Complications of endovascular aneurysm repair of the thoracic and abdominal aorta: evaluation and management
Introduction
Endovascular repair of the thoracic and abdominal aorta is an important advance in the treatment of aortic aneurysms and other aortic pathologies. Since the U.S. Food and Drug Administration (FDA) approved the use of endograft devices, there has been a 600 percent increase in the annual number of endovascular aneurysm (or aortic) repair (EVAR) procedures performed (1). Endovascular abdominal aortic aneurysm (AAA) repair and thoracic endovascular aneurysm repair (TEVAR) currently account for nearly 50% of all aortic aneurysm repairs that are performed in the U.S. (1). In recent years, there has been an overall decrease in the incidence of ruptured aneurysms, likely due a combination of improved AAA screening, and increased rates of elective endovascular repairs in patients who would not otherwise be surgical candidates (1). While these techniques were initially used for the treatment of patients who were deemed high-risk surgical candidates, emerging data in recent years proving their safety profile have made EVAR the preferred treatment techniques for many patients with aortic aneurysms due to the decreased perioperative morbidity and the comparable to improved outcomes of these procedures relative to open surgical repair (2-9).
EVAR involves the placement of a prosthetic endograft within the thoracic or abdominal aorta at the site of an aneurysm or other pathologic process that threatens the integrity of the aorta. The various endograft components are typically compressed within a delivery sheath and are introduced into the vascular system through the lumen of an access vessel, to be subsequently deployed at the site of the aneurysm. Once deployed at the target site of treatment, the endograft self-expands to contact the aortic wall thereby excluding the weakened aortic wall or aneurysm sac from the pathologic increased flow and pressure that might otherwise lead to aortic/aneurysm rupture. Among the most important determinants for the success of an endovascular repair are the anatomic suitability of the patient’s vasculature for device placement, and the choice of an endograft that is of appropriate size and configuration for the patient’s anatomy and aortic morphology. The device must provide adequate seals or fixation proximally and distally at the endograft landing zones in order to successfully exclude the aneurysm sac. To be a suitable candidate for EVAR, certain general anatomic criteria must be fulfilled including an aortic aneurysm proximal neck size that measures 18–32 mm in diameter and is greater than 10 mm in length, a neck angulation that is typically less than 45–60 degrees (depending on the device used), a common iliac artery diameter between 8–22 mm and an external iliac diameter greater than 7 mm (10). If the planned positioning of the endograft is expected to cover important aortic side branch vessels, debranching procedures may be needed prior to graft placement or fenestrated endografts may be required.
AAA repair is indicated in patients with symptomatic aneurysms, in those who have an aneurysm diameter greater than 5.5 cm, or in those whose aneurysm has expanded by more than 0.5 cm in a 6-month interval (2). Similarly, repair is indicated for thoracic aortic aneurysms in symptomatic patients, patients with an aortic size index equal to or greater than 2.75 cm/m2, patients with aortic diameters of 6 to 7 cm, patients with genetically-mediated conditions that are associated with aortic pathology or patients with aneurysm diameter expansion of greater than 10 mm per year (11-13). In patients whose anatomic criteria are suitable, EVAR is typically the preferred means of treatment. Absolute contraindications to EVAR include various unfavorable anatomic features such as excessive aortic tortuosity and angulation, a hostile proximal neck with circumferential calcification, excessive mural thrombus or an extremely conical configuration, and extremely small-caliber access vessels. There are also certain relative contraindications such as the inability or unwillingness to comply with post-procedural surveillance imaging.
EVAR of the abdominal aorta conveys a number of advantages when compared to open aneurysm repair. Available data show perioperative survival benefit as compared to open surgery. In a systematic review of 1,532 patients, endovascular repair was associated with a significantly lower 30-day mortality (1.6%) than open surgery (4.8%) (14). The survival advantage conveyed by endovascular repair is even greater in high-risk surgical candidates where the 30-day post-procedure mortality rate was found to be 4.7% compared to 19.2% in those who underwent open repair (15). To our knowledge, no randomized studies are available comparing open and endovascular repair in the thoracic aorta. However, observational studies suggest equivalent or better overall outcomes (16). EVAR of the abdominal aorta is also associated with a significant reduction in perioperative morbidity when compared to open surgery, with decreased blood loss, elimination of the need for cross-clamping the aorta intraprocedurally and shorter recovery periods (1,17-20). Specific to thoracic aneurysm repair, TEVAR provides the advantage of avoidance of sternotomy and thoracotomy, both of which carry high patient morbidity (20).
While EVAR is associated with improved short-term survival in patients with aortic aneurysms, it is important to note that available data do not show long-term improvement in survival benefit when compared to open repair. In a review of 22,830 matched Medicare patients who underwent endovascular and open repair, lower perioperative mortality was again demonstrated (21). However, the overall mortality was similar between the two groups at 3 to 4 years post-procedure (21). Because endograft imaging surveillance is mandatory for the remainder of a patient’s life after EVAR, the risk of the long-term radiation exposure associated with imaging makes the use of this technique somewhat controversial in young patients who are otherwise good surgical candidates, given the equivalent long-term survival outcomes of the two techniques. The decision to pursue endovascular or open repair should be personalized to each patient and should be based on the patient’s age, surgical risk and vascular anatomy.
With EVAR, the preferred intervention for the majority of patients with aortic aneurysms, an increasing number of complications are being reported as a result of the marked increase in the number of these procedures that are being performed (22). Emerging data show that endograft-related complications are relatively common. Following EVAR for AAA, the rate of complications has been reported to range between 16% and 30% with secondary interventions needed in up to 19% of patients (23-28). For TEVAR, late complications have been shown to occur in up to 38% of patients with secondary intervention required in approximately 24% of cases (9,29-32). In this article, we summarize the current surveillance recommendations for detecting and evaluating complications following EVAR of the thoracic and abdominal aorta. We also provide an overview of commonly reported complications and discuss the secondary interventions typically performed for treatment.
Endograft surveillance and evaluation
Current surveillance recommendations
Lifelong post procedure imaging surveillance is currently recommended in all patients following endovascular repair of the thoracic and abdominal aorta so as to evaluate the long-term performance of the endoprosthesis. Imaging is essential for assessment of the integrity of the endograft and for confirmation of the stability of or a decrease in the size of the excluded aneurysm sac. If a post-procedural complication or abnormality is detected by clinical or imaging surveillance, the latter can also be used to further evaluate and characterize the abnormal finding; commonly occurring post-procedural problems include endoleaks, endograft migration or collapse, limb kinking and/or stenosis and endograft infection. Imaging techniques that are used for surveillance include conventional radiography, computed tomography (CT), ultrasonography, nuclear imaging, magnetic resonance angiography (MRA) and conventional angiography, with CT considered as the gold standard modality. These techniques are summarized in Table 1. Current guidelines for surveillance imaging post-endovascular repair recommend imaging at 30 days, 6 and 12 months following the procedure and yearly thereafter, if no complications are detected (11,33).
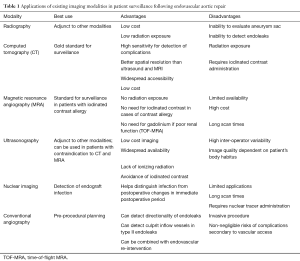
Full table
Conventional radiography
Conventional radiography can provide an overview of graft positioning and integrity and conveys the advantage of low surveillance cost and low radiation exposure (34). Anteroposterior (AP) radiographs can be helpful in detecting endograft migration and separation of modular endograft components (35). Supplemental oblique views can be used to detect wire fractures (35). However, conventional radiography is rarely used alone for post-procedural surveillance but may instead be used as a complement to other imaging modalities. There are multiple disadvantages of using conventional radiography for surveillance, such as the inability to evaluate the size of the residual aneurysm sac or to detect soft tissue and flow-related complications such as endoleaks and graft infections, many operators and institutions no longer routinely use this imaging technique for endograft evaluation.
CT and CT angiography (CTA)
CT is considered the gold standard technique for surveillance imaging in patients who have undergone EVAR. Typical CT imaging protocols include a non-contrast phase, an arterial imaging phase and a delayed imaging phase at 120–300 seconds. Non-contrast imaging is necessary so as to differentiate high density material such as calcification that may be present in the aneurysm sac from abnormalities such as endoleaks that may be seen on subsequent later phase imaging. Arterial and delayed-phase imaging are used to assess endograft integrity, to detect and characterize endoleaks and to assess for the presence of other abnormalities such as limb occlusion or endograft infection. The diameter or volume of the residual aneurysm sac should be measured on each surveillance scan in order to ensure stability or to demonstrate a decrease in the size of the excluded sac. We typically measure the largest diameter of the aneurysm sac using a double-oblique short-axis orientation, so as to improve measurement accuracy and increase inter-reader reproducibility. Many operators advocate calculation of the residual aneurysm sac volume as the most accurate measurement, if appropriate post-processing software is available.
CTA provides 92% sensitivity for the detection of endoleaks and offers better spatial resolution for the assessment of the endograft relative to ultrasonography and MRA (36,37). In addition, CT conveys the advantage of widespread accessibility and relatively low cost when compared to other imaging modalities. Despite these advantages, significant concerns remain about the cumulative radiation exposure and the need for the repetitive administration of iodinated contrast (34,38). Cumulative radiation exposure is of particular concern in younger patients undergoing yearly surveillance CT scans. Similarly, administration of iodinated contrast is problematic in patients who are at risk for contrast-induced nephropathy. Recent advances in dual-source dual-energy CT and other image reconstruction approaches are promising for radiation dose reduction (39,40). Reduced contrast-dose techniques are also being actively explored so as to reduce the risk of contrast-induced nephropathy (41).
MRA
MRA is considered an alternative to CTA for post-EVAR surveillance imaging (42). Typical imaging protocols include an axial T1-weighted gradient echo sequence, a single-shot fast spin echo sequence and pre- and post-contrast sequences. Non-contrast time-of-flight MRA (TOF-MRA) imaging can also be performed and is especially useful in patients with poor renal function or those who have a contraindication to gadolinium use. Unlike CT, TOF-MRA also allows the detection of the directionality of blood flow. Recent data suggest that MRA is superior for the imaging of nitinol endografts as compared to CT (43). Otherwise, gadolinium-enhanced MRA is equivalent to CTA in sensitivity for the detection of endoleaks. When TOF-MRA imaging is used alone in patients with a low estimated glomerular filtration rate (eGFR), its sensitivity for endoleak detection can be as low as 54% (44). However, when TOF is used in conjunction with gadolinium-enhanced MRA, it has 97% concordance with angiography for the detection of endoleaks (45).
MRA conveys several advantages when compared to CTA including the use of non-ionizing radiation for imaging and the avoidance of the administration of iodinated IV contrast. MRA is especially useful in patients who have an iodinated contrast allergy or who have other contraindications to receiving these contrast media. Potential drawbacks to MRA use include the more limited availability of MR as compared to CT, the higher imaging costs, longer scan acquisition times, use in claustrophobic patients and the inability to clear all patients for imaging by magnetic resonance. In patients undergoing contrast-enhanced magnetic resonance imaging (MRI), emerging evidence also suggests that there is gadolinium deposition and accumulation in the central nervous system even in patients with normal renal function (46,47). Although the clinical significance of gadolinium deposition in the brain remains unclear, care should be taken when using MRI for patient surveillance until further data is available about the long-term safety of gadolinium-based agents.
Ultrasonography
Ultrasound can be quite useful for the surveillance of a patient following EVAR. A typical post-procedure ultrasound protocol includes B-mode imaging of the abdominal aorta, iliac arteries and femoral arteries in transverse and longitudinal orientations in order to assess the endograft, the landing zones and the size of the residual aneurysm sac. The examination should also include the use of color and power Doppler so as to confirm endograft patency and to assess for flow directionality and the presence or absence of endoleaks. Emerging data suggest that contrast-enhanced ultrasound (CEUS) with non-targeted microbubbles can be used to enhance the sensitivity of ultrasound in endoleak detection (48). A recent systematic review also suggested that CEUS has high sensitivity for detecting endoleaks and can be introduced as a routine diagnostic modality to be followed by CTA only when the ultrasound is positive to further characterize an endoleak (49). Ultrasound is reported to have a specificity of 93–94% and a sensitivity of 70–82% for the detection of endoleaks (49-51).
Ultrasound imaging for surveillance following EVAR offers the advantage of low-cost imaging, widespread availability, a lack of ionizing radiation and the avoidance of iodinated contrast use. However, ultrasound suffers from high inter-operator variability and the quality of the collected images is highly dependent on the patient’s body habitus. Evaluation of endograft integrity and positioning is also limited with ultrasound. Accordingly, ultrasound remains an adjunctive technique in surveillance and is rarely used as the sole surveillance tool unless the patient has contraindications to both CT and MRA.
Nuclear imaging
Nuclear imaging techniques have been found to mostly be useful for the detection and characterization of an endograft infection, with labeled white blood cell (WBC) imaging, gallium scanning and FDG-PET imaging all having demonstrated roles (52,53). The sensitivity of these techniques for the detection of endograft infection has been reported to range between 60% and 100% (52,54). Nuclear imaging is especially useful in the immediate post-operative period when endograft infection is suspected. It has been shown that nuclear imaging during that period is more sensitive than CT for the detection of graft infection (52,55).
The use of 99mTc-labeled red blood cells and technetium-99m sulfur colloid has been proposed for the detection of endoleaks in post-endovascular repair surveillance imaging. However, available data show that these techniques exhibit significantly lower sensitivity when compared to CT (56,57).
Conventional angiography
CTA and MRA are more sensitive than digital subtraction angiography (DSA) for the detection of complications following EVAR (34,36). DSA is currently used for pre-procedural planning or intraprocedural guidance prior to or during secondary interventions. Post-procedural DSA also allows one to bypass immediate follow-up post-procedural imaging in patients who have clinically apparent post-EVAR complications. DSA is especially useful for the detection of the directionality of an endoleak and for identifying the culprit inflow vessel in type II endoleaks. DSA, however, is an invasive procedure and carries non-negligible risks including access site complications such as hematoma or pseudoaneurysm formation, and other adverse events like arterial dissection or thrombosis, retroperitoneal hemorrhage, and vessel rupture.
Endovascular repair complications and their management
Device-related complications
Endoleak
Endoleaks are the most commonly occurring complication following EVAR. The most common complications are summarized in Table 2. Endoleaks represent persistent blood flow perfusing the residual aneurysm sac thus indicating failure to completely exclude the aneurysm. There are five types of endoleaks that have been extensively described (58-61). Type I endoleaks occur because of an incompetent seal at the proximal (type IA) or distal (type IB) endograft attachment site. Type II endoleaks are characterized by persistent flow into and out of the residual aneurysm sac via patent aortic side branch vessels such as the inferior mesenteric artery, lumbar arteries, accessory renal arteries or the left subclavian artery. Type III endoleaks are caused by structural failure of the endograft itself. Examples include tears in the endograft fabric and separation or dehiscence of modular graft components. Type IV endoleaks are caused by graft porosity, while type V endoleaks are characterized by continued residual aneurysm sac expansion despite the lack of any evidence of an endoleak by imaging, which is a phenomenon that is known as endotension. The most commonly occurring types of endoleaks following both thoracic and abdominal EVAR are types I and II. Endoleaks are more commonly seen following endovascular repair of the abdominal aorta and occur in 15–30% of patients in the first 30 days after the procedure (34,62). They are seen less commonly with TEVAR, occurring in 4–15% of the cases (9,30,63). When present, endoleaks carry an increased risk for continued aneurysm expansion and eventual rupture.
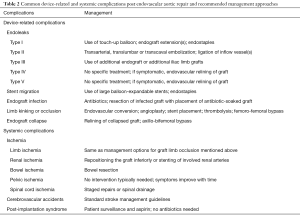
Full table
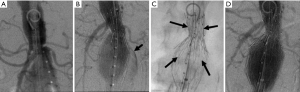
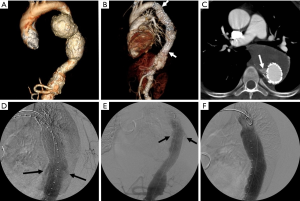
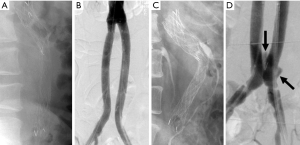
Because endoleaks are the most commonly occurring complication following EVAR, secondary interventions for their treatment, when necessary, represent the most frequently performed post-repair procedure. Techniques for treating type I endoleaks are aimed at securing the involved proximal or distal endograft attachment site or seal zone (Figures 1,2). Potential techniques include using a large caliber compliant balloon to more optimally distend the endograft at the attachment site or to extend the endograft proximally or distally with an endograft aortic extension cuff or limb (64). Other operators advocate using a high radial-force stent at the proximal attachment site to more securely seal the graft, while others have favored peri-graft embolization using a liquid embolic agent such as n-butyl cyanoacrylate (65,66). More recent techniques include the use of endostaples to secure the position of the primary aortic endograft or of an aortic extension cuff to the native aorta (67,68). The most commonly used strategy for treatment of a proximal type I endoleak involves placement of an aortic cuff extension.
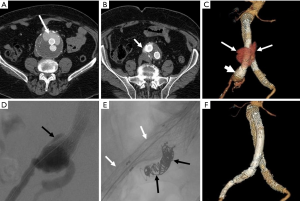
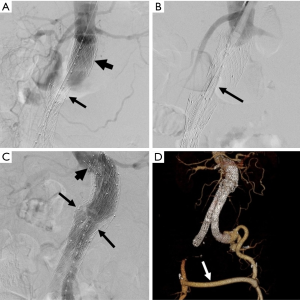
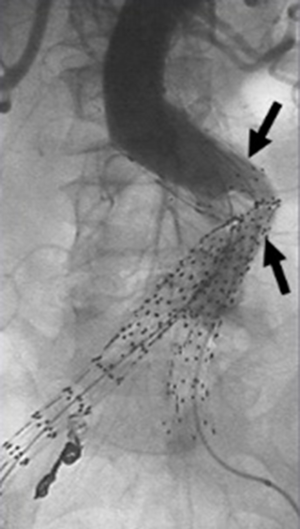
Secondary interventions for type II endoleaks almost always involve some type of embolization (Figures 3,4). Embolic occlusion of the patent aortic side branches that continue to perfuse the residual aneurysm sac or embolization of the nidus within the residual sac are the commonly employed techniques, with the latter favored. Routes of access for performing type II endoleak embolization include transarterial (69), percutaneous translumbar aortic (70), and transcaval approaches (71). Various embolic agents have been used including intravascular coils, liquid embolic agents such as n-butyl cyanoacrylate, thrombin and ethylene vinyl alcohol copolymer (Onyx®, Covidien-Medtronic, Minneapolis, MN, USA) (72). Other described techniques include surgical ligation of the culprit inflow vessel (73,74). These embolization procedures carry a risk for the development of ischemic colitis when the inferior mesenteric artery (IMA) is involved.
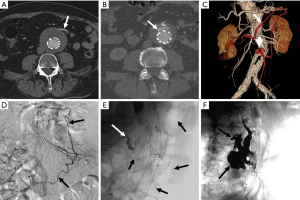
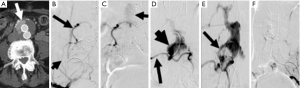
Management of type III endoleaks usually requires placement of additional modular endograft components to seal and re-establish the integrity of the affected portion(s) of the endograft (64) (Figures 5,6). No specific treatment is recommended for type IV and type V endoleaks. However, if treatment becomes necessary because of continued expansion of the residual aneurysm sac, endovascular re-lining of the original endograft or open surgical conversion may be necessary.
Endograft migration
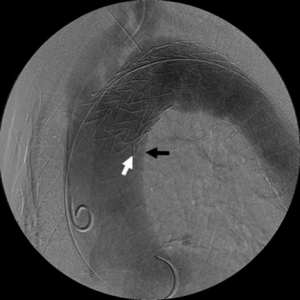
Device migration is a common complication that requires secondary intervention following EVAR (75). It is defined as displacement of the endograft by more than 5–10 mm from its original position (Figure 7). It is often due to progressive dilatation of the aneurysm neck but can also be related to aortic tortuosity, aortic wall degeneration after endograft placement or may be secondary to graft over- or under-sizing. Device migration is associated with endoleaks, aneurysm sac expansion and possible rupture. Device migration has been reported to occur following 1.0–2.8% of TEVAR procedures and 1–10% of endovascular repair of the abdominal aorta at 1 year post-intervention (6,9,30,76). In cases of aortic endograft migration, treatment is very similar to management of a type I endoleak. Endovascular treatment options include the use of aortic extension cuffs or placement of large balloon-expandable stents to augment the fixation of the endograft to the native aortic wall and thus extend the fixation zone. Another option is that of using endostaples to secure the graft to the aortic wall (77,78).
Endograft infection
Endograft infection has been reported to occur in 0.4–3.0% of cases following EVAR of the abdominal aorta (60,61,79,80). It is associated with high mortality rates that range from 25% to 50% and that are usually secondary to septic shock (60,80,81). Endograft infection may be caused by intraprocedural contamination, in which case the infection occurs early after the procedure. If the infection occurs at a later time after repair, it may be the result of a remote site of infection leading to colonization of the endograft. Rarely, endograft infection may lead to aortoenteric fistula formation (80). Patients typically present with fever, leukocytosis and back pain. Endograft infections may be managed conservatively with antibiotics or may be treated aggressively with endograft explantation and placement of an antibiotic-coated graft (37,82,83). The clinical approach is highly dependent on the clinical scenario and the patient’s comorbidities.
Limb kinking or occlusion
Kinking and/or occlusion of endograft limbs have been reported in 2–4% of patients following EVAR of the abdominal aorta (84,85) (Figure 8). Causes for these complications include progressive decrease of the size of the residual aneurysm sac over time, excessive aortic neck angulation and a narrow diameter to the distal aortic neck (86). Limb kinking can lead to type I and/or type III endoleaks as well as to endograft migration. It can also result in endograft limb thrombosis and occlusion which may in turn cause acute lower extremity ischemia. A number of treatment options are available for the treatment of limb kinking, stenosis or occlusion. Severe limb kinking can be treated by placement of reinforcing stents or additional endograft limbs within the original graft. Percutaneous angioplasty can be performed with or without additional endograft placement to treat limb stenosis or occlusion. Additionally, occluded endografts may sometimes be treated with thrombolysis or thrombectomy and new limb placement. With thrombectomy, care has to be taken to avoid distal embolization into outflow runoff vessels. Other options include a cross-femoral surgical bypass, which may often be the preferred procedure. Timely management of this complication is especially important so as to decrease the likelihood of distal limb ischemia and to improve patient outcomes.
Endograft collapse
Device infolding or collapse has been reported following TEVAR. It is thought to be related to a small proximal aortic curvature or may be associated with oversizing of the endograft relative to the native aorta (87,88). A bird-beak configuration of the endograft is significantly correlated with the risk of type IA endoleak formation, and is a potential risk factor for proximal endograft collapse or infolding (Figure 9). Endograft collapse is most commonly seen after endovascular repair of traumatic aortic injuries (89). Endograft collapse typically occurs within the first 30 days after the procedure, with a median time to collapse of 15 days, as reported in a review of 60 cases of endograft collapse following TEVAR (87). A high level of suspicion is needed for the diagnosis of endograft collapse in the first 30 days after the procedure. Patients typically present with symptoms of acute aortic occlusion.
The majority of patients undergo endovascular re-intervention with the repair achieved by relining the collapsed endograft (87). Relining is the preferred mode of re-intervention and provides for a more definitive solution. Available data shows that dilation of the collapsed endograft without stent placement is associated with early recurrence of the collapse (90,91). A small percentage of patients may require surgical intervention for repair by endograft removal and open aortic repair or by axillo-bifemoral bypass.
Systemic complications
Ischemia
Ischemic complications following EVAR have been reported in approximately 9% of cases, an incidence that is higher than is seen following open surgical repair (92). Ischemia may be caused by arterial thrombosis, embolism, arterial dissection or arterial obstruction occurring as a result of endograft malpositioning. Organs and vascular territories that may be affected by ischemia following EVAR of the abdominal aorta include the kidneys, bowel, pelvic organs/muscles and the lower extremities. Spinal cord ischemia is more commonly associated with TEVAR (93). A number of cases of left upper limb ischemia, left upper extremity claudication and subclavian steal syndrome have also been reported following TEVAR (94,95).
Lower limb ischemia is among the most common forms of ischemia seen following EVAR of the abdominal aorta with the majority occurring as a result of endograft limb occlusion (92), the management of which is addressed in the previous section. Limb ischemia can occur following TEVAR in the setting of inadvertent coverage of the left subclavian artery by the endograft (94-96). This is usually an infrequent complication and is rarely symptomatic (97). More often, based upon the individual patient anatomy, left subclavian artery coverage is a planned component of the procedure, for which carotid-subclavian bypass or transposition is performed prior to TEVAR.
Postprocedural renal ischemia may result from arterial thrombosis embolus or dissection, may be due to inadvertent intraprocedural coverage of the origin(s) of the renal arteries by the endograft or can result from endograft migration (98,99). A short aortic neck carries an increased risk of inadvertent coverage of the renal arteries by the endograft. If the kidneys are not visualized on completion arteriography, stenting of the involved renal artery(ies) may be attempted. If renal function continues to deteriorate, surgical bypass may be needed in order to revascularize the involved kidney.
Intestinal ischemia may occur following EVAR and, when present, most commonly involves the colon, where it is reported to occur in 1–3% of patients (100,101). Colonic ischemia is thought to result from endograft coverage of the inferior mesenteric artery origin, a phenomenon that occurs in all cases of EVAR of the abdominal aorta. If there are poorly developed mesenteric collateral arcades, left colonic ischemia may ensue. Small bowel or right colonic ischemia in the distribution of the superior mesenteric artery (SMA) is much less common and may be secondary to thromboembolism from catheter and/or guidewire manipulation, especially in a long procedure or by inadvertent coverage of the SMA origin by the endograft. Bowel ischemia is far less commonly seen with TEVAR and has been reported when there was inadvertent coverage of the celiac artery by the distal aspect of the endograft; these may often be less symptomatic if there is significant mesenteric collateralization. If there is inadvertent coverage of both the celiac trunk and SMA by the endograft, patients will likely present with ischemic colitis. Patients with ischemic colitis secondary to endovascular repair typically present with abdominal pain and bloody diarrhea less than 30 days post procedure. A history of prior embolization of one or both internal iliac arteries significantly increases the risk of this complication (101).
Pelvic ischemia has also been reported following EVAR of the abdominal aorta in the setting of internal iliac artery embolization. Intentional embolization of one or both internal iliac arteries has been used in patients with complex iliac arterial anatomy so as to allow extension of endograft limbs into the external iliac arteries or to exclude internal iliac artery aneurysms. Patient symptoms following internal iliac artery embolization include buttock claudication, rectal ischemia, erectile dysfunction and skin malperfusion and necrosis. Buttock claudication has been reported in 31–35% of cases and erectile dysfunction in 17–24% of patients. Symptoms tend to improve with time with no intervention needed. However, there is a higher risk of symptoms persisting in cases in which there has been bilateral internal iliac artery embolization. Intraoperative strategies to preserve perfusion of the internal iliac arterial territories in order to prevent these complications include investigational iliac branched devices (not currently approved by the FDA), surgical revascularization of the internal iliac artery, operator modification of currently existing endografts, and other techniques such as placement of parallel endografts.
Spinal cord ischemia occurs very rarely in association with EVAR of the abdominal aorta, with approximately 14 cases reported to date (92,102,103). Unfortunately, however, the incidence of spinal ischemia is much higher with TEVAR where it is estimated to occur in up to 12% of cases (93). Symptoms of spinal cord ischemia typically develop within 12 hours following repair and may lead to paraplegia (72). Risk factors include the extent of aortic coverage by the device, perioperative hypotension, long procedural durations, coverage of the left subclavian artery, previous open infrarenal aortic repair and renal insufficiency (16,104). Spinal drainage can be used in cases in which there is planned extensive coverage of the thoracic aorta to reduce the risk for spinal cord ischemia.
Cerebrovascular events
Embolic strokes have been reported to occur in 4% to 8% of the cases following TEVAR, an incidence rate that is comparable to open surgery (16,29,105). The relatively high risk is a result of the proximity of the proximal seal zone of the endovascular graft to the origins of the vertebral and carotid arteries. Risk factors for strokes complicating TEVAR include the presence of mobile atheromata in the aortic arch, a history of prior strokes, and the need for proximal graft deployment (105). Middle cerebral circulation strokes are most common, although posterior circulation strokes have been reported as a result of embolization of debris through the vertebral arteries.
Postimplantation syndrome
Postimplantation syndrome may occur after EVAR and has a reported incidence ranging between 13–60% (106,107). It is thought to represent an inflammatory immune-mediated response, with the release of inflammatory cytokines that occurs as a result of endothelial activation through a reaction to the endograft material (107). Symptoms are flu-like in nature and manifest clinically as a systemic inflammatory response that is characterized by fever, leukocytosis, and elevated inflammatory markers, including C-reactive protein (CRP), tumor necrosis factor (TNF)-alpha and interleukin (IL)-6 levels (92-94). Pleural effusions may occur with postimplantation syndrome and are seen in 37–73% of cases after TEVAR (108). Treatment consists of surveillance and aspirin administration to reduce inflammation, with no antibiotics indicated.
Open surgical conversion
Open surgical conversion involves surgical modification of an existing endovascular graft. Open conversion rates range from 0.6% to 4.5%. Surgical intervention is reserved to select cases where repair by endovascular means is not possible (16,109,110). Open surgical conversion is typically needed in select cases of symptomatic type V endoleaks, or in cases involving extensive endograft migration. Aneurysm rupture typically requires removal of the endograft and repair with synthetic grafts or homografts. Open surgical repair is usually of last resort especially with many patients treated with EVAR not being good surgical candidates.
Conclusions
EVAR is increasingly being used for the treatment for thoracic and AAAs and certain other aortic pathologies. It is minimally-invasive, is associated with decreased perioperative morbidity and conveys a short-term survival advantage when compared to open surgical repair. One of the disadvantages of EVAR is the relatively high incidence of post-procedural complications that thus necessitates lifelong imaging surveillance of patients. CT is the preferred method for post-procedural imaging surveillance. A number of patients require secondary re-interventions to address post-procedural endograft-related complications. Most re-interventions are pursued using an endovascular approach. With the increasing number of endovascular repair procedures performed, it is important for clinicians to gain familiarity with common complications and treatment strategies following this procedure.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Dillavou ED, Muluk SC, Makaroun MS. Improving aneurysm-related outcomes: nationwide benefits of endovascular repair. J Vasc Surg 2006;43:446-51; discussion 451-2. [Crossref] [PubMed]
- Chaikof EL, Brewster DC, Dalman RL, et al. The care of patients with an abdominal aortic aneurysm: The Society for Vascular Surgery practice guidelines. J Vasc Surg 2009;50:S2-49. [Crossref] [PubMed]
- Matsumura JS, Brewster DC, Makaroun MS, et al. A multicenter controlled clinical trial of open versus endovascular treatment of abdominal aortic aneurysm. J Vasc Surg 2003;37:262-71. [Crossref] [PubMed]
- Stone DH, Brewster DC, Kwolek CJ, et al. Stent-graft versus open-surgical repair of the thoracic aorta: mid-term results. J Vasc Surg 2006;44:1188-97. [Crossref] [PubMed]
- Najibi S, Terramani TT, Weiss VJ, et al. Endoluminal versus open treatment of descending thoracic aortic aneurysms. J Vasc Surg 2002;36:732-7. [Crossref] [PubMed]
- Bavaria JE, Appoo JJ, Makaroun MS, et al. Endovascular stent grafting versus open surgical repair of descending thoracic aortic aneurysms in low-risk patients: a multicenter comparative trial. J Thorac Cardiovasc Surg 2007;133:369-77. [Crossref] [PubMed]
- Makaroun MS, Dillavou ED, Kee ST, et al. Endovascular treatment of thoracic aortic aneurysms: results of the phase II multicenter trial of the GORE TAG thoracic endoprosthesis. J Vasc Surg 2005;41:1-9. [Crossref] [PubMed]
- Carpenter JP, Anderson WN, Brewster DC, et al. Multicenter pivotal trial results of the Lifepath System for endovascular aortic aneurysm repair. J Vasc Surg 2004;39:34-43. [Crossref] [PubMed]
- Makaroun MS, Dillavou ED, Wheatley GH, et al. Five-year results of endovascular treatment with the Gore TAG device compared with open repair of thoracic aortic aneurysms. J Vasc Surg 2008;47:912-8. [Crossref] [PubMed]
- Schanzer A, Greenberg RK, Hevelone N, et al. Predictors of abdominal aortic aneurysm sac enlargement after endovascular repair. Circulation 2011;123:2848-55. [Crossref] [PubMed]
- Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: executive summary. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association forThoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Catheter Cardiovasc Interv 2010;76:E43-86. [Crossref] [PubMed]
- Hiratzka LF, Creager MA, Isselbacher EM, et al. Surgery for Aortic Dilatation in Patients With Bicuspid Aortic Valves: A Statement of Clarification From the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2016;67:724-31. [Crossref] [PubMed]
- Davies RR, Gallo A, Coady MA, et al. Novel measurement of relative aortic size predicts rupture of thoracic aortic aneurysms. Ann Thorac Surg 2006;81:169-77. [Crossref] [PubMed]
- Davies RR, Gallo A, Coady MA, et al. Novel measurement of relative aortic size predicts rupture of thoracic aortic aneurysms. Ann Thorac Surg 2006;81:169-77. [Crossref] [PubMed]
- Lederle FA, Kane RL, MacDonald R, et al. Systematic review: repair of unruptured abdominal aortic aneurysm. Ann Intern Med 2007;146:735-41. [Crossref] [PubMed]
- Teufelsbauer H, Prusa AM, Wolff K, et al. Endovascular stent grafting versus open surgical operation in patients with infrarenal aortic aneurysms: a propensity score-adjusted analysis. Circulation 2002;106:782-7. [Crossref] [PubMed]
- Greenberg RK, Lu Q, Roselli EE, et al. Contemporary analysis of descending thoracic and thoracoabdominal aneurysm repair: a comparison of endovascular and open techniques. Circulation 2008;118:808-17. [Crossref] [PubMed]
- Giles KA, Pomposelli F, Hamdan A, et al. Decrease in total aneurysm-related deaths in the era of endovascular aneurysm repair. J Vasc Surg 2009;49:543-50; discussion 550-1. [Crossref] [PubMed]
- Giles KA, Schermerhorn ML, O'Malley AJ, et al. Risk prediction for perioperative mortality of endovascular vs open repair of abdominal aortic aneurysms using the Medicare population. J Vasc Surg 2009;50:256-62. [Crossref] [PubMed]
- Sadat U, Boyle JR, Walsh SR, et al. Endovascular vs open repair of acute abdominal aortic aneurysms--a systematic review and meta-analysis. J Vasc Surg 2008;48:227-36. [Crossref] [PubMed]
- Walsh SR, Tang TY, Sadat U, et al. Endovascular stenting versus open surgery for thoracic aortic disease: systematic review and meta-analysis of perioperative results. J Vasc Surg 2008;47:1094-8. [Crossref] [PubMed]
- Schermerhorn ML, O'Malley AJ, Jhaveri A, et al. Endovascular vs. open repair of abdominal aortic aneurysms in the Medicare population. N Engl J Med 2008;358:464-74. [Crossref] [PubMed]
- Walker TG, Kalva SP, Yeddula K, et al. Clinical practice guidelines for endovascular abdominal aortic aneurysm repair: written by the Standards of Practice Committee for the Society of Interventional Radiology and endorsed by the Cardiovascular and Interventional Radiological Society of Europe and the Canadian Interventional Radiology Association. J Vasc Interv Radiol 2010;21:1632-55. [Crossref] [PubMed]
- Prinssen M, Verhoeven EL, Buth J, et al. A randomized trial comparing conventional and endovascular repair of abdominal aortic aneurysms. N Engl J Med 2004;351:1607-18. [Crossref] [PubMed]
- Nordon IM, Karthikesalingam A, Hinchliffe RJ, et al. Secondary interventions following endovascular aneurysm repair (EVAR) and the enduring value of graft surveillance. Eur J Vasc Endovasc Surg 2010;39:547-54. [Crossref] [PubMed]
- Drury D, Michaels JA, Jones L, et al. Systematic review of recent evidence for the safety and efficacy of elective endovascular repair in the management of infrarenal abdominal aortic aneurysm. Br J Surg 2005;92:937-46. [Crossref] [PubMed]
- United Kingdom EVAR Trial Investigators, Greenhalgh RM, Brown LC, et al. Endovascular repair of aortic aneurysm in patients physically ineligible for open repair. N Engl J Med 2010;362:1872-80. [Crossref] [PubMed]
- Brown LC, Greenhalgh RM, Powell JT, et al. Use of baseline factors to predict complications and reinterventions after endovascular repair of abdominal aortic aneurysm. Br J Surg 2010;97:1207-17. [Crossref] [PubMed]
- Karthikesalingam A, Holt PJ, Hinchliffe RJ, et al. Risk of reintervention after endovascular aortic aneurysm repair. Br J Surg 2010;97:657-63. [Crossref] [PubMed]
- Dake MD, Miller DC, Mitchell RS, et al. The "first generation" of endovascular stent-grafts for patients with aneurysms of the descending thoracic aorta. J Thorac Cardiovasc Surg 1998;116:689-703; discussion 703-4. [Crossref] [PubMed]
- Matsumura JS, Cambria RP, Dake MD, et al. International controlled clinical trial of thoracic endovascular aneurysm repair with the Zenith TX2 endovascular graft: 1-year results. J Vasc Surg 2008;47:247-57; discussion 257. [Crossref] [PubMed]
- Scali ST, Beck AW, Butler K, et al. Pathology-specific secondary aortic interventions after thoracic endovascular aortic repair. J Vasc Surg 2014;59:599-607. [Crossref] [PubMed]
- Szeto WY, Desai ND, Moeller P, et al. Reintervention for endograft failures after thoracic endovascular aortic repair. J Thorac Cardiovasc Surg 2013;145:S165-70. [Crossref] [PubMed]
- Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 2006;113:e463-654. [Crossref] [PubMed]
- Shah A, Stavropoulos SW. Imaging Surveillance following Endovascular Aneurysm Repair. Semin Intervent Radiol 2009;26:10-6. [Crossref] [PubMed]
- Murphy M, Hodgson R, Harris PL, et al. Plain radiographic surveillance of abdominal aortic stent-grafts: the Liverpool/Perth protocol. J Endovasc Ther 2003;10:911-2. [Crossref] [PubMed]
- Armerding MD, Rubin GD, Beaulieu CF, et al. Aortic aneurysmal disease: assessment of stent-graft treatment-CT versus conventional angiography. Radiology 2000;215:138-46. [Crossref] [PubMed]
- Ducasse E, Calisti A, Speziale F, et al. Aortoiliac stent graft infection: current problems and management. Ann Vasc Surg 2004;18:521-6. [Crossref] [PubMed]
- Picel AC, Kansal N. Essentials of endovascular abdominal aortic aneurysm repair imaging: postprocedure surveillance and complications. AJR Am J Roentgenol 2014;203:W358-72. [PubMed]
- Stolzmann P, Frauenfelder T, Pfammatter T, et al. Endoleaks after endovascular abdominal aortic aneurysm repair: detection with dual-energy dual-source CT. Radiology 2008;249:682-91. [Crossref] [PubMed]
- Hansen NJ, Kaza RK, Maturen KE, et al. Evaluation of low-dose CT angiography with model-based iterative reconstruction after endovascular aneurysm repair of a thoracic or abdominal aortic aneurysm. AJR Am J Roentgenol 2014;202:648-55. [Crossref] [PubMed]
- Schindera ST, Graca P, Patak MA, et al. Thoracoabdominal-aortoiliac multidetector-row CT angiography at 80 and 100 kVp: assessment of image quality and radiation dose. Invest Radiol 2009;44:650-5. [Crossref] [PubMed]
- Haulon S, Lions C, McFadden EP, et al. Prospective evaluation of magnetic resonance imaging after endovascular treatment of infrarenal aortic aneurysms. Eur J Vasc Endovasc Surg 2001;22:62-9. [Crossref] [PubMed]
- Habets J, Zandvoort HJ, Reitsma JB, et al. Magnetic resonance imaging is more sensitive than computed tomography angiography for the detection of endoleaks after endovascular abdominal aortic aneurysm repair: a systematic review. Eur J Vasc Endovasc Surg 2013;45:340-50. [Crossref] [PubMed]
- Resta EC, Secchi F, Giardino A, et al. Non-contrast MR imaging for detecting endoleak after abdominal endovascular aortic repair. Int J Cardiovasc Imaging 2013;29:229-35. [Crossref] [PubMed]
- Cohen EI, Weinreb DB, Siegelbaum RH, et al. Time-resolved MR angiography for the classification of endoleaks after endovascular aneurysm repair. J Magn Reson Imaging 2008;27:500-3. [Crossref] [PubMed]
- Olchowy C, Cebulski K, Łasecki M, et al. The presence of the gadolinium-based contrast agent depositions in the brain and symptoms of gadolinium neurotoxicity - A systematic review. PLoS One 2017;12:e0171704. [Crossref] [PubMed]
- McDonald RJ, McDonald JS, Kallmes DF, et al. Intracranial Gadolinium Deposition after Contrast-enhanced MR Imaging. Radiology 2015;275:772-82. [Crossref] [PubMed]
- Millen A, Canavati R, Harrison G, et al. Defining a role for contrast-enhanced ultrasound in endovascular aneurysm repair surveillance. J Vasc Surg 2013;58:18-23. [Crossref] [PubMed]
- Abraha I, Luchetta M, De Florio R, et al. Ultrasonography versus computed tomography scan for endoleak detection after endoluminal abdominal aortic aneurysm repair. Cochrane Database Syst Rev 2017. [Crossref]
- Raman KG, Missig-Carroll N, Richardson T, et al. Color-flow duplex ultrasound scan versus computed tomographic scan in the surveillance of endovascular aneurysm repair. J Vasc Surg 2003;38:645-51. [Crossref] [PubMed]
- Ashoke R, Brown LC, Rodway A, et al. Color duplex ultrasonography is insensitive for the detection of endoleak after aortic endografting: a systematic review. J Endovasc Ther 2005;12:297-305. [Crossref] [PubMed]
- Mark AS, McCarthy SM, Moss AA, et al. Detection of abdominal aortic graft infection: comparison of CT and in-labeled white blood cell scans. AJR Am J Roentgenol 1985;144:315-8. [Crossref] [PubMed]
- Johnson KK, Russ PD, Bair JH, et al. Diagnosis of synthetic vascular graft infection: comparison of CT and gallium scans. AJR Am J Roentgenol 1990;154:405-9. [Crossref] [PubMed]
- Fiorani P, Speziale F, Rizzo L, et al. Detection of aortic graft infection with leukocytes labeled with technetium 99m-hexametazime. J Vasc Surg 1993;17:87-95; discussion 95-6. [Crossref] [PubMed]
- Orton DF, LeVeen RF, Saigh JA, et al. Aortic prosthetic graft infections: radiologic manifestations and implications for management. Radiographics 2000;20:977-93. [Crossref] [PubMed]
- Hovsepian DM, Siegel BA, Kimbiris G, et al. Tc-99m sulfur colloid scintigraphy for detecting perigraft flow following endovascular aortic aneurysm repair: A feasibility study. Cardiovasc Intervent Radiol 1999;22:447-51. [Crossref] [PubMed]
- Stavropoulos SW, Itkin M, Lakhani P, et al. Detection of endoleaks after endovascular aneurysm repair with use of technetium-99m sulfur colloid and (99m)Tc-labeled red blood cell scans. J Vasc Interv Radiol 2006;17:1739-43. [Crossref] [PubMed]
- Moll FL, Powell JT, Fraedrich G, et al. Management of abdominal aortic aneurysms clinical practice guidelines of the European society for vascular surgery. Eur J Vasc Endovasc Surg 2011;41 Suppl 1:S1-58. [Crossref] [PubMed]
- Chaikof EL, Blankensteijn JD, Harris PL, et al. Reporting standards for endovascular aortic aneurysm repair. J Vasc Surg 2002;35:1048-60. [Crossref] [PubMed]
- O'Connor S, Andrew P, Batt M, et al. A systematic review and meta-analysis of treatments for aortic graft infection. J Vasc Surg 2006;44:38-45. [Crossref] [PubMed]
- Cernohorsky P, Reijnen MM, Tielliu IF, et al. The relevance of aortic endograft prosthetic infection. J Vasc Surg 2011;54:327-33. [Crossref] [PubMed]
- Liaw JV, Clark M, Gibbs R, et al. Update: Complications and management of infrarenal EVAR. Eur J Radiol 2009;71:541-51. [Crossref] [PubMed]
- Parmer SS, Carpenter JP, Stavropoulos SW, et al. Endoleaks after endovascular repair of thoracic aortic aneurysms. J Vasc Surg 2006;44:447-52. [Crossref] [PubMed]
- Faries PL, Cadot H, Agarwal G, et al. Management of endoleak after endovascular aneurysm repair: cuffs, coils, and conversion. J Vasc Surg 2003;37:1155-61. [Crossref] [PubMed]
- Tzortzis E, Hinchliffe RJ, Hopkinson BR. Adjunctive procedures for the treatment of proximal type I endoleak: the role of peri-aortic ligatures and Palmaz stenting. J Endovasc Ther 2003;10:233-9. [Crossref] [PubMed]
- Kirby L, Goodwin J. Treatment of a primary type IA endoleak with a liquid embolic system under conditions of aortic occlusion. J Vasc Surg 2003;37:456-60. [Crossref] [PubMed]
- Deaton DH, Mehta M, Kasirajan K, et al. The phase I multicenter trial (STAPLE-1) of the Aptus endovascular repair system: results at 6 months and 1 year. J Vasc Surg 2009;49:851-7; discussion 857-8. [Crossref] [PubMed]
- Bail DH, Walker T, Giehl J. Vascular endostapling systems for vascular endografts (T)EVAR--systematic review--current state. Vasc Endovascular Surg 2013;47:261-6. [Crossref] [PubMed]
- Bonvini R, Alerci M, Antonucci F, et al. Preoperative embolization of collateral side branches: a valid means to reduce type II endoleaks after endovascular AAA repair. J Endovasc Ther 2003;10:227-32. [Crossref] [PubMed]
- Stavropoulos SW, Carpenter JP, Fairman RM, et al. Inferior vena cava traversal for translumbar endoleak embolization after endovascular abdominal aortic aneurysm repair. J Vasc Interv Radiol 2003;14:1191-4. [Crossref] [PubMed]
- Alvarez-Tostado JA, Moise MA, Bena JF, et al. The brachial artery: a critical access for endovascular procedures. J Vasc Surg 2009;49:378-85; discussion 385. [Crossref] [PubMed]
- Mansueto G, Cenzi D, D'Onofrio M, et al. Treatment of type II endoleaks after endovascular repair of abdominal aortic aneurysms: transcaval approach. Cardiovasc Intervent Radiol 2005;28:641-5. [Crossref] [PubMed]
- Ellis PK, Kennedy PT, Collins AJ, et al. The use of direct thrombin injection to treat a type II endoleak following endovascular repair of abdominal aortic aneurysm. Cardiovasc Intervent Radiol 2003;26:482-4. [Crossref] [PubMed]
- Wisselink W, Cuesta MA, Berends FJ, et al. Retroperitoneal endoscopic ligation of lumbar and inferior mesenteric arteries as a treatment of persistent endoleak after endoluminal aortic aneurysm repair. J Vasc Surg 2000;31:1240-4. [Crossref] [PubMed]
- Laheij RJ, Buth J, Harris PL, et al. Need for secondary interventions after endovascular repair of abdominal aortic aneurysms. Intermediate-term follow-up results of a European collaborative registry (EUROSTAR). Br J Surg 2000;87:1666-73. [Crossref] [PubMed]
- Tonnessen BH, Sternbergh WC 3rd, Money SR. Mid- and long-term device migration after endovascular abdominal aortic aneurysm repair: a comparison of AneuRx and Zenith endografts. J Vasc Surg 2005;42:392-400; discussion 400-1. [Crossref] [PubMed]
- Deaton DH. Improving proximal fixation and seal with the HeliFx Aortic EndoAnchor. Semin Vasc Surg 2012;25:187-92. [Crossref] [PubMed]
- Ohki T, Ouriel K, Silveira PG, et al. Initial results of wireless pressure sensing forendovascular aneurysm repair: the APEX Trial--Acute Pressure Measurement to Confirm Aneurysm Sac EXclusion. J Vasc Surg 2007;45:236-42. [Crossref] [PubMed]
- Murphy EH, Szeto WY, Herdrich BJ, et al. The management of endograft infections following endovascular thoracic and abdominal aneurysm repair. J Vasc Surg 2013;58:1179-85. [Crossref] [PubMed]
- Capoccia L, Speziale F, Menna D, et al. Preliminary Results from a National Enquiry of Infection in Abdominal Aortic Endovascular Repair (Registry of Infection in EVAR--R.I.EVAR). Ann Vasc Surg 2016;30:198-204. [Crossref] [PubMed]
- Smeds MR, Duncan AA, Harlander-Locke MP, et al. Treatment and outcomes of aortic endograft infection. J Vasc Surg 2016;63:332-40. [Crossref] [PubMed]
- Vogel TR, Symons R, Flum DR. The incidence and factors associated with graft infection after aortic aneurysm repair. J Vasc Surg 2008;47:264-9. [Crossref] [PubMed]
- Sharif MA, Lee B, Lau LL, et al. Prosthetic stent graft infection after endovascular abdominal aortic aneurysm repair. J Vasc Surg 2007;46:442-8. [Crossref] [PubMed]
- EVAR trial participants. Endovascular aneurysm repair versus open repair in patients with abdominal aortic aneurysm (EVAR trial 1): randomised controlled trial. Lancet 2005;365:2179-86. [Crossref] [PubMed]
- Fransen GA, Desgranges P, Laheij RJ, et al. Frequency, predictive factors, and consequences of stent-graft kink following endovascular AAA repair. J Endovasc Ther 2003;10:913-8. [Crossref] [PubMed]
- Jonker FH, Schlosser FJ, Geirsson A, et al. Endograft collapse after thoracic endovascular aortic repair. J Endovasc Ther 2010;17:725-34. [Crossref] [PubMed]
- Tadros RO, Lipsitz EC, Chaer RA, et al. A multicenter experience of the management of collapsed thoracic endografts. J Vasc Surg 2011;53:1217-22. [Crossref] [PubMed]
- Kasirajan K, Dake MD, Lumsden A, et al. Incidence and outcomes after infolding or collapse of thoracic stent grafts. J Vasc Surg 2012;55:652-8; discussion 658. [Crossref] [PubMed]
- Sze DY, Mitchell RS, Miller DC, et al. Infolding and collapse of thoracic endoprostheses: manifestations and treatment options. J Thorac Cardiovasc Surg 2009;138:324-33. [Crossref] [PubMed]
- Bandorski D, Brück M, Günther HU, et al. Endograft collapse after endovascular treatment for thoracic aortic disease. Cardiovasc Intervent Radiol 2010;33:492-7. [Crossref] [PubMed]
- Maldonado TS, Rockman CB, Riles E, et al. Ischemic complications after endovascular abdominal aortic aneurysm repair. J Vasc Surg 2004;40:703-9; discussion 709-10. [Crossref] [PubMed]
- Setacci F, Sirignano P, De Donato G, et al. Endovascular thoracic aortic repair and risk of spinal cord ischemia: the role of previous or concomitant treatment for aortic aneurysm. J Cardiovasc Surg (Torino) 2010;51:169-76. [PubMed]
- Si Y, Fu W, Liu Z, et al. Coverage of the left subclavian artery without revascularization during thoracic endovascular repair is feasible: a prospective study. Ann Vasc Surg 2014;28:850-9. [Crossref] [PubMed]
- Klocker J, Koell A, Erlmeier M, et al. Ischemia and functional status of the left arm and quality of life after left subclavian artery coverage during stent grafting of thoracic aortic diseases. J Vasc Surg 2014;60:64-9. [Crossref] [PubMed]
- Riesenman PJ, Farber MA, Mendes RR, et al. Coverage of the left subclavian artery during thoracic endovascular aortic repair. J Vasc Surg 2007;45:90-4; discussion 94-5. [Crossref] [PubMed]
- Maldonado TS, Dexter D, Rockman CB, et al. Left subclavian artery coverage during thoracic endovascular aortic aneurysm repair does not mandate revascularization. J Vasc Surg 2013;57:116-24. [Crossref] [PubMed]
- Chang CK, Chuter TA, Niemann CU, et al. Systemic inflammation, coagulopathy,and acute renal insufficiency following endovascular thoracoabdominal aortic aneurysm repair. J Vasc Surg 2009;49:1140-6. [Crossref] [PubMed]
- Brown LC, Brown EA, Greenhalgh RM, et al. Renal function and abdominal aortic aneurysm (AAA): the impact of different management strategies on long-term renal function in the UK EndoVascular Aneurysm Repair (EVAR) Trials. Ann Surg 2010;251:966-75. [Crossref] [PubMed]
- Becquemin JP, Majewski M, Fermani N, et al. Colon ischemia following abdominal aortic aneurysm repair in the era of endovascular abdominal aortic repair. J Vasc Surg 2008;47:258-63; discussion 263. [Crossref] [PubMed]
- Miller A, Marotta M, Scordi-Bello I, et al. Ischemic colitis after endovascular aortoiliac aneurysm repair: a 10-year retrospective study. Arch Surg 2009;144:900-3. [Crossref] [PubMed]
- Angiletta D, Marinazzo D, Guido G, et al. Spinal cord, bowel, and buttock ischemia after endovascular aneurysm repair. Ann Vasc Surg 2011;25:980.e15-9. [Crossref] [PubMed]
- Freyrie A, Testi G, Gargiulo M, et al. Spinal cord ischemia after endovascular treatment of infrarenal aortic aneurysm. Case report and literature review. J Cardiovasc Surg (Torino) 2011;52:731-4. [PubMed]
- Buth J, Harris PL, Hobo R, et al. Neurologic complications associated with endovascular repair of thoracic aortic pathology: Incidence and risk factors. a study from the European Collaborators on Stent/Graft Techniques for Aortic Aneurysm Repair (EUROSTAR) registry. J Vasc Surg 2007;46:1103-10; discussion 1110-1. [Crossref] [PubMed]
- Gutsche JT, Cheung AT, McGarvey ML, et al. Risk factors for perioperative stroke after thoracic endovascular aortic repair. Ann Thorac Surg 2007;84:1195-200; discussion 1200. [Crossref] [PubMed]
- Moulakakis KG, Dalainas I, Mylonas S, et al. Conversion to open repair after endografting for abdominal aortic aneurysm: a review of causes, incidence, results, and surgical techniques of reconstruction. J Endovasc Ther 2010;17:694-702. [Crossref] [PubMed]
- Gabriel EA, Locali RF, Romano CC, et al. Analysis of the inflammatory response in endovascular treatment of aortic aneurysms. Eur J Cardiothorac Surg 2007;31:406-12. [Crossref] [PubMed]
- Eggebrecht H, Mehta RH, Metozounve H, et al. Clinical implications of systemic inflammatory response syndrome following thoracic aortic stent-graft placement. J Endovasc Ther 2008;15:135-43. [Crossref] [PubMed]
- Lyden SP, McNamara JM, Sternbach Y, et al. Technical considerations for late removal of aortic endografts. J Vasc Surg 2002;36:674-8. [Crossref] [PubMed]
- Conner MS 3rd, Sternbergh WC 3rd, Carter G, et al. Secondary procedures after endovascular aortic aneurysm repair. J Vasc Surg 2002;36:992-6. [Crossref] [PubMed]


