Three-dimensional speckle-tracking echocardiography: benefits and limitations of integrating myocardial mechanics with three-dimensional imaging
Introduction
Over the past years, deformation imaging using two-dimensional speckle-tracking echocardiography (2DSTE) has emerged as a powerful technique for myocardial function quantification. Evidence supporting the role of 2DSTE-derived strain in daily clinical practice has quickly accumulated in a number of clinical scenarios (1-6). Moreover, efforts to establish reference values for 2D strain parameters have been increasingly done (7,8), allowing to establish robust abnormality thresholds and to incorporate them in recent guidelines (9).
However, because the left ventricular (LV) myofibers have a complex spatial orientation (10,11) and contract simultaneously in different directions, the LV mechanics is in nature a 3D phenomenon and its accurate assessment requires a 3D imaging method. In recent years, three-dimensional speckle-tracking (3DSTE) has been implemented for measuring 3D strain, and has emerged as a more physiologically sound tool for analyzing the complexity of LV mechanics, overcoming the inherent limitations of 2DSTE. Due to the superiority provided by the addition of the third dimension for myocardial deformation analysis (e.g., no through-plane motion of speckles, ability of tracking the speckles in two directions simultaneously for area strain quantification, etc.), 3DSTE has the potential to become the gold-standard technique for assessing LV systolic function by echocardiography in the near future. The present review aims to summarize the technical principles of 3DSTE, its advantages and limitations with respect to 2DSTE, and provide a framework for clinical applications and future research on this promising technology.
The road from 2D to 3D strain—an ongoing journey
2DSTE is based on the presence of distinctive patterns of gray scale values within the ultrasound images of myocardial tissue, commonly referred to as “speckles”. Speckles within a spatial unit (kernel) are arranged in distinct patterns which depend on the acoustic characteristics of the underlying myocardial tissue and are unique for each kernel within the ultrasound image (12), serving as a “fingerprint” that can be tracked frame-by-frame during the cardiac cycle by the 2DSTE software algorithm (1,13). 2DSTE relies on the assumption that speckles are moving within the scan plane of the 2D image in the consecutive frames of the cardiac cycle. However, since LV deformation involves a combination of apex-to-base shortening and thickening with simultaneous twisting (10,11), speckles have a complex motion in the 3D space, and thus are subject to through-plane motion from the scan planes of the 2D tomographic images during the cardiac cycle.
Recent developments of ultrasound transducer technology have allowed the possibility of 3D imaging of the LV almost real-time with satisfactory image and temporal resolution, thus making 3D strain measurement feasible for both research and potential future clinical purposes (14). The analysis sequence of the 3D data set begins with the automatic generation of a region of interest (ROI) from an endocardial and an epicardial mesh (used also for 3D LV volume and mass computation), followed by the automated segmentation of the LV into a 17-segment model. Each ROI contains cubes with specific 3D patterns of natural acoustic markers that are matched and searched through the cardiac cycle by the 3DSTE algorithm, a process called “block matching” (Figure 1). The 3DSTE algorithm calculates the quality of each match and identifies any outliers, removing them before performing the weighted spatial averaging of the results. Next, the results are mapped to an average myocardial mesh, so that the shape of the mesh model of the LV can be updated for all frames. Finally, quantitative results of LV deformation are derived from this mesh model (15) ( Figure 1) .
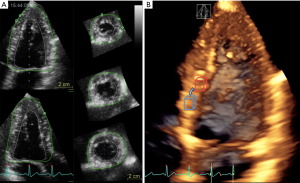
Because the blocks are tracked in a 3D volume, they can be followed in any direction and out-of-plane motion of the speckles does not occur with 3DSTE. Furthermore, 3DSTE is a time saving technique, as it allows the calculation of all 3D strain parameters of the LV from a single volumetric data set and avoids errors caused by heart rate variability that may occur when multiple acquisitions are needed as with 2DSTE (16,17). Table 1 summarizes the main differences between 3D and 2D STE.
Full table
Fundamentals on LV myocardial fibers and 3D mechanics
Knowledge of the anatomical structure and 3D mechanics of the LV myocardium is key to understand and interpret 3D strain in clinical practice. Briefly, LV myocardial fibers are oriented in a right-handed helix in the subendocardium and a left-handed helix in the subepicardium, with circumferential fibers lying between the two. This complex anatomical structure of the myocardium explains the various patterns of myocardial deformation. According to a simplified model of myocardial mechanics, during systole the LV shortens (longitudinal and circumferential dimension) and twists along its long axis, while its wall thickens (radial dimension). Strain is a measure of myocardial deformation of a segment in relation to its original dimension and it is expressed as a percentage. Thus, negative strain refers to the decrease in length (e.g., shortening-longitudinal and circumferential deformation), and positive strain to the increase in length (e.g., thickening-radial deformation) compared to initial length (Figure 2). Strain-rate is a measure of the rate (velocity) at which myocardial deformation occurs and it is expressed in seconds-1. Furthermore, the LV is characterized also by twist mechanics. Since mechanical activation occurs first in the right-handed helix, the base and apex rotate first in an anticlockwise direction during isovolumic contraction (19), followed by further anti-clockwise movement of the apex during ejection phase and clockwise rotation of the base due to the activation of the subepicardial left-handed helix (10,11). During isovolumic relaxation, the cardiac apex untwists by a clockwise rotation, generating active intraventricular suction forces that promote LV rapid filling (20). LV twist mechanics can be quantified using rotation, twist and torsion. Rotation refers to the angle of deformation measured on a short-axis LV plane and is expressed in degrees. Positive values represent anticlockwise rotation (as viewed from the apex), while negative values are used for clockwise rotation. Twist refers to the absolute difference of rotation between base and apex, and torsion is calculated by dividing LV twist by the length of the LV to allow comparability of LV twist in ventricles of different sizes.
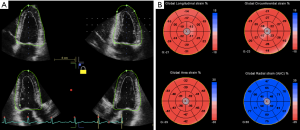
Using 2DSTE, all these deformation parameters of the LV can be quantified, however one needs to acquire multiple views of the LV from different approaches, at different times with no precise landmarks for ensuring their proper position and orientation. Therefore, the use of 3D strain is desirable, as it can avoid all these limitations in a more time-efficient manner (15).
Using 3DSTE analysis, area strain, a novel deformation parameter can be quantified that reflects the relative area change that combines the effect of both longitudinal and circumferential shortening. Since myocardium is uncompressible and according to the law of conservation of volume, thickening in the radial direction is necessary to accommodate this change. Area strain is thus inversely related to radial strain and is used to derive its value in commercially available systems. Area strain is calculated by measuring the segmental area at end diastole (Aed) and at end systole (Aes) using the formula Aes-Aed/Aed ×100 and thus normally has negative values. Since it results from the combination of longitudinal and circumferential strain, it can be regarded as an integrative parameter of deformation (21,22) making it attractive to study LV subclinical dysfunction. The commercially available echocardiographic equipments provide different software solutions, definitions and nomenclature for the analysis integrating myocardial deformation in multiple directions, emphasizing the current need for standardization in this area.
Validation
Validation of any strain imaging method and its application into clinical setting requires a complex process made of four steps: validation on synthetic data sets, in vitro and in vivo experiments, and finally a validation step into clinical practice.
Synthetic ultrasound images can be simulated using specific software packages. From this point of view, block matching-based (14) and elastic registration-based (23,24) 3DSTE approaches have been shown to perform well in these simulated models. These approaches provide reliable deformation curves throughout the cardiac cycle in the longitudinal, circumferential, and radial direction.
3DSTE algorithms have been validated also with phantoms and sonomicrometry in several animal models (21,25-27). Moderate to good correlations (r=0.49–0.91) with sonomicrometry data have been found for segmental strain components at baseline, during pharmacological stress, and with induction of myocardial ischemia by coronary artery occlusion in animal models (26). Among the different components of myocardial deformation, the radial strain showed the worst correlation and agreement with sonomicrometry for all the tested approaches. In order to overcome this shortcoming, a solution provided by some vendors was to compute radial strain from other strain components or from the endocardial surface area strain, which is more reliably measured. Area strain was also validated in similar experimental studies (21), and its measurement by 3DSTE was found to strongly correlate with that by sonomicrometry (r=0.87).
Image artifacts, often encountered in clinical practice, are usually absent in simulated models. Given the high susceptibility of 3DSTE to image quality issues, its application to real life patients can be challenging. When analyzing clinical validation studies one should keep in mind that there is no true gold standard method to measure myocardial deformation and comparisons can only be done with one of the currently established methods to assess LV myocardial function [e.g., tissue-Doppler imaging, 2DSTE, cardiac magnetic resonance (CMR) tagging and feature tracking]. These comparisons show the comparability of strain values among different imaging modalities and techniques, rather than indicating if one method is more accurate than the other (28). In clinical studies, 3D and 2D global longitudinal strain have shown close correlations (r=0.72–0.91), however segmental strain values have only shown moderate correlations (r=0.43–0.49) for longitudinal and circumferential values and low for radial strain (r=0.24) (16,29,30). 3D global circumferential strain has also shown good correlation with CMR tagging (31) and CMR feature tracking (32). However, it should be emphasized that strain values among various imaging methods are not interchangeable.
Reproducibility
Along with accuracy determined by comparisons with an independent reference technique, reproducibility represented by the variability of measurements repeated by the same operator (intraobserver variability), different operators (interobserver variability), and also by using different equipment and analysis techniques (intervendor variability), is an important factor in determining the clinical relevance and reliability of any diagnostic technique (33).
Reproducibility of 3DSTE has been reported as acceptable to excellent in several studies (34). Intraobserver variability ranges from 1% to 13%, while interobserver variability ranges from 2% to 14%. As with 2DSTE, variability of 3D radial strain values is higher than for 3D longitudinal or circumferential strain, and is also higher for segmental versus global strain values. Area strain, unique to 3DSTE, appears to have the best reproducibility among all 3D strain parameters (16). Compared to 2DSTE, the reproducibility of 3DSTE is equivalent or superior, particularly for radial and circumferential strain (16).
Three sources of temporal (test-retest) variability for 3D strain values should be taken into account: acquisition, post processing, and the hemodynamic status of the patient (e.g., blood pressure). The issue of the temporal resolution of LV data set is very important for the clinical application of 3DSTE, since temporal resolution is independently and inversely related with 3D strain magnitude (18). However, the most important aspect is probably to achieve the optimal trade-off between temporal and spatial resolution, since higher frame rates may lead to some compromise in spatial resolution because of the sacrifice in line density. This finding has important practical implications, since in addition to the specific equipment used for measuring the LV deformation, also the data set temporal resolution from which the 3D strain data is extracted must be taken into account when comparing sequential 3D strain measurements in longitudinal studies.
The biggest concern regarding the reproducibility of 3DSTE is related to vendor dependency. Since post-processing steps can differ among vendors, 3DSTE-derived strain parameters currently exhibit a wide variation in the measured values of 3D strain. Consequently, it remains difficult to compare strain values among several commercial systems, as shown by different studies (33,35,36). Reference values for each vendor and software should be used, and in longitudinal studies all clinicians should obtain the baseline and follow-up acquisitions and analyses from the same hardware and software equipment.
Feasibility
The accuracy of 3DSTE largely depends on optimal image quality, with sufficient frame rate, which requires a sufficient amount of dedicated training and skill. These basic technical requirements may dramatically limit the number of patients in whom 3DSTE is feasible in clinical practice (28).
Currently, the feasibility of 3DSTE in everyday practice is lower than the feasibility of 2DSTE. After excluding patients with irregular rhythm and unable to breathhold, the reported feasibility of 3DSTE in different studies ranges from 63% to 83% (16,29), being lower than for 2DSTE (80–97%) (37,38). Recently, we reported a feasibility of 3DGLS of 90% in a large cohort of healthy subjects (18), which was lower than the feasibility of 2DGLS (95%). As reported in other studies (23,30,39), LV basal segments are the most difficult to track by the 3DSTE software, because of their active excursion and their position in the far field, which adversely affects the spatial resolution of speckles.
Normal values
Despite a growing interest for 3DSTE and an increasing number of studies on pathologic conditions, reference values had only been assessed in small studies and solid normative data was lacking until recently, precluding its widespread use in clinical practice (17,18,34,40,41). Normative values of 2D strain cannot be used to interpret 3D strain results, as the results of 2DSTE and 3DSTE measurements differ considerably among them. This discrepancy has been attributed to the use of different algorithms in software packages for 2DSTE and 3DSTE, and the fact that 3D strain takes into account the complex 3D cardiac motion and twisting of the LV during systole (18,34,41,42), making it necessary to define method-specific reference values for LV strain measurements.
Recently, reference values for 3D strain parameters have been reported in four studies (18,40,41,43). Suggested reference values and the main characteristics of the study are summarized in Table 2.

Full table
It is worth noting that all normative studies report age and gender relationships of LV 3D strain. In general, women were found to have higher strain magnitudes than men, except for 3D circumferential strain, and young having higher magnitudes of 3D longitudinal strain than elderly groups (18,40,41). Age-related variations of 3D strain have also been recently confirmed in the pediatric population in a large group of normal children (44).
Among all 3D strain components, 3D radial strain shows the widest reference ranges, with the largest absolute values and with inhomogeneity across segments. The difficulty in estimating radial strain, as previously explained, is not unique to 3DSTE (45), and is likely related to the fact that radial strain must be calculated over a relatively small region due to the limited wall thickness, in combination with limited spatial resolution in the radial direction.
Reference values differ among studies due to the use of different scanners and software algorithms to compute 3D strain. This aspect confirms the suboptimal intervendor agreement for strain measurement and the resulting need of development of specific reference values of the strain components for each ultrasound system (35).
Clinical applications
According to the most recent literature, 3DSTE popularity is increasing and research studies continue to explore its clinical added value over conventional 2DSTE. In the following section, the current evidence supporting potential clinical applications of 3DSTE will be summarized, yet it is likely that they will continue to expand as more research is carried out.
Coronary artery disease
Assessment of regional wall motion continues to be an issue in the clinical setting, as a considerable amount of training is needed and interreader differences continue to exist in spite of good image quality (46). Area strain derived by 3DSTE, as an objective measure of regional wall motion abnormalities (WMA), has been shown to be accurate and reproducible when compared with the visual wall motion assessment by experienced echocardiographers (47). However, despite a good agreement between area strain values and expert wall motion score evaluation to discriminate normal and akinetic segments, there was a substantially lower agreement for hypokinetic segments, as expected. This fact may be attributable to the subjectivity in wall motion score evaluation or to the possibility that 3D area strain is a more sensitive index of regional ischemia than regional WMA (47). Because 3D area strain quantification is semi-automated, more objective and reproducible, and requires less training, it emerges as an attractive tool to identify regional WMA in patients with suspected or known coronary artery disease.
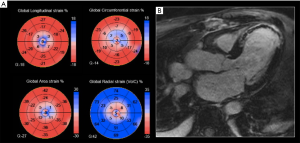
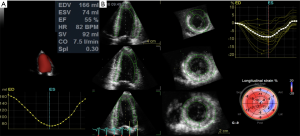
While LV ejection fraction (LVEF) is well known as an important prognostic factor in patients who survive a myocardial infarction (MI), myocardial strain, assessed by STE, has been found to further improve prognostic risk stratification (4). Recent data has shown that both 2DSTE and 3DSTE can be helpful in predicting LV remodeling and in stratifying risk after acute MI (48-50). The evaluation of 3D global longitudinal strain enabled to identify patients with massive MI size (>12% of the LV mass) by single photon emission computed tomography (SPECT) (51). This concept is further supported by comparisons with late gadolinium enhancement CMR, in which 3D global longitudinal strain and area strain have been found to closely correlate with infarct size (29,49) (Figure 3). 3D area strain has been found to be independently associated with increased risk of death or heart failure after acute MI, suggesting that it can be a useful prognostic parameter in this setting (48). 3D area strain was also the most accurate among the various 3D strain parameters for predicting myocardial viability identified by SPECT/PET imaging (52).
Valvular heart disease
3DSTE-derived strain may be useful to refine the assessment of cardiac performance, which is of outmost importance in patients with valvular heart disease, as the detection of subclinical LV myocardial dysfunction may aid in deciding the timing of surgical or interventional treatment. However, only a few published studies limited to left-sided valve diseases have reported on the added value of 3DSTE.
Aortic valve diseases
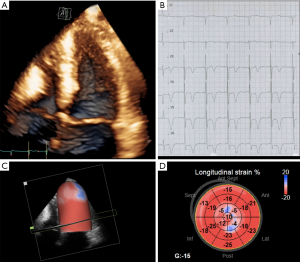
In aortic stenosis (AS), survival drops significantly after symptom onset (53). Thus, 2D longitudinal strain has been proposed as an early marker of LV dysfunction in asymptomatic patients with severe AS, in order to optimize the timing of intervention in these patients. LV strain parameters measured by 3DSTE, particularly global longitudinal strain (54), were shown to be useful indices of early-stage LV myocardial dysfunction in patients with AS. Recently, Nagata et al. studied 104 asymptomatic patients with severe AS and preserved LVEF, and reported that LV global longitudinal strain using either 2DSTE or 3DSTE was associated with increased risk of adverse events. However, after correcting for mean gradient and LV mass index at multivariable analysis, 3D longitudinal strain was found to be the only independent predictor of future adverse events, with an optimal cut-off value of −14.5% (55) (Figure 4).
In asymptomatic patients with moderate-severe aortic regurgitation, it was found that 3D global circumferential strain compensates for the reduction in 3D longitudinal strain for preserving the LVEF (56).
Further supporting the clinical use of 3DSTE, 3D strain was reported to be an independent predictor of acute and long-term clinical outcomes after cardiac surgery (57). 3DSTE was feasible, reproducible and time saving for assessing LV systolic function in patients before and after transcatheter aortic valve implantation (58).
Mitral valve diseases
In severe chronic mitral regurgitation (MR), guidelines recommend intervening on symptomatic patients or when LVEF drops (59,60). However, in patients with severe MR and LVEF still within normal range, subclinical LV dysfunction may go unrecognized (61,62). It was demonstrated that 2DSTE-derived LV global longitudinal strain may be useful to identify a maladaptive preload-related change associated with the risk for a substantial reduction in LVEF immediately following mitral valve repair (6,63,64). At present, few studies (62,65,66) have looked at the role of 3DSTE in patients with MR. In patients with MR with preserved LVEF, 3D area strain was shown to be an independent predictor of early symptoms or LV dysfunction (62). Preliminary results in patients with functional MR undergoing MitraClip procedure showed that 3DSTE could predict an unfavorable outcome and persistence of symptoms after the procedure (66).
Cardiac resynchronization therapy (CRT)
Despite the discouraging results of the PROSPECT trial (67), interest has built in the use of advanced echocardiographic imaging to select patients that would benefit from CRT. The systolic dyssynchrony index (SDI) derived from segmental 3D volume curves of all 16/17 segments of the LV was shown to be useful for this purpose (68). 3DSTE offers the advantage to directly assess and quantify myocardial deformation in individual LV segments, rather than the segmental volume changes provided by 3D echocardiography. By building 3DSTE time-strain curves of the 16/17 LV segments, quantitative information on both the timing and the magnitude of peak segmental strain can be obtained, which may improve prediction of response to CRT (69-71). The sites of latest mechanical activation of LV can also be identified, suggesting that 3DSTE might be useful to guide lead positioning in CRT patients (70).
Other emerging clinical applications
Due to the currently limited data on the role of 3DSTE in other clinical scenarios, 3D strain constitutes an attractive topic for ongoing research. In the following section, additional cardiac diseases will be addressed with the emerging evidence supporting a role for 3DSTE.
Hypertrophic cardiomyopathy
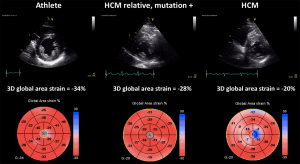
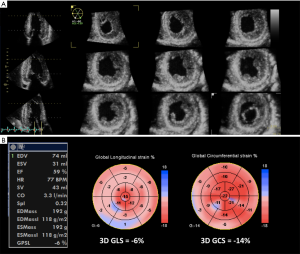
3DSTE has provided insights into LV mechanics, showing evidence of increased twist, preserved circumferential and decreased longitudinal strain in the setting of hypertrophic cardiomyopathy (72,73). Due to its ability to estimate separately LV 3D strain in different directions in a practical and clinically-feasible manner, 3DSTE could allow the early detection of myocardial function impairment, before a reduction in LVEF occurs. 3DSTE could help to differentiate between early hypertrophic cardiomyopathy and adaptative changes due to athletic training (Figure 5). Furthermore, as both hypertrophic cardiomyopathy and amyloidosis may present with increased LV wall thickness by conventional echocardiography, 3D strain might be used to identify characteristic strain patters to support in the differential diagnosis (Figure 6) (74). Since 3D echocardiography allows to avoid foreshortening of LV apex, 3D strain can also be useful to confirm the apical form of hypertrophic cardiomyopathy, by showing a decreased magnitude of myocardial deformation in the apical segments (Figure 7).
Cancer-related cardiac toxicity
Regarding the early detection and prediction of the development of cancer therapy-related cardiac dysfunction, a clear superiority of 3DSTE over 2DSTE has not yet been yet established. 3DSTE avoids the errors derived from the use of multiple 2D apical images of the LV to compute global longitudinal strain. Moreover, the clinical implementation of 3DSTE would allow to save time, since both LVEF and all strain components can be obtained from a single data set of the LV. Preliminary results have shown that 3DSTE is superior to both biomarkers and LVEF in predicting the development of cardiac toxicity, however further outcome studies are needed (75).
In cancer patients, feasibility of 3DSTE has been shown to be lower (66–78%), which might be related to the challenging acoustic windows due to chest radiation, mastectomy and breast prosthesis surgery (76).
Right ventricular (RV) function assessment
The quantitative analysis of RV function using conventional parameters and 2DSTE has been increasingly recognized to hold prognostic importance in a number of cardiac diseases (77-81). However, 2D methods are limited in their ability to quantify the RV function due to the complex anatomy of this chamber. Therefore, 2DSTE analysis is routinely applied on RV inflow to quantify the RV longitudinal strain only. The volumetric acquisition inherent to 3D echocardiography may allow to assess the RV volume and the true global RV systolic function independent of any geometric assumptions, as well as to evaluate the importance of radial/transversal mechanics or area strain for the overall RV performance. RV strain using 3DSTE has been shown to be feasible (82). Results from a single study support the prognostic usefulness of 3DSTE of the RV (using a vendor-specific software designed for the LV) to predict mortality among patients with pulmonary hypertension, although the superiority of 3DSTE over 2DSTE has not yet been proved (83). Recently, a software specifically designed for the assessment of RV 3DSTE has been validated (84). Future research is strongly needed to explore the potential benefits of using 3DSTE for RV functional analysis.
Limitations
Despite being around for more than 7 years, 3DSTE is still a research tool and not yet fully validated for clinical use. In addition, several limitations have not allowed its wide use in daily clinical practice.
A major disadvantage of 3DSTE is its reliance on good acoustic window and quality data sets, and on patient cooperation for breathholding, limiting its feasibility in a significant proportion of routine patients. The use of 3DSTE also requires an adequate temporal resolution to ensure the presence of recognizable natural acoustic markers. It has been shown that the optimal frame rate for 3DSTE is between 35–50 vps, and that 3D strain analysis performed at frame rates below 18 vps would lead to significant underestimation of strain magnitude (30,76,85). Thus, 3DSTE requires the use of multi-beat 3D acquisitions of the LV, limiting its use in patients with irregular rhythms. The current temporal resolution of 3D multi-beat data sets is insufficient to enable the evaluation of LV diastolic function. Recent advancements in 3D technology enable a single-beat full volume acquisition in real-time with satisfactory frame rate without stitching, making it easier to analyze patients with arrhythmias or unable to breathhold. However, the high frame rate single-beat capability often comes at the expense of a lower spatial resolution of 3D images than achievable by the routine multi-beat stitched 3D full-volume and is currently offered by a single vendor.
Other limitations pertain to individual 3DSTE software solutions commercially available and include: (I) the impossibility to manually re-adjust the ROI position after tracking, for optimizing the strain assessment in LV basal segments; (II) no automatic validation of speckle-tracking quality; (III) no option to exclude the poorly tracked segments from the computation of global strain values by the operator; (IV) no clear discrimination between poor tracking quality and poor function in abnormal segments, etc.
The large variability of strain measurements, algorithms and definitions used, and normative values among vendors is a major barrier against a more widespread use of 3DSTE. The EACVI/ASE/Industry Task Force to standardize deformation imaging (86) will continue its efforts by addressing the 3DSTE, and this will allow to solve the inconsistencies in definition and the variability of 3D strain measurements among the different commercially-available 3DSTE software algorithms. Finally, it is mandatory that educational initiatives should include practical courses focusing on 3D acquisition and 3DSTE analysis and interpretation, for a more uniform application of 3DSTE for clinical and research purposes.
Future research directions
The ultimate goal of implementing 3D strain in large-scale clinical studies is to assess its incremental prognostic value for predicting mortality and adverse events, and for arrhythmic risk stratification in comparison with LVEF and 2D longitudinal strain.
One of the greatest advantages of 3DSTE is that, after the semi-automatic definition of the ROI, the software is capable to derive simultaneously all strain components together with accurate measurements of LV geometry and displacement from the same 3D mesh (volume, mass, global and regional shape, regional wall thickness, mechanical dyssynchrony, etc.). This unique feature could enable to identify characteristic echocardiographic patterns for various conditions sharing similar phenotypes (athlete’s heart versus hypertrophic cardiomyopathy; hypertensive heart disease versus amyloidosis; apical hypertrophic cardiomyopathy versus other apical pathologies; ischemic regional wall hypokinesia versus non-ischemic abnormal regional motion, etc.) (Figures 5,7) based on complex multi-parametric computations from 3D volumetric data of the LV (“big data”).
Moreover, 3DSTE will allow to clarify the clinical added value of assessing strain in multiple directions rather than rely mainly on LV longitudinal function, as currently done with 2D strain. Emerging fields such as integration of 3D myocardial mechanics with blood flow patterns would supplement the imaging assessment of cardiac function in heart failure patients.
Finally, the development of commercially-available 3DSTE algorithms dedicated for the RV (84) would allow to obtain a true global measure of RV systolic performance, with less temporal variability and load-dependency than ejection fraction.
Conclusions
3DSTE is an advanced imaging technique for estimating myocardial deformation that holds significant promise to improve the accuracy and the reproducibility of LV function analysis by echocardiography, as well as to reduce the subjectivity in the visual interpretation of regional wall motion. However, further clinical testing and robust evidence with this technique are needed in order to optimize its clinical use and to identify its benefits in comparison with the established methods. The upcoming EACVI/ASE initiative for intervendor standardization focusing on 3D strain will allow to improve the current variability of 3D strain measurements among the different commercially-available 3DSTE software algorithms.
Acknowledgements
None.
Footnote
Conflicts of Interest: Drs. Muraru and Badano have received research equipment and speakers’ fee from GE Healthcare.
References
- Geyer H, Caracciolo G, Abe H, et al. Assessment of myocardial mechanics using speckle tracking echocardiography: fundamentals and clinical applications. J Am Soc Echocardiogr 2010;23:351-69. [Crossref] [PubMed]
- Smiseth OA, Torp H, Opdahl A, et al. Myocardial strain imaging: how useful is it in clinical decision making? Eur Heart J 2016;37:1196-207. [Crossref] [PubMed]
- Gorcsan J 3rd, Tanaka H.. Echocardiographic assessment of myocardial strain. J Am Coll Cardiol 2011;58:1401-13. [Crossref] [PubMed]
- Ersbøll M, Valeur N, Mogensen UM, et al. Prediction of all-cause mortality and heart failure admissions from global left ventricular longitudinal strain in patients with acute myocardial infarction and preserved left ventricular ejection fraction. J Am Coll Cardiol 2013;61:2365-73. [Crossref] [PubMed]
- Ternacle J, Berry M, Alonso E, et al. Incremental value of global longitudinal strain for predicting early outcome after cardiac surgery. Eur Heart J Cardiovasc Imaging 2013;14:77-84. [Crossref] [PubMed]
- Witkowski TG, Thomas JD, Debonnaire PJ, et al. Global longitudinal strain predicts left ventricular dysfunction after mitral valve repair. Eur Heart J Cardiovasc Imaging 2013;14:69-76. [Crossref] [PubMed]
- Kocabay G, Muraru D, Peluso D, et al. Normal left ventricular mechanics by two-dimensional speckle-tracking echocardiography. Reference values in healthy adults. Rev Esp Cardiol (Engl Ed) 2014;67:651-8. [Crossref] [PubMed]
- Yingchoncharoen T, Agarwal S, Popovi ZB, et al. Normal ranges of left ventricular strain: a meta-analysis. J Am Soc Echocardiogr 2013;26:185-91. [Crossref] [PubMed]
- Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16:233-70. [Crossref] [PubMed]
- Buckberg G, Hoffman JI, Mahajan A, et al. Cardiac mechanics revisited: the relationship of cardiac architecture to ventricular function. Circulation 2008;118:2571-87. [Crossref] [PubMed]
- Sengupta PP, Krishnamoorthy VK, Korinek J, et al. Left ventricular form and function revisited: applied translational science to cardiovascular ultrasound imaging. J Am Soc Echocardiogr 2007;20:539-51. [Crossref] [PubMed]
- Mondillo S, Galderisi M, Mele D, et al. Speckle-tracking echocardiography: a new technique for assessing myocardial function. J Ultrasound Med 2011;30:71-83. [Crossref] [PubMed]
- Blessberger H, Binder T.. NON-invasive imaging: Two dimensional speckle tracking echocardiography: basic principles. Heart 2010;96:716-22. [Crossref] [PubMed]
- Crosby J, Amundsen BH, Hergum T, et al. 3-D speckle tracking for assessment of regional left ventricular function. Ultrasound Med Biol 2009;35:458-71. [Crossref] [PubMed]
- Heimdal A. 4D Strain: Advanced research application for quantitative echocardiography. GE Healthcare White Paper 2011.
- Reant P, Barbot L, Touche C, et al. Evaluation of global left ventricular systolic function using three-dimensional echocardiography speckle-tracking strain parameters. J Am Soc Echocardiogr 2012;25:68-79. [Crossref] [PubMed]
- Pérez de Isla L, Balcones DV, Fernández-Golfín C, et al. Three-dimensional-wall motion tracking: a new and faster tool for myocardial strain assessment: comparison with two-dimensional-wall motion tracking. J Am Soc Echocardiogr 2009;22:325-30. [Crossref] [PubMed]
- Muraru D, Cucchini U, Mihaila S, et al. Left ventricular myocardial strain by three-dimensional speckle-tracking echocardiography in healthy subjects: reference values and analysis of their physiologic and technical determinants. J Am Soc Echocardiogr 2014;27:858-71.e1. [Crossref] [PubMed]
- Lorenz CH, Pastorek JS, Bundy JM. Delineation of normal human left ventricular twist throughout systole by tagged cine magnetic resonance imaging. J Cardiovasc Magn Reson 2000;2:97-108. [Crossref] [PubMed]
- Burns AT, La Gerche A, Prior DL, et al. Left ventricular untwisting is an important determinant of early diastolic function. JACC Cardiovasc Imaging 2009;2:709-16. [Crossref] [PubMed]
- Seo Y, Ishizu T, Enomoto Y, et al. Endocardial surface area tracking for assessment of regional LV wall deformation with 3D speckle tracking imaging. JACC Cardiovasc Imaging 2011;4:358-65. [Crossref] [PubMed]
- Li SN, Wong SJ, Cheung YF. Novel area strain based on three-dimensional wall motion analysis for assessment of global left ventricular performance after repair of tetralogy of Fallot. J Am Soc Echocardiogr 2011;24:819-25. [Crossref] [PubMed]
- Elen A, Choi HF, Loeckx D, et al. Three-dimensional cardiac strain estimation using spatio-temporal elastic registration of ultrasound images: a feasibility study. IEEE Trans Med Imaging 2008;27:1580-91. [Crossref] [PubMed]
- De Craene M, Piella G, Camara O, et al. Temporal diffeomorphic free-form deformation: application to motion and strain estimation from 3D echocardiography. Med Image Anal 2012;16:427-50. [Crossref] [PubMed]
- Hjertaas JJ, Fosså H, Dybdahl GL, et al. Accuracy of real-time single- and multi-beat 3-d speckle tracking echocardiography in vitro. Ultrasound Med Biol 2013;39:1006-14. [Crossref] [PubMed]
- Seo Y, Ishizu T, Enomoto Y, et al. Validation of 3-dimensional speckle tracking imaging to quantify regional myocardial deformation. Circ Cardiovasc Imaging 2009;2:451-9. [Crossref] [PubMed]
- Heyde B, Cygan S, Choi HF, et al. Regional cardiac motion and strain estimation in three-dimensional echocardiography: a validation study in thick-walled univentricular phantoms. IEEE Trans Ultrason Ferroelectr Freq Control 2012;59:668-82. [Crossref] [PubMed]
- Jasaityte R, Heyde B, D'hooge J. Current state of three-dimensional myocardial strain estimation using echocardiography. J Am Soc Echocardiogr 2013;26:15-28. [Crossref] [PubMed]
- Hayat D, Kloeckner M, Nahum J, et al. Comparison of real-time three-dimensional speckle tracking to magnetic resonance imaging in patients with coronary heart disease. Am J Cardiol 2012;109:180-6. [Crossref] [PubMed]
- Negishi K, Negishi T, Agler DA, et al. Role of temporal resolution in selection of the appropriate strain technique for evaluation of subclinical myocardial dysfunction. Echocardiography 2012;29:334-9. [Crossref] [PubMed]
- Kleijn SA, Brouwer WP, Aly MF, et al. Comparison between three-dimensional speckle-tracking echocardiography and cardiac magnetic resonance imaging for quantification of left ventricular volumes and function. Eur Heart J Cardiovasc Imaging 2012;13:834-9. [Crossref] [PubMed]
- Obokata M, Nagata Y, Wu VC, et al. Direct comparison of cardiac magnetic resonance feature tracking and 2D/3D echocardiography speckle tracking for evaluation of global left ventricular strain. Eur Heart J Cardiovasc Imaging 2016;17:525-32. [Crossref] [PubMed]
- Gayat E, Ahmad H, Weinert L, et al. Reproducibility and inter-vendor variability of left ventricular deformation measurements by three-dimensional speckle-tracking echocardiography. J Am Soc Echocardiogr 2011;24:878-85. [Crossref] [PubMed]
- Saito K, Okura H, Watanabe N, et al. Comprehensive evaluation of left ventricular strain using speckle tracking echocardiography in normal adults: comparison of three-dimensional and two-dimensional approaches. J Am Soc Echocardiogr 2009;22:1025-30. [Crossref] [PubMed]
- Badano LP, Cucchini U, Muraru D, et al. Use of three-dimensional speckle tracking to assess left ventricular myocardial mechanics: inter-vendor consistency and reproducibility of strain measurements. Eur Heart J Cardiovasc Imaging 2013;14:285-93. [Crossref] [PubMed]
- Yuda S, Sato Y, Abe K, et al. Inter-vendor variability of left ventricular volumes and strains determined by three-dimensional speckle tracking echocardiography. Echocardiography 2014;31:597-604. [Crossref] [PubMed]
- Marwick TH, Leano RL, Brown J, et al. Myocardial strain measurement with 2-dimensional speckle-tracking echocardiography: definition of normal range. JACC Cardiovasc Imaging 2009;2:80-4. [Crossref] [PubMed]
- Leitman M, Lysyansky P, Sidenko S, et al. Two-dimensional strain-a novel software for real-time quantitative echocardiographic assessment of myocardial function. J Am Soc Echocardiogr 2004;17:1021-9. [Crossref] [PubMed]
- Jasaityte R, Heyde B, Ferferieva V, et al. Comparison of a new methodology for the assessment of 3D myocardial strain from volumetric ultrasound with 2D speckle tracking. Int J Cardiovasc Imaging 2012;28:1049-60. [Crossref] [PubMed]
- Kaku K, Takeuchi M, Tsang W, et al. Age-related normal range of left ventricular strain and torsion using three-dimensional speckle-tracking echocardiography. J Am Soc Echocardiogr 2014;27:55-64. [Crossref] [PubMed]
- Kleijn SA, Pandian NG, Thomas JD, et al. Normal reference values of left ventricular strain using three-dimensional speckle tracking echocardiography: results from a multicentre study. Eur Heart J Cardiovasc Imaging 2015;16:410-6. [Crossref] [PubMed]
- Maffessanti F, Nesser HJ, Weinert L, et al. Quantitative evaluation of regional left ventricular function using three-dimensional speckle tracking echocardiography in patients with and without heart disease. Am J Cardiol 2009;104:1755-62. [Crossref] [PubMed]
- Pérez de Isla L, Millán M, Lennie V, et al. Area strain: normal values for a new parameter in healthy people. Rev Esp Cardiol 2011;64:1194-7. [PubMed]
- Zhang L, Gao J, Xie M, et al. Left ventricular three-dimensional global systolic strain by real-time three-dimensional speckle-tracking in children: feasibility, reproducibility, maturational changes, and normal ranges. J Am Soc Echocardiogr 2013;26:853-9. [Crossref] [PubMed]
- Langeland S, Wouters PF, Claus P, et al. Experimental assessment of a new research tool for the estimation of two-dimensional myocardial strain. Ultrasound Med Biol 2006;32:1509-13. [Crossref] [PubMed]
- Hoffmann R, von Bardeleben S, Kasprzak JD, et al. Analysis of regional left ventricular function by cineventriculography, cardiac magnetic resonance imaging, and unenhanced and contrast-enhanced echocardiography: a multicenter comparison of methods. J Am Coll Cardiol 2006;47:121-8. [Crossref] [PubMed]
- Ternacle J, Gallet R, Champagne S, et al. Changes in three-dimensional speckle-tracking-derived myocardial strain during percutaneous coronary intervention. J Am Soc Echocardiogr 2013;26:1444-9. [Crossref] [PubMed]
- Shin SH, Suh YJ, Baek YS, et al. Impact of area strain by 3D speckle tracking on clinical outcome in patients after acute myocardial infarction. Echocardiography 2016;33:1854-9. [Crossref] [PubMed]
- Zhu W, Liu W, Tong Y, et al. Three-dimensional speckle tracking echocardiography for the evaluation of the infarct size and segmental transmural involvement in patients with acute myocardial infarction. Echocardiography 2014;31:58-66. [Crossref] [PubMed]
- Abate E, Hoogslag GE, Antoni ML, et al. Value of three-dimensional speckle-tracking longitudinal strain for predicting improvement of left ventricular function after acute myocardial infarction. Am J Cardiol 2012;110:961-7. [Crossref] [PubMed]
- Wang Q, Zhang C, Huang D, et al. Evaluation of myocardial infarction size with three-dimensional speckle tracking echocardiography: a comparison with single photon emission computed tomography. Int J Cardiovasc Imaging 2015;31:1571-81. [Crossref] [PubMed]
- Ran H, Zhang PY, Zhang YX, et al. Assessment of Left Ventricular Myocardial Viability by 3-Dimensional Speckle-Tracking Echocardiography in Patients With Myocardial Infarction. J Ultrasound Med 2016;35:1631-8. [Crossref] [PubMed]
- Ross J Jr, Braunwald E.. Aortic stenosis. Circulation 1968;38:61-7. [Crossref] [PubMed]
- Li CM, Li C, Bai WJ, et al. Value of three-dimensional speckle-tracking in detecting left ventricular dysfunction in patients with aortic valvular diseases. J Am Soc Echocardiogr 2013;26:1245-52. [Crossref] [PubMed]
- Nagata Y, Takeuchi M, Wu VC, et al. Prognostic value of LV deformation parameters using 2D and 3D speckle-tracking echocardiography in asymptomatic patients with severe aortic stenosis and preserved LV ejection fraction. JACC Cardiovasc Imaging 2015;8:235-45. [Crossref] [PubMed]
- Broch K, de Marchi SF, Massey R, et al. Left Ventricular Contraction Pattern in Chronic Aortic Regurgitation and Preserved Ejection Fraction: Simultaneous Stress-Strain Analysis by Three-Dimensional Echocardiography. J Am Soc Echocardiogr 2017;30:422-30.e2. [Crossref] [PubMed]
- Howard-Quijano K, Salem A, Barkulis C, et al. Preoperative Three-Dimensional Strain Imaging Identifies Reduction in Left Ventricular Function and Predicts Outcomes After Cardiac Surgery. Anesth Analg 2017;124:419-28. [Crossref] [PubMed]
- Schueler R, Sinning JM, Momcilovic D, et al. Three-dimensional speckle-tracking analysis of left ventricular function after transcatheter aortic valve implantation. J Am Soc Echocardiogr 2012;25:827-34.e1. [Crossref] [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2438-88. [Crossref] [PubMed]
- Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC); European Association for Cardio-Thoracic Surgery (EACTS), Vahanian A, et al. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J 2012;33:2451-96. [Crossref] [PubMed]
- Enriquez-Sarano M, Schaff HV, Orszulak TA, et al. Congestive heart failure after surgical correction of mitral regurgitation. A long-term study. Circulation 1995;92:2496-503. [Crossref] [PubMed]
- Casas-Rojo E, Fernández-Golfin C, Moya-Mur JL, et al. Area strain from 3D speckle-tracking echocardiography as an independent predictor of early symptoms or ventricular dysfunction in asymptomatic severe mitral regurgitation with preserved ejection fraction. Int J Cardiovasc Imaging 2016;32:1189-98. [Crossref] [PubMed]
- de Isla LP, de Agustin A, Rodrigo JL, et al. Chronic mitral regurgitation: a pilot study to assess preoperative left ventricular contractile function using speckle-tracking echocardiography. J Am Soc Echocardiogr 2009;22:831-8. [Crossref] [PubMed]
- Pandis D, Sengupta PP, Castillo JG, et al. Assessment of longitudinal myocardial mechanics in patients with degenerative mitral valve regurgitation predicts postoperative worsening of left ventricular systolic function. J Am Soc Echocardiogr 2014;27:627-38. [Crossref] [PubMed]
- Scandura S, Dipasqua F, Gargiulo G, et al. Early results of MitraClip system implantation by real-time three-dimensional speckle-tracking left ventricle analysis. J Cardiovasc Med (Hagerstown) 2016;17:843-9. [Crossref] [PubMed]
- Vitarelli A, Mangieri E, Capotosto L, et al. Assessment of Biventricular Function by Three-Dimensional Speckle-Tracking Echocardiography in Secondary Mitral Regurgitation after Repair with the MitraClip System. J Am Soc Echocardiogr 2015;28:1070-82. [Crossref] [PubMed]
- Bax JJ, Gorcsan J 3rd. Echocardiography and noninvasive imaging in cardiac resynchronization therapy: results of the PROSPECT (Predictors of Response to Cardiac Resynchronization Therapy) study in perspective. J Am Coll Cardiol 2009;53:1933-43. [Crossref] [PubMed]
- Kleijn SA, Aly MF, Knol DL, et al. A meta-analysis of left ventricular dyssynchrony assessment and prediction of response to cardiac resynchronization therapy by three-dimensional echocardiography. Eur Heart J Cardiovasc Imaging 2012;13:763-75. [Crossref] [PubMed]
- Thebault C, Donal E, Bernard A, et al. Real-time three-dimensional speckle tracking echocardiography: a novel technique to quantify global left ventricular mechanical dyssynchrony. Eur J Echocardiogr 2011;12:26-32. [Crossref] [PubMed]
- Tanaka H, Hara H, Saba S, et al. Usefulness of three-dimensional speckle tracking strain to quantify dyssynchrony and the site of latest mechanical activation. Am J Cardiol 2010;105:235-42. [Crossref] [PubMed]
- Tatsumi K, Tanaka H, Tsuji T, et al. Strain dyssynchrony index determined by three-dimensional speckle area tracking can predict response to cardiac resynchronization therapy. Cardiovasc Ultrasound 2011;9:11. [Crossref] [PubMed]
- Urbano Moral JA, Arias Godinez JA, Maron MS, et al. Left ventricular twist mechanics in hypertrophic cardiomyopathy assessed by three-dimensional speckle tracking echocardiography. Am J Cardiol 2011;108:1788-95. [Crossref] [PubMed]
- Voilliot D, Huttin O, Hammache N, et al. Impact of Global and Segmental Hypertrophy on Two-Dimensional Strain Derived from Three-Dimensional Echocardiography in Hypertrophic Cardiomyopathy: Comparison with Healthy Subjects. J Am Soc Echocardiogr 2015;28:1093-102. [Crossref] [PubMed]
- Baccouche H, Maunz M, Beck T, et al. Differentiating cardiac amyloidosis and hypertrophic cardiomyopathy by use of three-dimensional speckle tracking echocardiography. Echocardiography 2012;29:668-77. [Crossref] [PubMed]
- Mornoş C, Manolis AJ, Cozma D, et al. The value of left ventricular global longitudinal strain assessed by three-dimensional strain imaging in the early detection of anthracyclinemediated cardiotoxicity. Hellenic J Cardiol 2014;55:235-44. [PubMed]
- Santoro C, Arpino G, Esposito R, et al. 2D and 3D strain for detection of subclinical anthracycline cardiotoxicity in breast cancer patients: a balance with feasibility. Eur Heart J Cardiovasc Imaging 2017;18:930-6. [Crossref] [PubMed]
- Dahou A, Clavel MA, Capoulade R, et al. Right ventricular longitudinal strain for risk stratification in low-flow, low-gradient aortic stenosis with low ejection fraction. Heart 2016;102:548-54. [Crossref] [PubMed]
- Park SJ, Park JH, Lee HS, et al. Impaired RV global longitudinal strain is associated with poor long-term clinical outcomes in patients with acute inferior STEMI. JACC Cardiovasc Imaging 2015;8:161-9. [Crossref] [PubMed]
- Lisi M, Cameli M, Righini FM, et al. RV Longitudinal Deformation Correlates With Myocardial Fibrosis in Patients With End-Stage Heart Failure. JACC Cardiovasc Imaging 2015;8:514-22. [Crossref] [PubMed]
- Galli E, Guirette Y, Feneon D, et al. Prevalence and prognostic value of right ventricular dysfunction in severe aortic stenosis. Eur Heart J Cardiovasc Imaging 2015;16:531-8. [Crossref] [PubMed]
- Wright LM, Dwyer N, Celermajer D, et al. Follow-Up of Pulmonary Hypertension With Echocardiography. JACC Cardiovasc Imaging 2016;9:733-46. [Crossref] [PubMed]
- Atsumi A, Ishizu T, Kameda Y, et al. Application of 3-dimensional speckle tracking imaging to the assessment of right ventricular regional deformation. Circ J 2013;77:1760-8. [Crossref] [PubMed]
- Smith BC, Dobson G, Dawson D, et al. Three-dimensional speckle tracking of the right ventricle: toward optimal quantification of right ventricular dysfunction in pulmonary hypertension. J Am Coll Cardiol 2014;64:41-51. [Crossref] [PubMed]
- Atsumi A, Seo Y, Ishizu T, et al. Right Ventricular Deformation Analyses Using a Three-Dimensional Speckle-Tracking Echocardiographic System Specialized for the Right Ventricle. J Am Soc Echocardiogr 2016;29:402-11.e2. [Crossref] [PubMed]
- Yodwut C, Weinert L, Klas B, et al. Effects of frame rate on three-dimensional speckle-tracking-based measurements of myocardial deformation. J Am Soc Echocardiogr 2012;25:978-85. [Crossref] [PubMed]
- Voigt JU, Pedrizzetti G, Lysyansky P, et al. Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging 2015;16:1-11. [Crossref] [PubMed]


