorporated in the diagnostic algorithm post EVAR surveillance for endoleak detection as with the purpose to increase the diagnostic accuracy of US. Patients with negative results could be safely discharged until the next follow-up date while further investigation with CTA can be performed in patients with clearly positive or suspicious results on CEUS (44,47,52,64). Furthermore, CEUS can be used as control imaging modality to document successful treatment after interventional embolization of an endoleak (Figures 3–5).

Full table
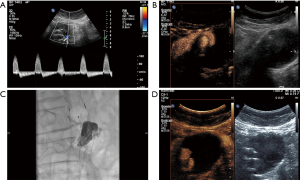
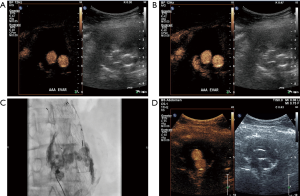
Aortic dissection
Aortic dissections account for the majority of aortic emergencies. An incidence of 2,000 new cases annually in the United States has been reported. The prognosis of this condition is based on prompt treatment after early diagnosis which largely relies on imaging (65). Clinical presentation of aortic dissection includes asymmetry in the blood pressure in the upper extremities, acute chest pain or signs of organ dysfunction caused by ischemia (66,67). The separation of the aortic wall layers results in the formation of an intimal flap dividing the aortic lumen into two compartments: the true lumen referring to the lumen containing circulating blood and the false lumen representing a blood-containing compartment situated within the aortic wall (65,68). CTA is the imaging modality of choice for the evaluation of aortic dissection, especially with the introduction of multi-detector technology allowing for faster image acquisition (65). US however, being the first-line imaging modality available in the emergency department still may hold a place in the initial investigation of suspected aortic pathology. In certain circumstances US may be able to pick up an aortic dissection and trigger a CTA exam. The addition of contrast may increase these incidentally detected aortic dissections thereby enabling early diagnosis and immediate treatment. There are some ultrasonographic findings which should point towards the diagnosis of dissection. Such findings include an intraluminal echogenic line on B-mode technique, corresponding to the intimal flap and bi-directional color flow signals on color-Doppler technique. In some cases though, these findings may be subtle, if for example the intimal flap is low-reflective and thus poorly visualized. In these cases, CEUS represents a valuable complementary technique with superior sensitivity for the diagnosis of aortic dissection. Intravenously administered microbubbles completely opacify both the true and the false lumen and readily visualize suspected intimal flaps, establishing the diagnosis of dissection. In addition to the visualization of intimal flaps, CEUS may demonstrate the presence of entry-or re-entry points of the false lumen, while it can also differentiate the true from the false lumen on the grounds that the enhancement of the false lumen is supposed to be later than the enhancement of the true lumen (18,41,69-71). Once a dissection is suspected based on CEUS, this should be followed by a CTA for further characterization of the dissection including Stanford classification.
Other applications/aortitis
The term aortitis is used to describe the inflammatory condition of either infectious or noninfectious origin affecting the aortic wall, similarly to any other type of vasculitis. The clinical presentation and laboratory testing findings of patients with aortitis are rather unspecific, leaving a significant role to imaging for the accurate diagnosis of this entity. The primary imaging modalities currently used for initial evaluation and follow-up of aortitis include CTA, MRA and nuclear medicine techniques, particularly 18F-fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT). Imaging in aortitis is not only valuable for initial investigation but also for monitoring the inflammation’s response to treatment (72,73). A series of rheumatoid diseases can be complicated by aortitis, including Takayasu arteritis, giant cell arteritis, rheumatoid arthritis, systematic lupus erythematosus (72).
Imaging findings of acute aortitis in CTA include circumferential aortic wall thickening, thrombosis, occlusion or lumen irregularities such as stenosis, pseudo-aneurysms or vascular ectasia and ulcerations (72). The “double ring” appearance refers to a thickened aortic wall showing poor enhancement in the internal part of the wall due to intimal edema but higher enhancement in the outer part of the vessel wall, namely the media and adventitia (72-76). A thickened hypoechoic circumferential vascular wall thickening has been described with color Doppler technique in inflamed temporal arteries with giant cell arteritis. Conventional US may also visualize the lumen irregularities including pseudo-aneurysm and stenosis (73,77).
Based on the inflammatory nature of aortitis, it is expected that molecular imaging techniques targeting inflammatory parameters like activated macrophages, neovascularization and increased metabolic activity of the inflamed vascular wall will be valuable means of imaging and grading disease activity. CEUS is a well-established modality for the evaluation of intraplaque neovascularization in carotid atherosclerotic disease (9,10). Similar to carotid plaques, CEUS can identify neovascularization within a thickened aortic wall, affected by any form of inflammatory process (73). CEUS visualizes luminal irregularities including stenotic segments of the vessel and ulcerations with increased sensitivity compared to the conventional technique. More importantly, CEUS provides an insight into the inflamed vascular wall, visualizing neovascularization in the form of moving microbubbles within the wall (1,9,12,78). CEUS has been used for evaluation of disease activity in large vessel vasculitis (Figure 6). For instance, carotid CEUS has been reported as a method of both diagnosis and monitoring of disease response to treatment for patients with Takayasu arteritis. Initial investigation with CEUS showed circumferential vascular wall thickening affecting the common carotid artery containing multiple enhancing vasa vasorum. Follow-up examination with CEUS after successful treatment demonstrated progressive decrease in arterial wall enhancement with decreased amount of vasa vasorum being opacified; findings suggestive of decreased inflammatory activity (79,80). Schinkel et al. have recently reproduced these findings, showing that CEUS is superior to color Doppler in terms of image quality and detection of vascularization in the arterial wall, when performed in patients with Takayasu arteritis or giant cell arteritis (81). CEUS has also been found useful in the evaluation of chronic peri-aortitis. In this condition, CEUS visualized enhancement of the circumferentially thickened aortic wall while the level of enhancement was shown to be lower post treatment (82).
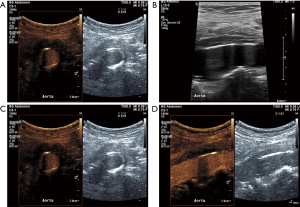
So far, these results have been reported using a common ultrasonographic agent, available for mere luminal opacification and visualization of the microcirculation. An interesting new field of active research in CEUS is that of targeted microbubbles. The latter are specifically designed so that they are binding to specific molecular targets on the surface of endothelium. Based on this affinity to certain endothelial molecules, once the luminal microbubbles are washed-out, enhancement can be still visualized in areas of the vascular wall where the molecular targets are identified. Examples of molecular targets visualized with targeted CEUS include leukocyte adhesion molecules such as ICAM-1, VCAM-1 and P-selectin. It is expected that in the future, CEUS using this technology will provide us with insights of the pathophysiologic events taking place within an inflamed arterial wall in a real-time approach (73).
Limitations of CEUS related to aortic imaging
US including CEUS has inherent well-known limitations which need to be considered when selecting the imaging modality to evaluate aortic diseases. Thorough evaluation of the aortic pathology can be hindered due to body habitus, the presence of overlying gas-containing bowel loops hiding the aorta and the fact that US in general and particular CEUS as a new technique are operator dependent. Moreover, the evaluation of luminal abnormalities can be limited in case of calcified atherosclerotic changes with acoustic shadowing. It should also be kept in mind that US as well as CEUS are primarily a two-dimensional technique evaluating three-dimensional structures like the carotid system or the aorta. As a consequence, abnormalities like superficial ulcerations may elude diagnosis if situated outside the imaging plane and important parameters like an aneurysm’s diameter may be falsely estimated due to poor imaging plane angulation in a conventional unenhanced US examination. Administration of US contrast agents and three-dimensional US can be used to address this issue (1,9,83).
Conclusions
The introduction of microbubbles as ultrasonographic contrast agents has augmented CEUS applications in aortic disease, offering benefits over conventional US results. CEUS revealed promising initial results in new applications like aortitis. Although superior to US, CEUS is by no means supposed to completely replace CTA, which is still regarded as the gold standard for evaluation of the aorta. However, it is a valuable complementary ultrasonographic technique which can be incorporated in workup algorithms in an effort to reduce unnecessary scanning with CTA. This is particularly essential for pediatric patients, patients with renal failure and patients in need of lifelong imaging surveillance.
Acknowledgements
Funding: D Staub received funding from the Swiss National Science Foundation (PZ00P3_142419) and unrestricted research grant from Bracco SA.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Piscaglia F, Nolsoe C, Dietrich CF, et al. The EFSUMB Guidelines and Recommendations on the Clinical Practice of Contrast Enhanced Ultrasound (CEUS): update 2011 on non-hepatic applications. Ultraschall Med 2012;33:33-59. [Crossref] [PubMed]
- Rübenthaler J, Reiser M, Clevert DA. Diagnostic vascular ultrasonography with the help of color Doppler and contrast-enhanced ultrasonography. Ultrasonography 2016;35:289-301. [Crossref] [PubMed]
- Claudon M, Dietrich CF, Choi BI, et al. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) in the liver--update 2012: a WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultraschall Med 2013;34:11-29. [PubMed]
- Cantisani V, Bertolotto M, Weskott HP, et al. Growing indications for CEUS: The kidney, testis, lymph nodes, thyroid, prostate, and small bowel. Eur J Radiol 2015;84:1675-84. [Crossref] [PubMed]
- D'Onofrio M, Romanini L, Serra C, et al. Contrast enhancement ultrasound application in focal liver lesions characterization: a retrospective study about guidelines application (SOCEUS-CEUS survey). J Ultrasound 2015;19:99-106. [Crossref] [PubMed]
- Cantisani V, Ricci P, Erturk M, et al. Detection of hepatic metastases from colorectal cancer: prospective evaluation of gray scale US versus SonoVue(R) low mechanical index real time-enhanced US as compared with multidetector-CT or Gd-BOPTA-MRI. Ultraschall Med 2010;31:500-5. [Crossref] [PubMed]
- Sidhu PS, Cantisani V, Deganello A, et al. Role of Contrast-Enhanced Ultrasound (CEUS) in Paediatric Practice: An EFSUMB Position Statement. Ultraschall Med 2017;38:33-43. [PubMed]
- Barr RG. How to Develop a Contrast-Enhanced Ultrasound Program. J Ultrasound Med 2017;36:1225-40. [Crossref] [PubMed]
- Rafailidis V, Charitanti A, Tegos T, et al. Contrast-enhanced ultrasound of the carotid system: a review of the current literature. J Ultrasound 2017;20:97-109. [Crossref] [PubMed]
- Clevert DA, Paprottka P, Sommer WH, et al. The role of contrast-enhanced ultrasound in imaging carotid arterial diseases. Semin Ultrasound CT MR 2013;34:204-12. [Crossref] [PubMed]
- Clevert DA, Sommer WH, Zengel P, et al. Imaging of carotid arterial diseases with contrast-enhanced ultrasound (CEUS). Eur J Radiol 2011;80:68-76. [Crossref] [PubMed]
- Rafailidis V, Chryssogonidis I, Tegos T, et al. Imaging of the ulcerated carotid atherosclerotic plaque: a review of the literature. Insights Imaging 2017;8:213-25. [Crossref] [PubMed]
- Blebea J, Zickler R, Volteas N, et al. Duplex imaging of the renal arteries with contrast enhancement. Vasc Endovascular Surg 2003;37:429-36. [Crossref] [PubMed]
- Blebea J, Volteas N, Neumyer M, et al. Contrast enhanced duplex ultrasound imaging of the mesenteric arteries. Ann Vasc Surg 2002;16:77-83. [Crossref] [PubMed]
- Schneider M. Characteristics of SonoVuetrade mark. Echocardiography 1999;16:743-6. [Crossref] [PubMed]
- Shalhoub J, Owen DR, Gauthier T, et al. The use of contrast enhanced ultrasound in carotid arterial disease. Eur J Vasc Endovasc Surg 2010;39:381-7. [Crossref] [PubMed]
- Staub D, Partovi S, Imfeld S, et al. Novel applications of contrast-enhanced ultrasound imaging in vascular medicine. Vasa 2013;42:17-31. [Crossref] [PubMed]
- Schinkel AF, Kaspar M, Staub D. Contrast-enhanced ultrasound: clinical applications in patients with atherosclerosis. Int J Cardiovasc Imaging 2016;32:35-48. [Crossref] [PubMed]
- Eisenbrey JR, Daecher A, Kramer MR, et al. Effects of Needle and Catheter Size on Commercially Available Ultrasound Contrast Agents. J Ultrasound Med 2015;34:1961-8. [Crossref] [PubMed]
- Forsberg F, Liu JB, Burns PN, et al. Artifacts in ultrasonic contrast agent studies. J Ultrasound Med 1994;13:357-65. [Crossref] [PubMed]
- Dietrich CF, Ignee A, Hocke M, et al. Pitfalls and artefacts using contrast enhanced ultrasound. Z Gastroenterol 2011;49:350-6. [Crossref] [PubMed]
- Forsberg F, Piccoli CW, Merton DA, et al. Breast lesions: imaging with contrast-enhanced subharmonic US--initial experience. Radiology 2007;244:718-26. [Crossref] [PubMed]
- Forsberg F, Goldberg BB, Liu JB, et al. On the feasibility of real-time, in vivo harmonic imaging with proteinaceous microspheres. J Ultrasound Med 1996;15:853-60. [Crossref] [PubMed]
- Piscaglia F, Bolondi L. Italian Society for Ultrasound in Medicine and Biology (SIUMB) Study Group on Ultrasound Contrast Agents. The safety of Sonovue in abdominal applications: retrospective analysis of 23188 investigations. Ultrasound Med Biol 2006;32:1369-75. [Crossref] [PubMed]
- ter Haar G. Safety and bio-effects of ultrasound contrast agents. Med Biol Eng Comput 2009;47:893-900. [Crossref] [PubMed]
- Parker JM, Weller MW, Feinstein LM, et al. Safety of ultrasound contrast agents in patients with known or suspected cardiac shunts. Am J Cardiol 2013;112:1039-45. [Crossref] [PubMed]
- Kent KC. Clinical practice. Abdominal aortic aneurysms. N Engl J Med 2014;371:2101-8. [Crossref] [PubMed]
- Moll FL, Powell JT, Fraedrich G, et al. Management of Abdominal Aortic Aneurysms Clinical Practice Guidelines of the European Society for Vascular Surgery. Eur J Vasc Endovasc Surg 2011;41 Suppl 1:S1-58. [Crossref] [PubMed]
- Brown LC, Powell JT. Risk Factors for Aneurysm Rupture in Patients Kept Under Ultrasound Surveillance. UK Small Aneurysm Trial Participants. Ann Surg 1999;230:289-96; discussion 296-7. [Crossref] [PubMed]
- Assar AN, Zarins CK. Ruptured abdominal aortic aneurysm: a surgical emergency with many clinical presentations. Postgrad Med J 2009;85:268-73. [Crossref] [PubMed]
- Clevert DA, Horng A, Reiser MF. Ultrasound imaging of the abdominal aorta. Radiologe 2009;49:1024-32. [Crossref] [PubMed]
- Hatakeyama T, Shigematsu H, Muto T. Risk factors for rupture of abdominal aortic aneurysm based on three-dimensional study. J Vasc Surg 2001;33:453-61. [Crossref] [PubMed]
- Singh K, Bonaa KH, Solberg S, et al. Intra- and interobserver variability in ultrasound measurements of abdominal aortic diameter. The Tromso Study. Eur J Vasc Endovasc Surg 1998;15:497-504. [Crossref] [PubMed]
- Health Quality Ontario. Ultrasound screening for abdominal aortic aneurysm: an evidence-based analysis. Ont Health Technol Assess Ser 2006;6:1-67. [PubMed]
- Rajiah P, Reiber JH, Partovi S. Population based ultrasonographic screening of abdominal aortic aneurysms. Int J Cardiovasc Imaging 2016;32:1605-7. [Crossref] [PubMed]
- Rafailidis V, Godosis D, Kouskouras K, et al. Man With Abdominal Pain. Ann Emerg Med 2016;68:e1-2. [Crossref] [PubMed]
- Catalano O, Lobianco R, Cusati B, et al. Contrast-enhanced sonography for diagnosis of ruptured abdominal aortic aneurysm. AJR Am J Roentgenol 2005;184:423-7. [Crossref] [PubMed]
- Catalano O, Sandomenico F, Raso MM, et al. Real-time, contrast-enhanced sonography: a new tool for detecting active bleeding. J Trauma 2005;59:933-9. [Crossref] [PubMed]
- Schwartz SA, Taljanovic MS, Smyth S, et al. CT Findings of Rupture, Impending Rupture, and Contained Rupture of Abdominal Aortic Aneurysms. AJR Am J Roentgenol 2007;188:W57-62. [Crossref] [PubMed]
- Voitle E, Hofmann W, Cejna M. Aortic emergencies—diagnosis and treatment: a pictorial review. Insights into Imaging 2015;6:17-32. [Crossref] [PubMed]
- Clevert DA, Stickel M, Flach P, et al. Contrast-enhanced ultrasound in detection and follow-up of an infrarenal abdominal aortic aneurysm with aorto-caval fistula and endovascular treatment. Cardiovasc Intervent Radiol 2007;30:480-4. [Crossref] [PubMed]
- Shah A, Stavropoulos SW. Imaging Surveillance following Endovascular Aneurysm Repair. Semin Intervent Radiol 2009;26:10-6. [Crossref] [PubMed]
- White RA. Endograft surveillance: a priority for long-term device performance. J Endovasc Ther 2000;7:522. [Crossref] [PubMed]
- Cantisani V, Grazhdani H, Clevert DA, et al. EVAR: Benefits of CEUS for monitoring stent-graft status. Eur J Radiol 2015;84:1658-65. [Crossref] [PubMed]
- Pandey N, Litt HI. Surveillance Imaging Following Endovascular Aneurysm Repair. Semin Intervent Radiol 2015;32:239-48. [Crossref] [PubMed]
- Schlösser FJ, Gusberg RJ, Dardik A, et al. Aneurysm rupture after EVAR: can the ultimate failure be predicted? Eur J Vasc Endovasc Surg 2009;37:15-22. [Crossref] [PubMed]
- Iezzi R, Cotroneo AR, Basilico R, et al. Endoleaks after endovascular repair of abdominal aortic aneurysm: value of CEUS. Abdom Imaging 2010;35:106-14. [Crossref] [PubMed]
- White GH, Yu W, May J. Endoleak--a proposed new terminology to describe incomplete aneurysm exclusion by an endoluminal graft. J Endovasc Surg 1996;3:124-5. [Crossref] [PubMed]
- Baum RA, Stavropoulos SW, Fairman RM, et al. Endoleaks after endovascular repair of abdominal aortic aneurysms. J Vasc Interv Radiol 2003;14:1111-7. [Crossref] [PubMed]
- Manning BJ, O'Neill SM, Haider SN, et al. Duplex ultrasound in aneurysm surveillance following endovascular aneurysm repair: a comparison with computed tomography aortography. J Vasc Surg 2009;49:60-5. [Crossref] [PubMed]
- Schmieder GC, Stout CL, Stokes GK, et al. Endoleak after endovascular aneurysm repair: Duplex ultrasound imaging is better than computed tomography at determining the need for intervention. J Vasc Surg 2009;50:1012-7; discussion 1017-8. [Crossref] [PubMed]
- Clevert DA, Minaifar N, Weckbach S, et al. Color duplex ultrasound and contrast-enhanced ultrasound in comparison to MS-CT in the detection of endoleak following endovascular aneurysm repair. Clin Hemorheol Microcirc 2008;39:121-32. [PubMed]
- Clevert DA, Horng A, Kopp R, et al. Imaging of endoleaks after endovascular aneurysm repair (EVAR) with contrast-enhanced ultrasound (CEUS). Radiologe 2009;49:1033-9. [Crossref] [PubMed]
- Bendick PJ, Bove PG, Long GW, et al. Efficacy of ultrasound scan contrast agents in the noninvasive follow-up of aortic stent grafts. J Vasc Surg 2003;37:381-5. [Crossref] [PubMed]
- Giannoni MF, Palombo G, Sbarigia E, et al. Contrast-enhanced ultrasound imaging for aortic stent-graft surveillance. J Endovasc Ther 2003;10:208-17. [Crossref] [PubMed]
- David E, Cantisani V, Grazhdani H, et al. What is the role of contrast-enhanced ultrasound in the evaluation of the endoleak of aortic endoprostheses? A comparison between CEUS and CT on a widespread scale. J Ultrasound 2016;19:281-7. [Crossref] [PubMed]
- Yang X, Chen YX, Zhang B, et al. Contrast-enhanced Ultrasound in Detecting Endoleaks with Failed Computed Tomography Angiography Diagnosis after Endovascular Abdominal Aortic Aneurysm Repair. Chin Med J (Engl) 2015;128:2491-7. [Crossref] [PubMed]
- Dill-Macky MJ, Wilson SR, Sternbach Y, et al. Detecting Endoleaks in Aortic Endografts Using Contrast-Enhanced Sonography. AJR Am J Roentgenol 2007;188:W262-8. [Crossref] [PubMed]
- Pfister K, Rennert J, Uller W, et al. Contrast harmonic imaging ultrasound and perfusion imaging for surveillance after endovascular abdominal aneurysm repair regarding detection and characterization of suspected endoleaks. Clin Hemorheol Microcirc 2009;43:119-28. [PubMed]
- Chung J, Kordzadeh A, Prionidis I, et al. Contrast-enhanced ultrasound (CEUS) versus computed tomography angiography (CTA) in detection of endoleaks in post-EVAR patients. Are delayed type II endoleaks being missed? A systematic review and meta-analysis. J Ultrasound 2015;18:91-9. [Crossref] [PubMed]
- Guo Q, Zhao J, Huang B, et al. A Systematic Review of Ultrasound or Magnetic Resonance Imaging Compared With Computed Tomography for Endoleak Detection and Aneurysm Diameter Measurement After Endovascular Aneurysm Repair. J Endovasc Ther 2016;23:936-43. [Crossref] [PubMed]
- Jung EM, Rennert J, Fellner C, et al. Detection and characterization of endoleaks following endovascular treatment of abdominal aortic aneurysms using contrast harmonic imaging (CHI) with quantitative perfusion analysis (TIC) compared to CT angiography (CTA). Ultraschall Med 2010;31:564-70. [Crossref] [PubMed]
- Gargiulo M, Gallitto E, Serra C, et al. Could four-dimensional contrast-enhanced ultrasound replace computed tomography angiography during follow up of fenestrated endografts? Results of a preliminary experience. Eur J Vasc Endovasc Surg 2014;48:536-42. [Crossref] [PubMed]
- Partovi S, Kaspar M, Aschwanden M, et al. Contrast-enhanced ultrasound after endovascular aortic repair—current status and future perspectives. Cardiovasc Diagn Ther 2015;5:454-63. [PubMed]
- McMahon MA, Squirrell CA. Multidetector CT of Aortic Dissection: A Pictorial Review. Radiographics 2010;30:445-60. [Crossref] [PubMed]
- Spittell PC, Spittell JA Jr, Joyce JW, et al. Clinical features and differential diagnosis of aortic dissection: experience with 236 cases (1980 through 1990). Mayo Clin Proc 1993;68:642-51. [Crossref] [PubMed]
- Khan IA, Nair CK. Clinical, diagnostic, and management perspectives of aortic dissection. Chest 2002;122:311-28. [Crossref] [PubMed]
- Williams DM, Lee DY, Hamilton BH, et al. The dissected aorta: part III. Anatomy and radiologic diagnosis of branch-vessel compromise. Radiology 1997;203:37-44. [Crossref] [PubMed]
- Clevert DA, Stickel M, Johnson T, et al. Imaging of aortic abnormalities with contrast-enhanced ultrasound. A pictorial comparison with CT. Eur Radiol 2007;17:2991-3000. [Crossref] [PubMed]
- Clevert DA, Weckbach S, Kopp R, et al. Imaging of aortic lesions with color coded duplex sonography and contrast-enhanced ultrasound versus multislice computed tomography (MS-CT) angiography. Clin Hemorheol Microcirc 2008;40:267-79. [PubMed]
- Clevert DA, Horng A, Clevert DA, et al. Contrast-enhanced ultrasound versus conventional ultrasound and MS-CT in the diagnosis of abdominal aortic dissection. Clin Hemorheol Microcirc 2009;43:129-39. [PubMed]
- Litmanovich DE, Yildirim A, Bankier AA. Insights into imaging of aortitis. Insights Imaging 2012;3:545-60. [Crossref] [PubMed]
- Ammirati E, Moroni F, Pedrotti P, et al. Non-Invasive Imaging of Vascular Inflammation. Front Immunol 2014;5:399. [Crossref] [PubMed]
- Restrepo CS, Ocazionez D, Suri R, et al. Aortitis: Imaging Spectrum of the Infectious and Inflammatory Conditions of the Aorta. RadioGraphics 2011;31:435-51. [Crossref] [PubMed]
- Yajima M, Numano F, Park YB, et al. Comparative studies of patients with Takayasu arteritis in Japan, Korea and India--comparison of clinical manifestations, angiography and HLA-B antigen. Jpn Circ J 1994;58:9-14. [Crossref] [PubMed]
- Gornik HL, Creager MA. Aortitis. Circulation 2008;117:3039-51. [Crossref] [PubMed]
- Schmidt WA, Kraft HE, Vorpahl K, et al. Color Duplex Ultrasonography in the Diagnosis of Temporal Arteritis. N Engl J Med 1997;337:1336-42. [Crossref] [PubMed]
- Kono Y, Pinnell SP, Sirlin CB, et al. Carotid Arteries: Contrast-enhanced US Angiography—Preliminary Clinical Experience. Radiology 2004;230:561-8. [Crossref] [PubMed]
- Giordana P, Baque-Juston MC, Jeandel PY, et al. Contrast-enhanced ultrasound of carotid artery wall in Takayasu disease: first evidence of application in diagnosis and monitoring of response to treatment. Circulation 2011;124:245-7. [Crossref] [PubMed]
- Magnoni M, Dagna L, Coli S, et al. Assessment of Takayasu arteritis activity by carotid contrast-enhanced ultrasound. Circ Cardiovasc Imaging 2011;4:e1-2. [Crossref] [PubMed]
- Schinkel AF, van den Oord SC, van der Steen AF, et al. Utility of contrast-enhanced ultrasound for the assessment of the carotid artery wall in patients with Takayasu or giant cell arteritis. Eur Heart J Cardiovasc Imaging 2014;15:541-6. [Crossref] [PubMed]
- Partovi S, Imfeld S, Aschwanden M, et al. The Use of Contrast-enhanced Ultrasound (CEUS) in Chronic Periaortitis. Ultraschall Med 2012. [Epub ahead of print]. [PubMed]
- Ten Kate GL, van den Oord SC, Sijbrands EJ, et al. Current status and future developments of contrast-enhanced ultrasound of carotid atherosclerosis. J Vasc Surg 2013;57:539-46. [Crossref] [PubMed]


