Tumor thrombus: incidence, imaging, prognosis and treatment
Introduction
Intravascular tumor thrombus is defined as tumor extension into a vessel. Its presence changes stage, prognosis, and treatment. It occurs in a wide variety of malignancies, most frequently in renal cell carcinoma (RCC), Wilms tumor, adrenal cortical carcinoma (ACC), and hepatocellular carcinoma (HCC). Imaging plays a crucial role both in the detection of tumor thrombus and is essential in differentiating it from bland thrombus. The incidence, diagnosis and approach to treatment of tumor thrombus are discussed in this article.
RCC
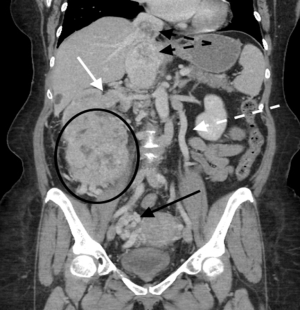
RCC is the 9th most common type of cancer diagnosed in the US and is projected to result in 14,400 deaths in 2017. RCC has a known proclivity for vascular invasion, which occurs in approximately 10% of cases (1-3) (Figure 1). The presence of tumor thrombus changes staging, prognosis and surgical approach (4-8).
Clinical symptoms, staging and prognosis
Tumor thrombus may be asymptomatic or may cause a variety of symptoms including varicocele, lower extremity swelling, cardiac dysfunction, pulmonary embolism, or Budd-Chiari syndrome (9-12). In the absence concurrent invasion of Gerota’s fascia or the ipsilateral adrenal gland, RCC with tumor thrombus is staged as T3 (2) (Table 1). When the tumor thrombus is present only in a segmental renal vein branch or the main renal vein, it is staged as T3a. When the tumor thrombus extends into the IVC but below the level of the diaphragm, it is staged as T3b. When the tumor thrombus extends above the diaphragm or invades the wall of the IVC, it is staged as T3c. An alternative classification system of tumor thrombus level is the Mayo Clinic Classification, which is useful for surgical planning (Table 2) (13-16). While it is controversial whether higher-level thrombus is associated with increased cancer related mortality, surgical morbidity and mortality does increase. Perioperative complications of 78% and mortality of 13% have been reported for T3c tumors (16-20).

Full table

Full table
Imaging
Both MRI and CT have high accuracy in detecting tumor thrombus, assessing its extension and distinguishing it from bland thrombus (21-24). Tumor thrombus is differentiated from bland thrombus by the presence of enhancement, vessel expansion and uptake of fluorodeoxyglucose on positron emission tomography (18F-FDG-PET) (Table 3) (25). On angiography, the “streak and thread” sign may be seen (26). Venography will show filling defects within the affected vessel.

Full table
Invasion into and through the wall of the IVC occurs infrequently (27,28). A definitive sign of invasion of the IVC wall is presence of tumor on both sides of the caval wall. Vessel wall invasion is suggested when tumor thrombus causes complete occlusion and expansion of the IVC.
Treatment/surgical approach
Complete surgical resection of the parenchymal tumor and tumor thrombus results in a 5-year survival of greater than 50% (29-32). With incomplete tumor resection, 5-year survival drops to about 10% (10,31). In the presence of known metastatic disease, surgical tumor debulking not only improves symptoms but can also prolong survival, especially when targeted chemotherapy is subsequently employed (33-35). Surgery becomes more complex and has a higher morbidity and mortality rate the higher the tumor thrombus extends.
Prior to surgery, the patient should undergo anesthesia and/or cardiology assessment to evaluate if the patient is an appropriate surgical candidate given the relatively high rates of perioperative morbidity and mortality. Depending on the extension of tumor and/or need for vascular reconstruction, multidisciplinary discussion between urology, vascular surgery and cardiothoracic surgery may be necessary to determine optimal surgical approach.
If bland thrombus is present in addition to tumor thrombus, anticoagulation should be considered (36). IVC filter placement, however, should be avoided. The presence of an IVC filter can convert a challenging surgery to something nearly impossible. The presence of a supra-renal filter makes it difficult to obtain control cranial to the tumor thrombus at the time of surgery. Tumor thrombus may also become incorporated into the filter making removal of both the filter and the tumor thrombus very difficult (36).
Traditional neo-adjuvant chemotherapy and radiation are ineffective against RCC. Small studies of targeted molecular therapies (e.g., sorafenib, erlotinib and temsirolimus) have shown their ability to down stage tumor thrombus level to make subsequent surgery safer and easier (37-39). More investigation is needed to see if these targeted neo-adjuvant chemotherapy agents should be recommended in cases of tumor thrombus (20).
Pre-surgical trans-arterial embolization is sometimes performed. While it does decrease operative blood loss and shorten operative time, it has not been shown to improve overall survival (40-42). Therefore, its role and use is debated.
To prevent tumor thrombus from embolizing to the lungs during surgery, control central to the tumor thrombus must be gained (10,43). The approach to gain central control varies according to the level of intravascular tumor extension (Table 2). For level 0 tumor thrombus, the renal vein is ligated central to the tumor thrombus and en bloc nephrectomy is performed. For level I tumor thrombus, no clamping or control of the IVC is necessary; tumor thrombus is milked back into the renal vein while removing the kidney. The renal vein is then ligated and nephrectomy completed en bloc (20).
Surgery for level II tumor thrombus is more involved. Tumor thrombus extending >2 cm into IVC cannot be milked back into the renal vein. To gain control of the IVC cephalad to the tumor thrombus, the liver is mobilized by ligating the accessory hepatic veins draining the caudate lobe. The intrahepatic IVC, below the level of the hepatic veins, is then clamped. Additionally, inflow control is necessary. Both the infrarenal IVC and contralateral renal vein need to be clamped. Fortunately, cardiopulmonary or venovenous bypass is not necessary for level II thrombus as clamping as the hepatic veins provide approximately 1/3rd of IVC flow. Therefore, adequate venous return can be maintained. After adequate proximal and distal control is obtained, an L-shaped cavotomy is made extending into the affected renal vein (16). Through this incision the tumor thrombus is removed from the IVC. The kidney and remaining tumor thrombus are removed en bloc.
Surgical resection for level III tumor thrombus, which extends above the level of the hepatic vein-IVC confluence but stays below the diaphragm, is complex. First, intraoperative transesophageal echocardiogram may be necessary to evaluate the precise cranial extent of the tumor. Once it is shown to be subdiaphragmatic, the liver needs to be extensively mobilized in order to gain control of the IVC above the hepatic veins. This is done by dividing the liver from its diaphragmatic attachments and ligating the accessory hepatic veins. This mobilization is often done in conjunction with a hepatobiliary or transplant surgeon (44). Cardiopulmonary bypass or venovenous bypass are necessary given that clamping the suprahepatic IVC obstructs all venous return from the IVC, which accounts for 2/3rds of all venous return.
Level IV tumor thrombus extends above the diaphragm into the right atrium (10). Involvement of cardiothoracic surgery and cardiopulmonary bypass are necessary. TEE helps predict adherence of the tumor to the myocardium and tricuspid valve, which helps one decide whether or not incision into the heart is necessary (45).
When tumor has invaded into the wall of the IVC, resection of the IVC is necessary to completely remove the tumor. When tumor has invaded the wall of the IVC, there is a high risk of local tumor recurrence. Bovine pericardium, autologous pericardium and expanded polytetrafluoroethylene (ePTFE) have all been used for IVC reconstruction.
HCC
HCC is the 6th most common cancer and is the 3rd leading cause of cancer related deaths (46,47). Macrovascular invasion is a well-recognized characteristic of HCC; portal vein tumor thrombus (PVVT) occurs in 35% of cases and hepatic vein tumor thrombus occurs in 2% of cases (48,49). Macrovascular invasion tends to affect younger patients, those with aggressive tumor biology and patients with poor underlying liver function (49). The prognosis of HCC with tumor thrombus is a poor and depends on the extent of PVVT (48,49). Of note, upwards of 25% of all patients with cirrhosis have bland portal vein thrombus (50,51). Bland and tumor thrombus can be distinguished by imaging characteristics (Table 3).
Staging, clinical symptoms and prognosis
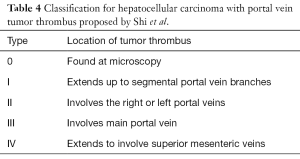
The overall prognosis in HCC tumor is poor. Without PVTT, median survival is approximately 16 months. This prognosis drops to 6 months if PVTT is present (52). The presence of PVTT has been shown to have a more significant effect on prognosis than tumor size (53). The extent of PVTT is also very important in predicting survival (54-56). Patients with segmental PVTT are found to have twice the survival times as patients with main portal vein invasion (9 vs. 4.6 months) (54). Shi et al. proposed a PVTT classification system based on the extent of tumor involvement (Table 4) (56). Tumor thrombus may cause specific symptoms. PVTT may cause portal hypertension through obstruction of flow and/or due to arterioportal shunting. Hepatic vein tumor thrombus may lead to Budd-Chiari syndrome.
Full table
HCC patients with any macroscopic vascular invasion are classified as Barcelona Clinic Liver Cancer (BCLC) stage C (advanced stage) (Table 5) (57-59). Patients with extrahepatic spread and patients with reduced performance status (Eastern Cooperative Oncology Group 1 and 2) are also classified as BCLC stage C. BCLC stage C therefore encompasses a heterogeneous group of patients with wide ranging survival (54). Therefore, some suggest sub-classifying stage C (54,60).

Full table
Imaging
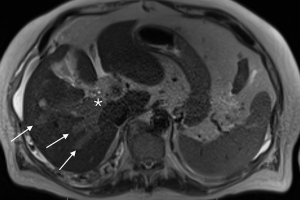
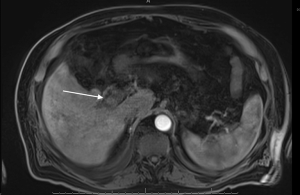
PVVT can be diagnosed and differentiated from bland thrombus by many different imaging modalities, including conventional angiography, color Doppler ultrasound, contrast enhanced ultrasound, contrast enhanced CT scan, MRI, and FDG PET imaging (61-64) (Table 3, Figures 2-4). Contrast enhanced ultrasound is reported to be 100% sensitive and specific (65). Differentiating bland from tumor thrombus is necessary as the former occurs in up to 25% of patients with chronic liver disease (51). The key feature for diagnosing portal vein tumor thrombus is the presence of enhancement or color Doppler flow within the thrombus (63,64). Subtraction imaging may be helpful to detect subtle enhancement within the thrombus. Diffusion imaging on MRI can be a useful adjunctive imaging finding (62). Tumor thrombus has an increased cellular density and nuclear to cytoplasm ratios and will show restricted diffusion, appearing bright on diffusion weighted imaging (DWI) and dark on apparent diffusion coefficient (ADC) mapping (66). Nonetheless, care must be taken not to evaluate diffusion sequences in isolation as bland thrombus may also demonstrate low ADC values. This is due to the high viscosity of thrombus as well as paramagnetic effects of intracellular deoxyhemoglobin and methemoglobin present in bland thrombus (67). Other imaging characteristics that favor tumor thrombus include expansion of the involved vessel and increased uptake on FDG-PET (63,64,68-70). On angiography, portal vein tumor thrombosis may be recognized by the characteristic streak and thread appearance due to parallel opacification of small vessels within the tumor and arterial venous shunting (26).
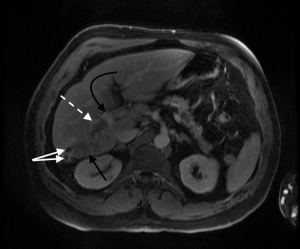
Treatment
Sorafenib is a multi-kinase inhibitor, targeting VEGFR, PDGFR and Raf family kinases (71). According to the American Association for the Study of Liver disease (AASLD), the European Association for the Study of the Liver (EASL), and the BCLC treatment guidelines, it is the recommended treatment for BCLC stage C patients (72,73). There are many potential side effects of this drug; one characteristic side effect is hand-foot syndrome, which consists of redness, swelling, numbness and peeling of the palms of the hands and soles of the feet (74). Survival of those treated with Sorafenib is improved by only a modest 2–3 months (75,76). Limiting patients with tumor thrombus to treatment with Sorafenib may be too conservative. In clinical practice, other more aggressive treatments are employed (54).
In western countries, surgical resection is rarely performed in the setting of HCC tumor thrombus given poor underlying liver function. In the east, cirrhosis is by and large due to Hepatitis B. These patients have better underlying hepatic function compared to alcohol or hepatitis C cirrhosis seen in the west. Therefore, surgical resection is performed for patients with branch of portal vein tumor thrombus (77). Reflecting this practice, the Hong Kong Liver Staging does not consider hepatic vascular invasion to be a contraindication for surgical resection (78).
Due to the high rate of recurrence after transplant, the presence of tumor thrombus is considered an absolute contraindication to liver transplantation (79,80).
Thermal ablation of portal vein tumor thrombus is rarely considered given the portal vein’s the tumor’s oblong shape as well as its proximity to critical structures, like the hepatic artery and bile ducts. However, one recent report has shown good results after endoluminal ablation followed by stenting (81).
Given the risk of hepatic necrosis and worsening of liver function, the presence portal vein tumor thrombus is considered a relative contraindication to transarterial chemoembolization (TACE) (82,83). EASL, AASLD and BCLC guidelines all discourage TACE in patients with macroscopic vascular invasion (72,73). However, in patients with non-occlusive thrombus, preserved liver function and small tumor burden, super-selective TACE can be safely and effectively performed with improvement in patient survival (83-85). The Asian Pacific Association for the Study of the Liver (APASL) guidelines recommend TACE in the setting of branch vessel thrombus in Child-Pugh A and B patients (86). While hepatic necrosis is not a worry in those with hepatic vein tumor thrombus, particulate embolization to the pulmonary vasculature is a potential sequela given hepatic arteriovenous shunting within the hepatic vein tumor thrombus (87).
Selective internal radio-embolization (SIRT) using Yittrium-90 (Y-90) is considered by many to be the preferred treatment of patients with HCC and portal vein tumor invasion (88,89). EASL guidelines state that that although SIRT appears to be safe and demonstrate promising results, more studies are needed before it can be recommended as standard of care (73). When treating patients with hepatic vein tumor thrombus, one should be cognizant of the propensity for hepatopulmonary shunting (90). The presence of hepatic vein tumor thrombus is associated with a 3–4× elevated lung shunt fraction, as detected by Technetium-99m microaggregated albumin (Tc-99m MAA) (91). As the limit for radiation exposure to the lungs is set at 30 Gy in a single session and 50 Gy in a cumulative dose reduction is often necessary (92).
There are emerging therapies for portal vein thrombus. External beam radiation therapy in combination with intra-arterial 5-FU and interferon-alpha infusion has shown positive results (93,94). Percutaneous portal vein stenting followed by TACE and external beam radiation has also shown promising results (95). Irradiation stents (self-expandable stent loaded with iodine-125 seeds) in combination with TACE has shown benefit in a small cohort of patients with partially obstructing portal vein tumor thrombus (96).
ACC
ACC is a rare and aggressive malignancy. ACC also has a propensity to lead to tumor thrombus which effects about 1/4th of patients (Figure 5). Similar to RCC with tumor thrombus, patients can present with a varicocele or lower extremity edema (97-99). Invasion of the wall of the IVC has been reported with ACC (100). Although there are some cases of long-term survival in cases of ACC with vascular invasion after complete resection, this is the exception rather than the rule (101-103). Systemic chemotherapy is the preferred treatment modality.
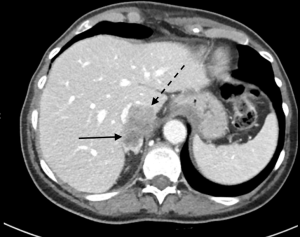
Wilm’s tumor
Wilm’s tumor is the most common kidney mass in children and accounts for 6% of all childhood cancers. Its peak incidence is 3–4 years old. It has a marked propensity for macrovascular invasion which occurs in up to 35% of cases. Extension into the IVC occurs in up to 10% of cases (104). Intracardiac extension is rare but does occur (105). While pre-operative chemotherapy is generally recommended, this is a decision that should be made on a case-by-case basis. If the tumor thrombus appears to be at high risk of embolizing, surgery prior to chemotherapy is preferred (106,107).
Other tumors
Many other tumors can develop tumor thrombus, but do so less frequently. These include intravenous leiomyomatosis, islet cell tumors of the pancreas, thyroid cancer.
Intravenous leiomyomatosis is a rare benign smooth muscle tumor of uterine origin that grows into veins pelvic veins. It often can cause lower extremity swelling but has been reported to cause death if they extend into the heart (108-110).
Islet cell tumors of the pancreas can develop tumor thrombus. This tumor thrombus can involve the splenic, the portal and/or the superior mesenteric veins (111-115). Splenic vein tumor thrombus can cause sinistral portal hypertension, with development of isolated gastric varices (116). As with other tumor thrombi, one will see heterogeneous arterial enhancement and expansion of the involved veins in contiguity with the primary mass. FDG avidity of the tumor thrombus may also be seen on PET scan (117).
While the incidence is unknown, there are reports of macrovascular invasion of thyroid cancer with extension into the jugular vein, SVC and right atrium (118-120). As with other tumor thrombi, proximal and distal control of the thrombus is necessary during surgical excision. This is illustrated in one case report which describes tumor thrombus embolizing to the pulmonary artery during surgery in the absence of adequate central control (121). Thyroid tumor thrombus can first be detected by ultrasound and may be further imaged with by contrast enhanced CT or MRI (122). Good long-term outcome can be achieved with complete excision.
Testicular cancer may rarely cause tumor thrombus. While autopsy reports have suggested the incidence of tumor thrombus to be as high as 11%, it is only seen in 1% of cases by imaging (123,124). In addition to tumor thrombus originating from the primary site and extending into the gonadal veins, retroperitoneal metastatic lymphadenopathy may grow directly into the IVC (125). There are variable approaches to treatment of testicular cancer in the setting of tumor thrombus; some advocate retrievable IVC filter placement prior to definitive therapy, others employ neoadjuvant chemotherapy, still others advocate chemotherapy alone and finally some support early primary surgical resection (126-128).
Lung cancer is the second most common cancer and is responsible for the most number of cancer related deaths both in US and worldwide (129). While rare, lung cancer tumor thrombus can occur. It may extend to involve the pulmonary veins, extending towards and even into the left atrium. Tumor thrombus in the pulmonary veins may cause symptoms distinct from systemic venous tumor thrombus. Cases of embolization causing stroke, bowel infarction and an ischemic leg have been reported (130). Left ventricular obstruction even cause sudden death (131,132). Given these potentially devastating complications, surgery prior to chemotherapy is generally advocated (133).
Colorectal cancer is the 4th leading cause of cancer in the US (129). Venous tumor thrombus is a rare finding in colorectal cancer, seen in only 1–2% of cases (134). When it occurs, venous invasion into the portal venous system occurs from the cecum to the sigmoid colon. Given its dual venous drainage via the portal and internal iliac systems, tumor thrombus from rectal cancer can involve either the inferior mesenteric vein (portal venous system) or the internal iliac veins (systemic venous system) (135). Preoperative diagnosis of portal vein tumor thrombus is important, allowing the surgeon to ligate the involved vein above the thrombus can help one avoid tumor embolism to the liver. Open rather than laparoscopic approach may be indicated as the surgeon can directly palpate the involved vein to determine where to ligate and resect. The rate of liver metastasis occurs at a higher rate in the presence of tumor thrombus (134).
Conclusions
Intravascular tumor extension can occur in many different types of cancer. Those with the highest proclivity include Wilm’s tumor, RCC, adrenal cortical carcinoma and hepatocellular carcinoma. Tumor thrombus occurs less frequently in a myriad of other tumor types, including benign and malignant histologies. Imaging plays a central role in its diagnosis. The presence of tumor thrombus markedly worsens prognosis and impacts treatment approach.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Zisman A, Wieder JA, Pantuck AJ, et al. Renal cell carcinoma with tumor thrombus extension: biology, role of nephrectomy and response to immunotherapy. J Urol 2003;169:909-16. [Crossref] [PubMed]
- Lam JS, Klatte T, Breda A. Staging of renal cell carcinoma: Current concepts. Indian J Urol 2009;25:446-54. [Crossref] [PubMed]
- Turun S, Banghua L, Zheng S, et al. Is tumor size a reliable predictor of histopathological characteristics of renal cell carcinoma? Urol Ann 2012;4:24-8. [Crossref] [PubMed]
- Dall'Oglio MF, Ribeiro-Filho LA, Antunes AA, et al. Microvascular tumor invasion, tumor size and Fuhrman grade: a pathological triad for prognostic evaluation of renal cell carcinoma. J Urol 2007;178:425-8; discussion 428. [Crossref] [PubMed]
- Qi J, Gu Z, Chen F, et al. Management of renal cell carcinoma with tumor thrombus in renal vein and the inferior vena cava. Ann Vasc Surg 2010;24:1089-93. [Crossref] [PubMed]
- Nesbitt JC, Soltero ER, Dinney CP, et al. Surgical management of renal cell carcinoma with inferior vena cava tumor thrombus. Ann Thorac Surg 1997;63:1592-600. [Crossref] [PubMed]
- Parekh DJ, Cookson MS, Chapman W, et al. Renal cell carcinoma with renal vein and inferior vena caval involvement: clinicopathological features, surgical techniques and outcomes. J Urol 2005;173:1897-902. [Crossref] [PubMed]
- Tsuji Y, Goto A, Hara I, et al. Renal cell carcinoma with extension of tumor thrombus into the vena cava: surgical strategy and prognosis. J Vasc Surg 2001;33:789-96. [Crossref] [PubMed]
- Sunela KL, Kataja MJ, Kellokumpu-Lehtinen PL. Changes in symptoms of renal cell carcinoma over four decades. BJU Int 2010;106:649-53. [Crossref] [PubMed]
- Noguchi K, Hori D, Nomura Y, et al. Renal cell carcinoma with tumor-thrombus extension into the right ventricle. Ann Vasc Dis 2012;5:376-80. [Crossref] [PubMed]
- El Abiad Y, Qarro A. IMAGES IN CLINICAL MEDICINE. Acute varicocele revealing renal cancer. N Engl J Med 2016;374:2075. [Crossref] [PubMed]
- Shirodkar SP, Soloway MS, Ciancio G. Budd-Chiari syndrome in urology: Impact on nephrectomy for advanced renal cell carcinoma. Indian J Urol 2011;27:351-6. [Crossref] [PubMed]
- Nouh MA, Inui M, Kakehi Y. Renal cell carcinoma with IVC thrombi; current concepts and future perspectives. Clin Med Oncol 2008;2:247-56. [Crossref] [PubMed]
- Sweeney P, Wood CG, Pisters LL, et al. Surgical management of renal cell carcinoma associated with complex inferior vena caval thrombi. Urol Oncol 2003;21:327-33. [Crossref] [PubMed]
- Neves RJ, Zincke H. Surgical treatment of renal cancer with vena cava extension. Br J Urol 1987;59:390-5. [Crossref] [PubMed]
- Blute ML, Leibovich BC, Lohse CM, et al. The Mayo Clinic experience with surgical management, complications and outcome for patients with renal cell carcinoma and venous tumour thrombus. BJU Int 2004;94:33-41. [Crossref] [PubMed]
- Martinez-Salamanca JI, Linares E, González J, et al. Lessons learned from the International Renal Cell Carcinoma-Venous Thrombus Consortium (IRCC-VTC). Curr Urol Rep 2014;15:404. [Crossref] [PubMed]
- Wagner B, Patard JJ, Méjean A, et al. Prognostic value of renal vein and inferior vena cava involvement in renal cell carcinoma. Eur Urol 2009;55:452-9. [Crossref] [PubMed]
- Dedeilias P, Koletsis E, Rousakis AG, et al. Deep hypothermia and circulatory arrest in the surgical management of renal tumors with cavoatrial extension. J Card Surg 2009;24:617-23. [Crossref] [PubMed]
- Psutka SP, Leibovich BC. Management of inferior vena cava tumor thrombus in locally advanced renal cell carcinoma. Ther Adv Urol 2015;7:216-29. [Crossref] [PubMed]
- Zhang L, Yang G, Shen W, et al. Spectrum of the inferior vena cava: MDCT findings. Abdom Imaging 2007;32:495-503. [Crossref] [PubMed]
- Oto A, Herts BR, Remer EM, et al. Inferior vena cava tumor thrombus in renal cell carcinoma: staging by MR imaging and impact on surgical treatment. AJR Am J Roentgenol 1998;171:1619-24. [Crossref] [PubMed]
- Lawrentschuk N, Gani J, Riordan R, et al. Multidetector computed tomography vs magnetic resonance imaging for defining the upper limit of tumour thrombus in renal cell carcinoma: a study and review. BJU Int 2005;96:291-5. [Crossref] [PubMed]
- Hockley NM, Foster RS, Bihrle R, et al. Use of magnetic resonance imaging to determine surgical approach to renal cell carcinoma with vena caval extension. Urology 1990;36:55-60. [Crossref] [PubMed]
- Kaufman LB, Yeh BM, Breiman RS, et al. Inferior vena cava filling defects on CT and MRI. AJR Am J Roentgenol 2005;185:717-26. [Crossref] [PubMed]
- Raab BW. The thread and streak sign. Radiology 2005;236:284-5. [Crossref] [PubMed]
- Psutka SP, Boorjian SA, Thompson RH, et al. Clinical and radiographic predictors of the need for inferior vena cava resection during nephrectomy for patients with renal cell carcinoma and caval tumour thrombus. BJU Int 2015;116:388-96. [Crossref] [PubMed]
- Aslam Sohaib SA, Teh J, Nargund VH, et al. Assessment of tumor invasion of the vena caval wall in renal cell carcinoma cases by magnetic resonance imaging. J Urol 2002;167:1271-5. [Crossref] [PubMed]
- Kirkali Z, Van Poppel H. A critical analysis of surgery for kidney cancer with vena cava invasion. Eur Urol 2007;52:658-62. [Crossref] [PubMed]
- Skinner DG, Pritchett TR, Lieskovsky G, et al. Vena caval involvement by renal cell carcinoma. Surgical resection provides meaningful long-term survival. Ann Surg 1989;210:387-92; discussion 392-4. [Crossref] [PubMed]
- Ioannis V, Panagiotis S, Anastasios A, et al. Tumor extending through inferior vena cava into the right atrium. A late recurrence of renal cell carcinoma. Int J Cardiovasc Imaging 2003;19:179-82. [Crossref] [PubMed]
- Haddad AQ, Wood CG, Abel EJ, et al. Oncologic outcomes following surgical resection of renal cell carcinoma with inferior vena caval thrombus extending above the hepatic veins: a contemporary multicenter cohort. J Urol 2014;192:1050-6. [Crossref] [PubMed]
- Flanigan RC, Mickisch G, Sylvester R, et al. Cytoreductive nephrectomy in patients with metastatic renal cancer: a combined analysis. J Urol 2004;171:1071-6. [Crossref] [PubMed]
- Mickisch GH, Garin A, van Poppel H, et al. Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: a randomised trial. Lancet 2001;358:966-70. [Crossref] [PubMed]
- Vergho DC, Loeser A, Kocot A, et al. Tumor thrombus of inferior vena cava in patients with renal cell carcinoma - clinical and oncological outcome of 50 patients after surgery. BMC Res Notes 2012;5:5. [Crossref] [PubMed]
- Woodruff DY, Van Veldhuizen P, Muehlebach G, et al. The perioperative management of an inferior vena caval tumor thrombus in patients with renal cell carcinoma. Urol Oncol 2013;31:517-21. [Crossref] [PubMed]
- Bigot P, Fardoun T, Bernhard JC, et al. Neoadjuvant targeted molecular therapies in patients undergoing nephrectomy and inferior vena cava thrombectomy: is it useful? World J Urol 2014;32:109-14. [Crossref] [PubMed]
- Kwon T, Lee JL, Kim JK, et al. The Choi response criteria for inferior vena cava tumor thrombus in renal cell carcinoma treated with targeted therapy. J Cancer Res Clin Oncol 2014;140:1751-8. [Crossref] [PubMed]
- Borregales LD, Adibi M, Thomas AZ, et al. The role of neoadjuvant therapy in the management of locally advanced renal cell carcinoma. Ther Adv Urol 2016;8:130-41. [Crossref] [PubMed]
- Wotkowicz C, Wszolek MF, Libertino JA. Resection of renal tumors invading the vena cava. Urol Clin North Am 2008;35:657-71. viii. [Crossref] [PubMed]
- Zielinski H, Szmigielski S, Petrovich Z. Comparison of preoperative embolization followed by radical nephrectomy with radical nephrectomy alone for renal cell carcinoma. Am J Clin Oncol 2000;23:6-12. [Crossref] [PubMed]
- May M, Brookman-Amissah S, Pflanz S, et al. Pre-operative renal arterial embolisation does not provide survival benefit in patients with radical nephrectomy for renal cell carcinoma. Br J Radiol 2009;82:724-31. [Crossref] [PubMed]
- Fukui K, Narita J, Takahashi S, et al. A case report of a massive pulmonary tumor embolism occurring during surgery for renal cell carcinoma. Kyobu Geka 1992;45:529-32. [PubMed]
- Zhang JP, Zhu Y, Liu YJ, et al. Temporary filters and liver mobilization technique improve the safety and prognosis of radical nephrectomy and inferior vena cava thrombectomy in renal cell carcinoma with subdiaphragmatic thrombosis. Urol Int 2013;91:279-84. [Crossref] [PubMed]
- Karnes RJ, Blute ML. Surgery insight: management of renal cell carcinoma with associated inferior vena cava thrombus. Nat Clin Pract Urol 2008;5:329-39. [PubMed]
- Waller LP, Deshpande V, Pyrsopoulos N. Hepatocellular carcinoma: A comprehensive review. World J Hepatol 2015;7:2648-63. [Crossref] [PubMed]
- Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet 2012;379:1245-55. [Crossref] [PubMed]
- Kokudo T, Hasegawa K, Yamamoto S, et al. Surgical treatment of hepatocellular carcinoma associated with hepatic vein tumor thrombosis. J Hepatol 2014;61:583-8. [Crossref] [PubMed]
- Lee YH, Hsu CY, Huang YH, et al. Vascular invasion in hepatocellular carcinoma: prevalence, determinants and prognostic impact. J Clin Gastroenterol 2014;48:734-41. [Crossref] [PubMed]
- Amitrano L, Guardascione MA, Brancaccio V, et al. Risk factors and clinical presentation of portal vein thrombosis in patients with liver cirrhosis. J Hepatol 2004;40:736-41. [Crossref] [PubMed]
- Ogren M, Bergqvist D, Björck M, et al. Portal vein thrombosis: prevalence, patient characteristics and lifetime risk: a population study based on 23,796 consecutive autopsies. World J Gastroenterol 2006;12:2115-9. [Crossref] [PubMed]
- Greten TF, Papendorf F, Bleck JS, et al. Survival rate in patients with hepatocellular carcinoma: a retrospective analysis of 389 patients. Br J Cancer 2005;92:1862-8. [Crossref] [PubMed]
- Takayasu K, Arii S, Ikai I, et al. Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patients. Gastroenterology 2006;131:461-9. [Crossref] [PubMed]
- Sinn DH, Cho JY, Gwak GY, et al. Different survival of Barcelona clinic liver cancer stage C hepatocellular carcinoma patients by the extent of portal vein invasion and the type of extrahepatic spread. PLoS One 2015;10:e0124434. [Crossref] [PubMed]
- Park KW, Park JW, Choi JI, et al. Survival analysis of 904 patients with hepatocellular carcinoma in a hepatitis B virus-endemic area. J Gastroenterol Hepatol 2008;23:467-73. [Crossref] [PubMed]
- Shi J, Lai EC, Li N, et al. Surgical treatment of hepatocellular carcinoma with portal vein tumor thrombus. Ann Surg Oncol 2010;17:2073-80. [Crossref] [PubMed]
- Terzis I, Haritanti A, Economou I. Fibrolamellar hepatocellular carcinoma: a case report with distinct radiological features. J Gastrointest Cancer 2010;41:2-5. [Crossref] [PubMed]
- Chan SL, Chong CC, Chan AW, et al. Management of hepatocellular carcinoma with portal vein tumor thrombosis: Review and update at 2016. World J Gastroenterol 2016;22:7289-300. [Crossref] [PubMed]
- Forner A, Reig ME, de Lope CR, et al. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis 2010;30:61-74. [Crossref] [PubMed]
- Bolondi L, Burroughs A, Dufour JF, et al. Heterogeneity of patients with intermediate (BCLC B) Hepatocellular Carcinoma: proposal for a subclassification to facilitate treatment decisions. Semin Liver Dis 2012;32:348-59. [PubMed]
- Ueno N, Kawamura H, Takahashi H, et al. Characterization of portal vein thrombus with the use of contrast-enhanced sonography. J Ultrasound Med 2006;25:1147-52. [Crossref] [PubMed]
- Catalano OA, Choy G, Zhu A, et al. Differentiation of malignant thrombus from bland thrombus of the portal vein in patients with hepatocellular carcinoma: application of diffusion-weighted MR imaging. Radiology 2010;254:154-62. [Crossref] [PubMed]
- Tublin ME, Dodd GD 3rd, Baron RL. Benign and malignant portal vein thrombosis: differentiation by CT characteristics. AJR Am J Roentgenol 1997;168:719-23. [Crossref] [PubMed]
- Shah ZK, McKernan MG, Hahn PF, et al. Enhancing and expansile portal vein thrombosis: value in the diagnosis of hepatocellular carcinoma in patients with multiple hepatic lesions. AJR Am J Roentgenol 2007;188:1320-3. [Crossref] [PubMed]
- Rossi S, Ghittoni G, Ravetta V, et al. Contrast-enhanced ultrasonography and spiral computed tomography in the detection and characterization of portal vein thrombosis complicating hepatocellular carcinoma. Eur Radiol 2008;18:1749-56. [Crossref] [PubMed]
- Taouli B, Koh DM. Diffusion-weighted MR imaging of the liver. Radiology 2010;254:47-66. [Crossref] [PubMed]
- Ahn JH, Yu JS, Cho ES, et al. Diffusion-Weighted MRI of Malignant versus Benign Portal Vein Thrombosis. Korean J Radiol 2016;17:533-40. [Crossref] [PubMed]
- Nguyen XC, Nguyen DS, Ngo VT, et al. FDG-Avid Portal Vein Tumor Thrombosis from Hepatocellular Carcinoma in Contrast-Enhanced FDG PET/CT. Asia Ocean J Nucl Med Biol 2015;3:10-7. [PubMed]
- Pozniak MA, Baus KM. Hepatofugal arterial signal in the main portal vein: an indicator of intravascular tumor spread. Radiology 1991;180:663-6. [Crossref] [PubMed]
- Sorrentino P, D'Angelo S, Tarantino L, et al. Contrast-enhanced sonography versus biopsy for the differential diagnosis of thrombosis in hepatocellular carcinoma patients. World J Gastroenterol 2009;15:2245-51. [Crossref] [PubMed]
- Wilhelm SM, Carter C, Tang L, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 2004;64:7099-109. [Crossref] [PubMed]
- Heimbach J, Kulik LM, Finn R, et al. Aasld guidelines for the treatment of hepatocellular carcinoma. Hepatology 2017. [Epub ahead of print]. [Crossref] [PubMed]
- European Association For The Study Of The Liver, European Organisation For Research, Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56:908-43. [Crossref] [PubMed]
- Berk V, Kaplan MA, Tonyali O, et al. Efficiency and side effects of sorafenib therapy for advanced hepatocellular carcinoma: a retrospective study by the anatolian society of medical oncology. Asian Pac J Cancer Prev 2013;14:7367-9. [Crossref] [PubMed]
- Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009;10:25-34. [Crossref] [PubMed]
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-90. [Crossref] [PubMed]
- Wu CC, Hsieh SR, Chen JT, et al. An appraisal of liver and portal vein resection for hepatocellular carcinoma with tumor thrombi extending to portal bifurcation. Arch Surg 2000;135:1273-9. [Crossref] [PubMed]
- Liu PH, Hsia CY, Lee YH, et al. Surgical resection versus transarterial chemoembolization for BCLC stage C hepatocellular carcinoma. J Surg Oncol 2015;111:404-9. [Crossref] [PubMed]
- Figueras J, Ibanez L, Ramos E, et al. Selection criteria for liver transplantation in early-stage hepatocellular carcinoma with cirrhosis: results of a multicenter study. Liver Transpl 2001;7:877-83. [Crossref] [PubMed]
- Mazzaferro V, Bhoori S, Sposito C, et al. Milan criteria in liver transplantation for hepatocellular carcinoma: an evidence-based analysis of 15 years of experience. Liver Transpl 2011;17 Suppl 2:S44-57. [Crossref] [PubMed]
- Mizandari M, Azrumelashvili T, Paksashvili N, et al. Tumor Regression in HCC Patient with Portal Vein Tumor Thrombosis after Intraportal Radiofrequency Thermal Ablation. Case Reports Hepatol 2016;2016:6843121. [PubMed]
- Woo HY, Heo J. New perspectives on the management of hepatocellular carcinoma with portal vein thrombosis. Clin Mol Hepatol 2015;21:115-21. [Crossref] [PubMed]
- Clark TW. Complications of hepatic chemoembolization. Semin Intervent Radiol 2006;23:119-25. [Crossref] [PubMed]
- Prajapati HJ, Dhanasekaran R, El-Rayes BF, et al. Safety and efficacy of doxorubicin drug-eluting bead transarterial chemoembolization in patients with advanced hepatocellular carcinoma. J Vasc Interv Radiol 2013;24:307-15. [Crossref] [PubMed]
- Xue TC, Xie XY, Zhang L, et al. Transarterial chemoembolization for hepatocellular carcinoma with portal vein tumor thrombus: a meta-analysis. BMC Gastroenterol 2013;13:60. [Crossref] [PubMed]
- Omata M, Lesmana LA, Tateishi R, et al. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int 2010;4:439-74. [Crossref] [PubMed]
- Mei Q, Li Y. Transcatheter arterial embolization of hepatic arteriovenous shunts in patients with hepatocellular carcinoma. Semin Intervent Radiol 2012;29:237-40. [Crossref] [PubMed]
- Salem R, Lewandowski RJ, Mulcahy MF, et al. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology 2010;138:52-64. [Crossref] [PubMed]
- Salem R, Lewandowski R, Roberts C, et al. Use of Yttrium-90 glass microspheres (TheraSphere) for the treatment of unresectable hepatocellular carcinoma in patients with portal vein thrombosis. J Vasc Interv Radiol 2004;15:335-45. [Crossref] [PubMed]
- Leung WT, Lau WY, Ho SK, et al. Measuring lung shunting in hepatocellular carcinoma with intrahepatic-arterial technetium-99m macroaggregated albumin. J Nucl Med 1994;35:70-3. [PubMed]
- Olorunsola OG, Kohi MP, Behr SC, et al. Imaging Predictors of Elevated Lung Shunt Fraction in Patients Being Considered for Yttrium-90 Radioembolization. J Vasc Interv Radiol 2015;26:1472-8. [Crossref] [PubMed]
- Wright CL, Werner JD, Tran JM, et al. Radiation pneumonitis following yttrium-90 radioembolization: case report and literature review. J Vasc Interv Radiol 2012;23:669-74. [Crossref] [PubMed]
- Katamura Y, Aikata H, Takaki S, et al. Intra-arterial 5-fluorouracil/interferon combination therapy for advanced hepatocellular carcinoma with or without three-dimensional conformal radiotherapy for portal vein tumor thrombosis. J Gastroenterol 2009;44:492-502. [Crossref] [PubMed]
- Yoon SM, Lim YS, Won HJ, et al. Radiotherapy plus transarterial chemoembolization for hepatocellular carcinoma invading the portal vein: long-term patient outcomes. Int J Radiat Oncol Biol Phys 2012;82:2004-11. [Crossref] [PubMed]
- Zhang XB, Wang JH, Yan ZP, et al. Hepatocellular carcinoma with main portal vein tumor thrombus: treatment with 3-dimensional conformal radiotherapy after portal vein stenting and transarterial chemoembolization. Cancer 2009;115:1245-52. [Crossref] [PubMed]
- Lu J, Guo JH, Zhu HD, et al. Safety and Efficacy of Irradiation Stent Placement for Malignant Portal Vein Thrombus Combined with Transarterial Chemoembolization for Hepatocellular Carcinoma: A Single-Center Experience. J Vasc Interv Radiol 2017;28:786-94.e3. [Crossref] [PubMed]
- Cheungpasitporn W, Horne JM, Howarth CB. Adrenocortical carcinoma presenting as varicocele and renal vein thrombosis: a case report. J Med Case Rep 2011;5:337. [Crossref] [PubMed]
- Nader S, Hickey RC, Sellin RV, et al. Adrenal cortical carcinoma. A study of 77 cases. Cancer 1983;52:707-11. [Crossref] [PubMed]
- Chiche L, Dousset B, Kieffer E, et al. Adrenocortical carcinoma extending into the inferior vena cava: presentation of a 15-patient series and review of the literature. Surgery 2006;139:15-27. [Crossref] [PubMed]
- Mezhir JJ, Song J, Piano G, et al. Adrenocortical carcinoma invading the inferior vena cava: case report and literature review. Endocr Pract 2008;14:721-5. [Crossref] [PubMed]
- Wei CY, Chen KK, Chen MT, et al. Adrenal cortical carcinoma with tumor thrombus invasion of inferior vena cava. Urology 1995;45:1052-4. [Crossref] [PubMed]
- Reyes MA, Ciancio G, Singal R, et al. Adrenocortical carcinoma with tumor thrombus in the right hepatic vein. Int J Urol 2006;13:1233-5. [Crossref] [PubMed]
- Haghdani S, Kafash Nayeri R, Zargar H, et al. Adrenocortical carcinoma with renal vein tumor thrombus extension. Urol J 2015;12:2037-9. [PubMed]
- Emir S. Wilms tumor with intravascular tumor thrombus. Transl Pediatr 2014;3:29-33. [PubMed]
- Abdullah Y, Karpelowsky J, Davidson A, et al. Management of nine cases of Wilms' tumour with intracardiac extension - a single centre experience. J Pediatr Surg 2013;48:394-9. [Crossref] [PubMed]
- Shamberger RC, Ritchey ML, Haase GM, et al. Intravascular extension of Wilms tumor. Ann Surg 2001;234:116-21. [Crossref] [PubMed]
- Vaideeswar P, Chaudhari JP. Wilms' tumor with right heart extension: report of a post-chemotherapeutic fatality. Indian J Pathol Microbiol 2012;55:381-3. [Crossref] [PubMed]
- Baert J, Vandamme B, Sciot R, et al. Benign angiomyolipoma involving the renal vein and vena cava as a tumor thrombus: case report. J Urol 1995;153:1205-7. [Crossref] [PubMed]
- Morales MM, Anacleto A, Leal JC, et al. Intravascular leiomyoma with heart extension. Clinics (Sao Paulo) 2012;67:83-7. [Crossref] [PubMed]
- Gunderson CC, Parsons B, Penaroza S, et al. Intravenous leiomyomatosis disguised as a large deep vein thrombosis. J Radiol Case Rep 2016;10:29-35. [Crossref] [PubMed]
- Bedirli A, Patiroglu TE, Sakrak O, et al. Portal vein resection for a portal vein thrombus caused by nonfunctioning islet cell carcinoma: report of a case. Surg Today 2004;34:802-4. [Crossref] [PubMed]
- Rodriguez RA, Overton H, Morris KT. Pancreatic neuroendocrine tumor with splenic vein tumor thrombus: A case report. Int J Surg Case Rep 2014;5:1271-4. [Crossref] [PubMed]
- Obuz F, Bora S, Sarioğlu S. Malignant islet cell tumor of the pancreas associated with portal venous thrombus. Eur Radiol 2001;11:1642-4. [Crossref] [PubMed]
- Smith TM, Semelka RC, Noone TC, et al. Islet cell tumor of the pancreas associated with tumor thrombus in the portal vein. Magn Reson Imaging 1999;17:1093-6. [Crossref] [PubMed]
- Geehan DM, Kapcala LP, Saberinia M, et al. Intravascular tumor: a previously unreported finding of glucagonoma. South Med J 1997;90:743-7. [Crossref] [PubMed]
- Dalvi AN, Rege SA, Bapat MR, et al. Nonfunctioning islet cell tumor presenting with ascites and portal hypertension. Indian J Gastroenterol 2002;21:227-8. [PubMed]
- Nguyen BD. Pancreatic neuroendocrine tumor with portal vein tumor thrombus: PET demonstration. Clin Nucl Med 2005;30:628-9. [Crossref] [PubMed]
- Yamagami Y, Tori M, Sakaki M, et al. Thyroid carcinoma with extensive tumor thrombus in the atrium. Gen Thorac Cardiovasc Surg 2008;56:555-8. [Crossref] [PubMed]
- Onoda N, Nakamura M, Hosono M, et al. Successful surgical treatment of advanced follicular thyroid carcinoma with tumor thrombus infiltrating the superior vena cava: report of a case. Surg Today 2012;42:185-90. [Crossref] [PubMed]
- Al-Jarrah Q, Abou-Foul A, Heis H. Intravascular extension of papillary thyroid carcinoma to the internal jugular vein: A case report. Int J Surg Case Rep 2014;5:551-3. [Crossref] [PubMed]
- Kawano F, Tomita M, Tanaka H, et al. Thyroid carcinoma with extensive tumor thrombus in the superior vena cava: A case report. Int J Surg Case Rep 2016;29:25-9. [Crossref] [PubMed]
- Kobayashi K, Hirokawa M, Yabuta T, et al. Tumor thrombus of thyroid malignancies in veins: importance of detection by ultrasonography. Thyroid 2011;21:527-31. [Crossref] [PubMed]
- Johnson DE, Appelt G, Samuels ML, et al. Metastases from testicular carcinoma. Study of 78 autopsied cases. Urology 1976;8:234-9. [Crossref] [PubMed]
- Husband JE, Bellamy EA. Unusual thoracoabdominal sites of metastases in testicular tumors. AJR Am J Roentgenol 1985;145:1165-71. [Crossref] [PubMed]
- Dusaud M, Bayoud Y, Desfemmes FR, et al. Unusual presentation of testicular cancer with tumor thrombus extending to the inferior vena cava. Case Rep Urol 2015;2015:160560. [PubMed]
- Masui S, Onishi T, Arima K, et al. Successful management of inferior vena cava thrombus complicating advanced germ cell testicular tumor with temporary inferior vena cava filter. Int J Urol 2005;12:513-5. [Crossref] [PubMed]
- Savarese DM, Rohrer MJ, Pezzella AT, et al. Successful management of intracardiac extension of tumor thrombus in a patient with advanced nonseminomatous germ cell testicular cancer. Urology 1995;46:883-7. [Crossref] [PubMed]
- Geffen DB, Kaneti J, Hendler N, et al. Testicular carcinoma with inferior vena cava thrombosis extending into the right atrium treated with chemotherapy and anticoagulation. Eur Urol 1992;21:82-4. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Sadat U, Noor N, See TC, et al. Peripheral arterial ischemia by a primary lung tumour invading left atrium. Lung Cancer 2007;57:237-9. [Crossref] [PubMed]
- Capdeville M, Hearn C, Rice TW, et al. Left atrial metastasis of a large cell carcinoma of the lung in an asymptomatic patient: transesophageal echocardiographic evaluation. J Cardiothorac Vasc Anesth 1997;11:492-4. [Crossref] [PubMed]
- Brandt RR, Rubin J, Reeder GS. Intracardiac extension of a lung tumor causing left ventricular inflow obstruction. J Am Soc Echocardiogr 1995;8:930-3. [Crossref] [PubMed]
- Ucak A, Inan K, Onan B, et al. Free-floating tumor thrombus in the left atrium associated with non-small cell lung cancer. J Card Surg 2009;24:686-9. [Crossref] [PubMed]
- Otani K, Ishihara S, Hata K, et al. Colorectal cancer with venous tumor thrombosis. Asian J Surg 2016. pii: S1015-9584(16)30149-X.
- Gupta P, Kramer EL, Ponzo F. FDG uptake in tumor thrombus in inferior vena cava from rectal cancer on positron emission tomography. Clin Nucl Med 2005;30:342-3. [Crossref] [PubMed]


