Endovascular management of iliac aneurysmal disease with hypogastric artery preservation
Introduction
As the field of endovascular aneurysm repair continues to evolve, more complex and dynamic treatment options are becoming available to tailor repairs for individual patients. The one-size-fits-all approach of aneurysm repair is being supplanted by advanced techniques to treat even the most perplexing aneurysms. Common iliac artery aneurysms (CIAAs) have posed a particular challenge in the realm of endovascular therapies. Due to complex anatomical constraints of CIAA, repair has traditionally been performed with sacrifice of the ipsilateral hypogastric artery (HA) using embolization or covering the origin of the HA with an endograft limb (1,2). This, however, carries the potential for significant morbidity, including unremitting buttock claudication, bowel ischemia, renal failure, sexual dysfunction, gluteal necrosis and paralysis (3,4).
The most common of these is claudication. In fact, rates of buttock claudication are reported to be as high as 50% when the HA is embolized or covered by an endograft limb (1,5,6). Risk factors for development of symptoms of pelvic ischemia revolve around the patient’s collateral circulation, age, activity level, and cardiac function (7,8). Younger patients who are more active are more prone to developing claudication and patients with decreased cardiac function are also at increased risk. Iliopoulos et al. showed the greater importance of collateral flow through the ipsilateral profunda and circumflex femoral arteries rather than the contralateral HA with respect to arterial occlusive disease (8). Symptoms of buttock claudication are not always self-limiting; continued complications of claudication may last >6 months in up to 34% of patients (7).
New-onset sexual dysfunction is reported in up to 33% of patients with persistent symptoms found in 15% (7,9). Taken together, the complications of claudication and sexual dysfunction result in marked decrease in quality of life following aneurysm repair. With the advent of new endografts and novel endovascular techniques, it is feasible to significantly decrease the rates of these complications associated with CIAA repair. Herein we discuss the various treatment methods of endovascular repair for CIAA while preserving flow to the HA.
Indications
Most CIAAs are asymptomatic and found incidentally. These are often found in patients with abdominal aortic aneurysms at a rate of 20% or greater (10). As with aneurysms elsewhere, the goal of treatment for CIAAs is to prevent aneurysm rupture. A common iliac artery is considered aneurysmal at 1.5 cm or >50% larger than the adjacent vessel. However, aneurysms can be safely followed with sizes below 3 cm. Average growth rates are reported at 1.1 mm/year at sizes below 3 cm and 2.6 mm/year at 3–5 cm (11). Huang et al. reported their experience with 438 patients with 715 CIAAs (12). On average, CIAAs ruptured at 6 cm (range, 3.8–8.5 cm) although smaller sizes were reported. The growth rate for CIAAs is relatively slow at 0.29 cm/year. Although there is variability among authors, current recommendations are to repair CIAAs when they exceed 3.0–3.5 cm.
Techniques
Tube grafts and bell-bottoms
In rare instances when isolated CIAAs present as saccular or fusiform within the mid segment of CIA, aneurysm treatment can be carried out with placement of a tube graft using an iliac limb or covered balloon-expandable stent, such as the Viabahn VBX (Gore Flagstaff, AZ, USA). This requires adequate proximal and distal landing zones at the aortic bifurcation and iliac bifurcation, typically greater than 1 cm. Reports have also described combined procedures of placing tube grafts through the common and external iliac arteries (EIA) with surgical re-implantation or bypass to the ipsilateral HA (13-16). Retrograde HA preservation (REHAP) has also been described (17). Using this technique, a covered stent is placed from the HA to the ipsilateral EIA. This excludes flow from the CIA and requires a femorofemoral bypass.
Alternatively, most standard endografts have common iliac limbs offering flared extensions of various sizes to treat small and medium sized CIAAs (1.6–2.8 cm) (Figure 1). Torsello et al. reported their 5-year data on bell-bottom extensions in 89 patients showing no change in CIAA diameter at last follow-up (18). When compared with embolization of the HA and extension of the endograft into the EIA versus placement of a bell-bottom graft, the combined incidence of perioperative complications and reintervention favors bell-bottom treatment (19).
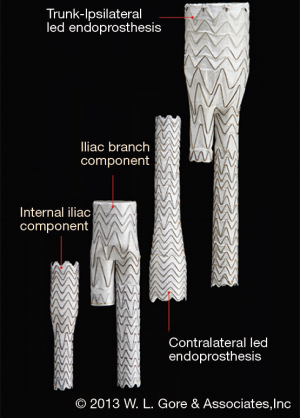
Iliac branch devices
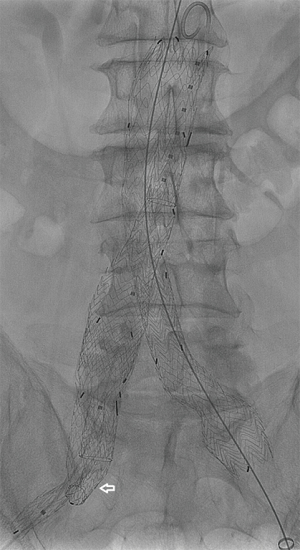
In March 2016, the US Food and Drug Administration (FDA) approved the GORE EXCLUDER Iliac Branch Endoprosthesis (IBE). This is the first commercially approved device for treating CIAAs in the US. The GORE IBE device consists of a modified 23-mm main body endograft and a HA iliac branch component (reversed iliac extension limb) placed into the HA (Figure 1). The GORE IBE is positioned and deployed in a two-stage system to facilitate accuracy of placement. The HA gate has a hypotube mounted for advancement of a hydrophilic wire. The wire is snared from the contralateral groin to allow for through-and-through femoral access. A sheath is advanced through the contralateral groin to the HA gate where the reversed iliac extension is deployed (Figure 2). As part of the instructions for use, a standard Gore endograft is then deployed with a bridging bell-bottom limb from the contralateral gate of the main body endograft to the GORE IBE. Off-label use without placement of the main body endograft has also been reported (20).
A number of limitations exist with all iliac branch devices (IBD), including the GORE IBE device. First, adequate length is necessary; distance from the lowest renal artery to the HA origin must be >165 mm in order to have sufficient minimum overlap of the main body and iliac components. Secondly, the common iliac diameter must have a flow lumen >17 mm at the proximal implantation zone and >14 mm at the common iliac bifurcation to appropriately seat the device. Lastly, HA diameters must be between 6.5 and 13.5 mm with at least 10-mm landing zones for adequate HA seal. If the length of the landing zone is inadequate, the HA limb is deployed into the larger of the anterior or posterior divisions with embolization of the other branch. The EIA diameter must be 6.5–25.0 mm.
Reports of use of the GORE IBE in large series of patients are lacking, as is data on mid- and long-term results. A retrospective analysis of 51 IBE implantations for 46 patients from 13 sites in The Netherlands showed procedural success of 93.5% with two type Ib endoleaks which resolved on follow-up imaging (21). Primary patency was reported at 94% at 6 months with two interventions each, one for EIA limb stenosis and the other for placement of an occlusion device in the ipsilateral HA for a new type Ib endoleak. Four patients developed buttock claudication with two on the contralateral side. One patient treated with bilateral IBE had symptoms due to unclear reasons as the HA were patent on follow-up imaging. The fourth patient had interval occlusion of the HA limb on short-term follow-up resulting in symptoms; this patient was treated with a walking program. Ferrer et al. (22) described their series of seven patients demonstrating a technical success rate and branch patency rate of 100% with 1 of 2 bilateral CIAA cases requiring re-intervention due to compression of the iliac legs at the aortic bifurcation. Similarly, Millon et al. (23) reported on ten patients with a technical success rate of 100% and one branch occlusion at 6 months. No type Ib or III endoleaks were reported.
Most recently, prospective results from the US pivotal trial for FDA approval of the device were reported on 63 patients who underwent unilateral IBE (24). In the setting of bilateral CIAA (39.1%), the contralateral HA was embolized. Technical success was 95.2% with three failures each due to type Ib or type III endoleaks or implantation of a standard Gore limb extension rather than the approved iliac branch component. However, both endoleaks resolved at 1 month follow-up. Three patients had asymptomatic occlusion of the HA at 1-month follow-up for a primary patency rate of 95.1% at 6 months. There were no instances of buttock claudication on the IBE side, but 6 of 21 (28.6%) patients with bilateral CIAA developed symptoms on the side where the HA was embolized. Although there were no type I or III endoleaks at 3 months, a high number of type II endoleaks were reported at 54.7% for unclear reasons.
In addition to the GORE IBE, other IBD have been commercially available in Europe and have proven results of safety and efficacy (25-27). These are not available in the US at the time of this publication, but include versions of the Zenith IBD (Cook Medical, Brisbane, Australia) and the Jotec E-liac device (Jotec, Hechingen, Germany). Two generations of the Zenith IBD have been used since 2006. The most recent iteration of the device has a fixed proximal diameter of 12 mm and a distal diameter of 10, 12, or 16 mm, a common iliac segment of 45 or 61 mm, and an EIA segment length of 41 or 58 mm. The deployment mechanism consists of a preloaded catheter for an 0.035” guidewire. The wire is snared from the contralateral side and a sheath is advanced over the wire into the reinforced stump of an internal iliac artery (IIA) fenestration. A bridging balloon-expandable covered stent is then advanced and inserted into the HA with a minimum 10-mm landing zone. Similar to the GORE IBE, if the length of the landing zone is inadequate, the stent is deployed into the larger of the anterior or posterior divisions with embolization of the other branch. Successful deployment of the IBD is then followed by placement of a main-body Zenith endograft in the absence of an adequate proximal landing zone in the common iliac artery or when aortic aneurysmal disease is present.
The Zenith IBD is also limited by anatomical constraints. EIA length must be ≥20 mm with diameters 8–11 mm and the HA diameters must be 6–9 mm. CIA length must be >50 mm with diameters of ≥16 mm.
Parlani et al. reported their single-center experiences with the Zenith IBD in 120 patients over 5 years (25). Success rate was reported at 95% with 5 intra-operative IBD occlusions—4 due to embolization of thrombus into the HA during difficult advancement of the contralateral sheath. The fifth occlusion was due to failure to adequately deploy the covered stent in a tortuous HA. During the 30-day perioperative period, two patients had external iliac limb occlusion treated with thrombolysis or thrombectomy. Mean follow-up was 17 months with no aneurysm ruptures reported and only two additional HA limb occlusions reported. IBD patency rate was 91.4%. Two type I endoleaks were noted at 1 and 15 months, and one type III endoleak occurred during the first month. These were successfully treated with extensions into the HA via brachial access. Rates of buttock claudication were 4% and due to either occlusion of the HA limb or due to contralateral HA embolization in the case of bilateral CIA aneurysms (when only one side was repaired).
In a similar report, Pratesi et al. detailed their work at two centers in Italy with 85 patients with 37.1% of patients having bilateral CIAAs (26). Only 4.9% of these were treated with bilateral IBDs while the remaining underwent unilateral treatment with embolization or overstenting of the contralateral HA. Technical success was 98.7% and only one IBD occlusion occurred during the first 30 days. Patency of the IBD at 48 months was 98% with no aneurysm-related deaths. Three (3.7%) type I IBD endoleaks were noted without aneurysm growth and are under surveillance. Seven patients (8.6%) experienced buttock claudication—6 of these were reported on patients with bilateral disease who had contralateral HA embolization, and the seventh was due to occlusion of the IBD.
A new device on the European market is the Jotec E-liac device. The E-liac device has similar design to other IBD and can be used as a stand-alone device or in combination with the Jotec E-vita AAA system. In this endograft, an asymmetric spring device allows for conformability and potentially less kinking in tortuous vessels. Anatomical constraints associated with this device include a CIA diameter of ≥18 mm with at least a 40-mm length, 10-mm length of normal HA with diameters ≥4 mm and EIA diameter ≥8 mm. The device is deployed through an 18 F hydrophilic sheath after snaring a through-and-through hydrophilic wire from the contralateral groin. Once the device is deployed, a covered balloon-expandable stent is placed into the HA.
Mylonas et al. reported 12-month prospective data on 82 implantations on 70 patients with use of the Jotec E-liac device (28). Technical success was achieved in 100%, although two type Ib endoleaks occurred from the HA. These were each treated with covered-stent extensions. By 12-month follow-up, one HA, two EIA, and two CIA occlusions occurred, and freedom from occlusion at 12 months was 92%. Initially, type II endoleaks were noted in 15 patients in the first 30 days but only 1 persisted at 12 months. All were without aneurysm enlargement. No instances of buttock claudication were noted.
Snorkels, periscopes, and sandwiches
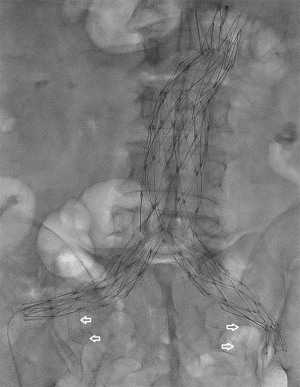
Due to the limitations associated with IBDs, only 35% of patients have anatomy suitable for repair (29). When CIAA fall outside the instructions for use (IFU) of the above devices, several creative methods of endovascular aneurysm repair have been developed. The sandwich or snorkel technique consists of placing a main body endograft into the aorta and inserting a stent-graft—typically a self-expanding Viabahn (Gore Flagstaff, AZ, USA) or less commonly a Fluency (Bard, Flagstaff, AZ, USA) or a balloon-expandable iCast (Atrium, Hudson, NH, USA)—in parallel with either a second stent-graft or a limb extension from the HA and EIA. The two endoprostheses are placed within the main body endograft to form a “double-D” configuration within the ipsilateral gate. This can be achieved with a bilateral groin approach with an AFX device (Endologix, Irvine, CA, USA) (Figure 3) or with groin and brachial approaches for other devices.
Placement of parallel endograft components has been reported. In this technique, off-the-shelf iliac limbs are selected and deployed from bilateral common femoral artery accesses concomitantly with a self-expanding stent-graft in the HA from a brachial approach (30,31). The stent-graft is deployed 5–10 mm above the iliac limbs to prevent compression. The sum of the diameter of the iliac limbs and stent-graft are measured to achieve a 20% oversize of the neck of the normal aorta. This approach is limited to treatment of isolated CIAA or distal aortoiliac aneurysms. Due to length constraints of iliac limb extensions, the limbs are sized to seal in the mid-to-distal aorta so a long neck is needed below the renal arteries.
Cross-over chimney techniques have been reported (32). In this instance, HA preservation during aortic aneurysm repair is achieved by placing a self-expanding stent-graft from the ipsilateral HA over the aortic bifurcation with the distal landing zone in the contralateral CIA or EIA (if contralateral CIA aneurysm exists). The stent-graft is deployed in parallel with a standard bifurcated endograft.
Although these innovative methods of iliac aneurysm repair are technically interesting and clinically necessary in select instances, the “off-label” use of these devices pose unknown risks of patency and gutter-related type I and type III endoleaks, especially in light of the extreme variability in techniques. Long-term data are lacking to prove durability. Lobato et al. reported their mid-term analysis of 40 patients who underwent sandwich technique procedures with placement of a main body endograft and a limb extension with a self-expanding covered stent (33). At the time of completion angiogram, 2 (5%) type I, 4 (10%) type II, and 1 (2.5%) type III endoleaks were noted. The two type I endoleaks were treated successfully with proximal cuffs and the type III endoleaks resolved spontaneously. Three type II endoleaks resolved and the fourth was followed. Buttock claudication initially developed in 4.2% of patients and resolved on mid-term follow-up, and erectile dysfunction developed in 2.5% which did not resolve.
Endovascular sealing (EVAS) and multilayered stents
A more recent technical advancement is the concept of EVAS. With this technique, two balloon-expandable covered stents, surrounded by polyurethane endobags, are placed across the aneurysms within the aorta and CIAs. Once the stents are deployed, the endobags are filled with a polyethylene glycol polymer, displacing blood within the flow lumen of the aneurysm. Adequate proximal and distal landing zones are required in the aorta and CIAs in order to preserve flow into the HAs. Although the endobags can be landed precisely above the origins of the HA in the setting of CIAA, if the aneurysm involves the HA origin, EVAS alone is often not possible if HA preservation is desired. Youssef et al. reported their experiences with EVAS for treatment of CIAAs either as a primary treatment or in conjunction with other methods described above with conventional endografts (i.e., IBD) (34). In this study, 17 patients underwent EVAS with an IBD. No type III endoleaks were noted, and technical success was 100%. One type II endoleak was noted from an ectatic HA on final angiogram that resolved on follow-up CT. No endoleaks were seen at 12-month follow-up and all IBDs remained patent.
Flow-diverting multilayered stents have been proposed as a treatment alternative to embolization or parallel grafting in patients that are not candidates for IBD/IBE. These devices are not commercially available in the US. The non-covered, interwoven stent design allows for a decrease in wall shear stress on the aneurysm (encouraging aneurysm thrombosis) while preserving blood flow to branch vessels. Data on iliac artery aneurysm treatment is scarce and generally limited to case reports. In the largest published series to date, Pieper et al. reported their results on eight patients treated for ten iliac artery aneurysms (35). Two stent occlusions occurred. Four CIAAs thrombosed and branch vessels remained patent at 15 months. In the remaining four patients, one CIAA decreased slightly in diameter while the other CIAAs were stable in size.
Conclusions
With the advent of new techniques and devices, hypogastric artery preservation during repair of CIAA has become feasible in most cases. Rates of complications are low and high technical success has been shown. To prevent the possible complications of pelvic ischemia, hypogastric preservation should be offered to all patients when technically achievable.
Acknowledgements
None.
Footnote
Conflicts of Interest: BJ Schiro, RT Gandhi, CS Peña, AR Geronemus, A Powell and JF Benenati are speakers for WL Gore. CS Peña and RT Gandhi are also speakers for Cook Medical and Medtronic.
References
- Lee C, Dougherty M, Calligaro K. Concomitant unilateral internal iliac artery embolization and endovascular infrarenal aortic aneurysm repair. J Vasc Surg 2006;43:903-7. [Crossref] [PubMed]
- Papazoglou KO, Sfyroeras GS, Zambas N, et al. Outcomes of endovascular aneurysm repair with selective internal iliac artery coverage without coil embolization. J Vasc Surg 2012;56:298-303. [Crossref] [PubMed]
- Mehta M, Veith FJ, Ohki T, et al. Unilateral and bilateral hypogastric artery interruption during aortoiliac aneurysm repair in 154 patients: a relatively innocuous procedure. J Vasc Surg 2001;33:S27-32. [Crossref] [PubMed]
- Farivar BS, Kalsi R, Drucker CB, et al. Implications of concomitant hypogastric artery embolization with endovascular repair of infrarenal abdominal aortic aneurysms. J Vasc Surg 2017;66:95-101. [Crossref] [PubMed]
- Rayt HS, Sutton AJ, London NJ, et al. A systematic review and meta-analysis of endovascular repair (EVAR) for ruptured abdominal aortic aneurysm. Eur J Vasc Endovasc Surg 2008;36:536-44. [Crossref] [PubMed]
- Razavi MK, DeGroot M, Olcott C 3rd, et al. Internal iliac artery embolization in the stent-graft treatment of aortoiliac aneurysms: analysis of outcomes and complications. J Vasc Interv Radiol 2000;11:561-6. [Crossref] [PubMed]
- Farahmand P, Becquemin JP, Desgranges P, et al. Is hypogastric artery embolization during endovascular aortoiliac aneurysm repair (EVAR) innocuous and useful? Eur J Vasc Endovasc Surg 2008;35:429-35. [Crossref] [PubMed]
- Iliopoulos JI, Howanitz PE, Pierce GE, et al. The critical hypogastric circulation. Am J Surg 1987;154:671-5. [Crossref] [PubMed]
- Rayt HS, Bown MJ, Lambert KV, et al. Buttock claudication and erectile dysfunction after internal iliac artery embolization in patients prior to endovascular aortic aneurysm repair. Cardiovasc Intervent Radiol 2008;31:728-34. [Crossref] [PubMed]
- Baskin KM, Jimenez RM, Cahill AM, et al. Cavoatrial junction and central venous anatomy: implications for central venous access tip position. J Vasc Interv Radiol 2008;19:359-65. [Crossref] [PubMed]
- Santilli SM, Wernsing SE, Lee ES. Expansion rates and outcomes for iliac artery aneurysms. J Vasc Surg 2000;31:114-21. [Crossref] [PubMed]
- Huang Y, Gloviczki P, Duncan AA, et al. Common iliac artery aneurysm: expansion rate and results of open surgical and endovascular repair. J Vasc Surg 2008;47:1203-10; discussion 1210-1. [Crossref] [PubMed]
- Arko FR, Lee WA, Hill BB, et al. Hypogastric artery bypass to preserve pelvic circulation: improved outcome after endovascular abdominal aortic aneurysm repair. J Vasc Surg 2004;39:404-8. [Crossref] [PubMed]
- Faries PL, Morrissey N, Burks JA, et al. Internal iliac artery revascularization as an adjunct to endovascular repair of aortoiliac aneurysms. J Vasc Surg 2001;34:892-9. [Crossref] [PubMed]
- Hosaka A, Kato M, Kato I, et al. Outcome after concomitant unilateral embolization of the internal iliac artery and contralateral external-to-internal iliac artery bypass grafting during endovascular aneurysm repair. J Vasc Surg 2011;54:960-4. [Crossref] [PubMed]
- Lee WA, Nelson PR, Berceli SA, et al. Outcome after hypogastric artery bypass and embolization during endovascular aneurysm repair. J Vasc Surg 2006;44:1162-8; discussion 1168-9. [Crossref] [PubMed]
- Ayerdi J, McLafferty RB, Solis MM, et al. Retrograde endovascular hypogastric artery preservation (REHAP) and aortouniiliac (AUI) endografting in the management of complex aortoiliac aneurysms. Ann Vasc Surg 2003;17:329-34. [Crossref] [PubMed]
- Torsello G, Schönefeld E, Osada N, et al. Endovascular treatment of common iliac artery aneurysms using the bell-bottom technique: long-term results. J Endovasc Ther 2010;17:504-9. [Crossref] [PubMed]
- Naughton PA, Park MS, Kheirelseid EA, et al. A comparative study of the bell-bottom technique vs hypogastric exclusion for the treatment of aneurysmal extension to the iliac bifurcation. J Vasc Surg 2012;55:956-62. [Crossref] [PubMed]
- Ardita V, Giaquinta A, Veroux M, et al. Endovascular repair of bilateral common iliac artery aneurysms using GORE Excluder iliac branch endoprosthesis without aortobi-iliac stent graft conjunction: A case report. Medicine (Baltimore) 2017;96:e5977. [Crossref] [PubMed]
- van Sterkenburg SM, Heyligers JM, van Bladel M, et al. Experience with the GORE EXCLUDER iliac branch endoprosthesis for common iliac artery aneurysms. J Vasc Surg 2016;63:1451-7. [Crossref] [PubMed]
- Ferrer C, De Crescenzo F, Coscarella C, et al. Early experience with the Excluder® iliac branch endoprosthesis. J Cardiovasc Surg (Torino) 2014;55:679-83. [PubMed]
- Millon A, Della Schiava N, Arsicot M, et al. Preliminary Experience with the GORE(®) EXCLUDER(®) Iliac Branch Endoprosthesis for Common Iliac Aneurysm Endovascular Treatment. Ann Vasc Surg 2016;33:11-7. [Crossref] [PubMed]
- Schneider DB, Matsumura JS, Lee JT, et al. Prospective, multicenter study of endovascular repair of aortoiliac and iliac aneurysms using the Gore Iliac Branch Endoprosthesis. J Vasc Surg 2017;66:775-85. [Crossref] [PubMed]
- Parlani G, Verzini F, De Rango P, et al. Long-term results of iliac aneurysm repair with iliac branched endograft: a 5-year experience on 100 consecutive cases. Eur J Vasc Endovasc Surg 2012;43:287-92. [Crossref] [PubMed]
- Pratesi G, Fargion A, Pulli R, et al. Endovascular treatment of aorto-iliac aneurysms: four-year results of iliac branch endograft. Eur J Vasc Endovasc Surg 2013;45:607-9. [Crossref] [PubMed]
- Karthikesalingam A, Hinchliffe RJ, Holt PJ, et al. Endovascular aneurysm repair with preservation of the internal iliac artery using the iliac branch graft device. Eur J Vasc Endovasc Surg 2010;39:285-94. [Crossref] [PubMed]
- Mylonas SN, Rümenapf G, Schelzig H, et al. A multicenter 12-month experience with a new iliac side-branched device for revascularization of hypogastric arteries. J Vasc Surg 2016;64:1652-9.e1. [Crossref] [PubMed]
- Pearce BJ, Varu VN, Glocker R, et al. Anatomic suitability of aortoiliac aneurysms for next generation branched systems. Ann Vasc Surg 2015;29:69-75. [Crossref] [PubMed]
- Lepidi S, Piazza M, Scrivere P, et al. Parallel endografts in the treatment of distal aortic and common iliac aneurysms. Eur J Vasc Endovasc Surg 2014;48:29-37. [Crossref] [PubMed]
- Frigatti P, Lepidi S, Piazza M, et al. A New Endovascular Approach to Exclude Isolated Bilateral Common Iliac Artery Aneurysms. EJVES Extra 2010;19:e55-7. [Crossref]
- Guo X, Li P, Liu GR, et al. Treating patients with abdominal aortic aneurysm with endovascular repair and the crossover chimney technique in the internal iliac artery to protect the unilateral internal iliac artery. Int J Clin Exp Med 2015;8:21737-45. [PubMed]
- Lobato AC, Camacho-Lobato L. The sandwich technique to treat complex aortoiliac or isolated iliac aneurysms: results of midterm follow-up. J Vasc Surg 2013;57:26S-34S. [Crossref] [PubMed]
- Youssef M, Zerwes S, Jakob R, et al. Treating iliac aneurysm using the Nellix Endovascular Sac Sealing System. Semin Vasc Surg 2016;29:114-9. [Crossref] [PubMed]
- Pieper CC, Meyer C, Rudolph J, et al. Interventional exclusion of iliac artery aneurysms using the flow-diverting multilayer stent. Cardiovasc Intervent Radiol 2013;36:917-25. [Crossref] [PubMed]


