Re-intervention for occluded iliac vein stents
Introduction
Iliac vein stenting is performed when adequate venous patency is not obtained with angioplasty and/or lysis alone in the treatment of three common conditions: non-thrombotic iliac vein lesions (NIVLs), i.e., compression—also known as May-Thurner syndrome; thrombotic occlusions and outflow obstructions in patients with symptomatic reflux—chronic venous insufficiency (CVI). Stenting of the venous system has been a natural extension of arterial stent technique and technology. Unfortunately, the stents are typically designed for the arterial system and do not possess the ideal characteristics for treatment of venous pathology. Despite this limitation, iliac vein stenting is known to be effective and successful in the treatment of all three disease processes, however, stent failure in the form of in-stent stenosis does occasionally occur. Before discussing the options for re-intervention and the technique, it is best to review why stent placement was initially performed.
May-Thurner syndrome
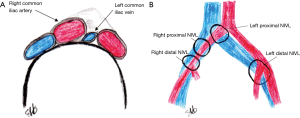
The prevalence of symptomatic left iliac vein compression by the right common femoral artery is unknown, however, it is estimated to occur in 18–49% of patients with a left lower extremity deep venous thrombosis (DVT) (1). The existence of iliac vein compressions and intraluminal webs is in itself more pathogenic than previously thought as they were thought to be of little clinical importance. NIVLs are found as often as post-thrombotic obstruction in the setting of chronic venous disease involving the iliofemoral venous segment. NIVLs may involve the common and external iliac veins of either lower limb in both sexes at any age (2). Some degree of iliac vein compression is present as a normal anatomic variant in otherwise healthy patients [>50% compression in up to 25% of patients (3)] and can be considered a permissive lesion (4) (Figure 1).
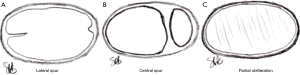
As early as 1851, Virchow noted that DVT was 5 times more common in the left leg versus the right leg. It was not until 1957 when May and Thurner described thickening of the left common iliac vein where it was crossed and compressed by the right common iliac artery as focal segmental venous fibrosis or “spurring” as a causative factor for left iliofemoral DVT. Their study of 430 cadavers demonstrated obstructive lesions in 22% of left iliac veins. They described three types of lesions (Figure 2):
- Lateral “spur”—a coulisse-like tender membrane protruding velum-like from the medial or lateral wall towards the lumen, like a pier, which it narrows;
- Central “spur” —the lumen can be divided into a large number of fields, but only in a dorso-anterior fashion;
- Partial obliteration—the lumen is obliterated almost entirely, as if “quilted,” and the vessel appears fenestrated (5).
The prevailing thought is that the “spur” forms as a result of microvascular trauma to the vein endothelium from the pressure of the overlying artery pushing the vein posteriorly into the lumbar vertebrae. As a result, elastin & collagen are deposited in the vein.
A female predominance is observed in May-Thurner syndrome with two common presentations. The “classic” presentation is the female between 20–40 years of age with an acute DVT, with or without known risk factors for DVT. The less common presentation is in a patient with progressive pain, unilateral left leg swelling, varicose veins +/− venous ulcerations without an identifiable DVT.
Diagnosis requires contrast venography and will help identify the three common angiographic patterns of lesions associated with May-Thurner syndrome. These include focal stenosis or collateralized short segment occlusion of the left common iliac vein, acute iliofemoral venous thrombosis with the underlying spur/lesion being revealed after thrombolysis, and chronic isolated thrombosis of the left common and external iliac veins with collaterals arising from the common femoral vein (6).
Treatment should be performed only in symptomatic individuals. Revascularization is necessary to resolve the venous hypertension and prevent the dreaded sequelae of post-thrombotic syndrome (PTS) whose symptoms can include: CVI, venous stasis ulcerations, venous eczema, and venous claudication. Ideally, after the vein is patent, IVUS combined with contrast venography guide further therapy which may include angioplasty with possible stenting.
Thrombotic occlusions
Iliofemoral vein thromboses account for up to 25% of all DVTs and are associated with an increased risk of embolic and post-thrombotic complications when compared to more distal DVTs. Iliofemoral DVTs are most frequently diagnosed with ultrasound, however, they can also be identified with computed tomography venogram, magnetic resonance venogram, and on routine cross-sectional imaging with intra-venous contrast. Unfortunately, more than 30% of patients with symptomatic iliofemoral DVT will have residual thrombus following a 3-month course of anticoagulation. Residual thrombus is a strong risk factor for recurrent DVT, which occurs nearly 3 times as often following iliofemoral thrombosis as compared to a DVT below the knee. Residual thrombus and recurrent DVT are strong predictors of subsequent post thrombotic syndrome. With the use of anticoagulation and adjuvant compression stockings alone (no procedural intervention), over 50% of patients with iliofemoral DVT will go on to develop PTS (7). Treatment may include systemic anticoagulation with possible thrombolysis +/− angioplasty and stenting.
CVI
CVI is persistent ambulatory venous hypertension—defined as elevated venous pressure during exercise. Ambulatory venous pressure in the lower leg and foot generally drops to less than 50% of the standing venous pressure in normal individuals. In patients with PTS, the ambulatory venous pressure drops very little, and in patients with persistent venous occlusion, the ambulatory venous pressure may actually rise above standing pressure.
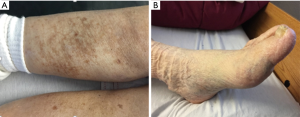
Varicose veins (including telangiectasias and reticular veins) affect more than 25 million adults in the United States and greater than 6 million have “advanced” venous disease with symptoms including: hyperpigmentation, venous eczema, lipodermatosclerosis, atrophie blanche, and healed or active ulcers (8). CVI is thought to be the 7th most common indication for medical referral in the United States (9). There is great difficulty in identifying the exact incidence due to varying definitions for varicosities and subjective evaluations of advanced disease. The National Venous Screening Program conducted by the American Venous Forum in the United States identified varicose veins in >30% of participants and more advanced disease in >10% (5). Approximately 20% of those with CVI will develop venous ulcers (10). Prognosis is poor for these patients as delayed healing and recurrent ulceration are very common. Disability related to venous ulcers leads to loss of productive work hours, estimated at 2 million workdays per year, and may cause early retirement, found in >12% of workers with venous ulcers (11). The financial burden of venous ulcer disease alone is great, with an estimated $1 billion spent annually on treatment of chronic wounds in the United States (8).
Multiple studies have demonstrated the prevalence to be approximately twice as high in women, and it increases with age (9). Predisposing factors for venous insufficiency include: gender, age, heredity, parity, obesity, employment, and lifestyle. The two most widely accepted theories of primary varicose vein pathophysiology are (I) primary valvular incompetence and (II) primary, or congenital, vein wall weakness. There is increasing evidence that primary varicose veins result from an intrinsic genetic defect of collagen synthesis (9).
The primary pathophysiology is also affected by superimposed DVT. Multiple studies have shown that patients with the most severe PTS have valvular incompetence accompanied by luminal obstruction (12,13). Venous obstruction is not synonymous to occlusion. Obstruction is a relative narrowing of the venous lumen whereas occlusion is complete closure of the venous lumen.
The diagnosis of CVI requires a complete history and physical exam and a thorough ultrasound of the legs including standing evaluation. The presence of reflux is determined by the direction of flow, because any significant pedal flow is suggestive of reflux. The reflux time, or duration of visualized reflux, is used to diagnose the presence of reflux: >0.5 seconds for superficial veins and ≥1.0 seconds for deep veins. Treatment will vary depending upon the severity. If central obstruction is identified as a causal factor, angioplasty with possible stenting is recommended (Table 1) (Figures 3,4) .

Full table
In order to standardize the reporting and treatment of the diverse manifestations of chronic venous disorders, a comprehensive classification system (CEAP) has been developed to allow uniform diagnosis so physicians can attempt to be more accurate and consistent in their workups across a variety of settings and specialties. CEAP is an abbreviation for: Clinical, Etiologic, Anatomic, and Pathophysiologic (Table 2).

Full table
Initial treatment strategy
The degree of lysis is a major predictor of early and long-term patency for all disease states. More complete clearance of thrombus leads to patency rates in excess of 75% in most studies, versus incomplete lysis (≥50% residual clot remaining) leads to patency rates of less than 40% (14). Stents should be placed in the iliac vein when any non-dissolved lesion is identified. Per Bækgaard et al., ballooning alone has no role in management of iliac vein obstructive lesions (15). The National Venous Registry in 1999 demonstrated that post-procedural re-thrombosis with anticoagulation was significantly more frequent in the group with untreated lesions compared with the group of stented segments. Iliac patency after 1 year was significantly better in the stented group compared to the limbs without stent placement (74% versus 53%, P<0.001) (14). The stents need to be self-expandable, flexible due to the complex curves of the pelvis, and have enough radial force to overcome the low pressure of the venous system (Figure 5).
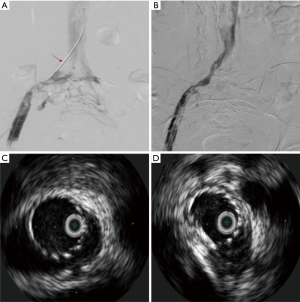
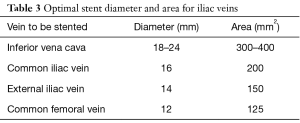
Initial stent sizing can have a significant impact on the need for re-intervention. Initial deployment of undersized stents for fear of rupture is misguided and may result in poor symptomatic relief due to inadequate decompression of the vein. Management can be limited with angioplasty at a later date due to the size of the stent. Use of IVUS to evaluate inflow and outflow is particularly important in these cases to ensure no other concomitant problems (Table 3).
Full table
Long-Term outcomes of venous outflow stenting
Neglen et al. reviewed 982 non-malignant obstructive lesions of the femoro-ilio-caval vein treated with stents placed with fluoroscopic guidance and IVUS. The mean age was 54 years with a female/male ratio of 2.6:1, left/right symptoms of 2.4:1, and primary/secondary etiology of 518:464. The clinical CEAP scores were 2–7%, 3–47%, 4–24%, 5–5%, and 6–17%. Among these patients, 94% were monitored for a mean of 22 months. At 5 years, cumulative rates of complete relief of pain and swelling were 62% and 32%, respectively, and 58% of ulcers healed. The cumulative rate of severe in-stent restenosis (ISR) (<50%) occurred in 5% of limbs at 72 months (10% thrombotic limbs, 1% NIVL). The chief risk factors associated with stent occlusion were the presence and severity of thrombotic disease. Thrombophilia by itself was not a risk factor. Stent occlusion appears to be caused by a recurrent thrombotic event rather than slowly evolving narrowing of the stent (2). Overall, the findings indicate that iliofemoral stenting can be performed with a low morbidity and mortality, high long-term patency rates, and low rate of ISR. Long lesions requiring multiple stents, and extension of the stent into the common femoral vein were associated with stent occlusion. The median time to re-intervention after initial stent placement was 15 months (16).
Re-interventions in the setting of malfunctioning iliac vein stents
As demonstrated by the study from Neglen et al., re-intervention is rarely needed. Early thrombosis (<30 days) of the stent occurred in 0.009% (8/870) of treated limbs—all in patients with post-thrombotic limbs. Six patients were treated with thrombolysis—only 3 of which were successful. Other interventions included bypass and thrombectomy. Late stent occlusion occurred in 3% of limbs at a median of 13 months. Acute re-thrombosis was the most common presentation and those patients were treated with thrombolysis. The thrombolysis was successful in only 31% of limbs with the remainder left occluded. Patients identified with occlusion at routine follow-up were successfully treated with anticoagulation (2).
A study performed by Raju et al. evaluated re-interventions for non-occlusive stent malfunction (16). In their series, 1,085 limbs underwent femoral-iliac-caval stenting over 10 years. Disease was NIVL in 577 limbs and post-thrombotic in 508 limbs. Thirteen percent of limbs [137] required 177 re-intervention procedures for non-occlusive stent malfunction. Stent malfunction was discovered on routine surveillance imaging in 31% of limbs while 69% of limbs demonstrated residual/recurrent symptoms. Presenting symptoms prompting re-intervention included: pain, swelling, pain with swelling, and venous dermatitis/ulceration. Intra-stent lesions alone occurred in 42% of procedures with concurrent extra-stent stenosis in 18%. Stenotic lesions outside the original stent territory were seen in 40% of procedures. Extra-stent stenosis occurred in 58% of re-interventions overall.
Differences were noted between the types of extra-stent lesions when comparing NIVL limbs to post-thrombotic limbs. In NIVL limbs, the outflow lesions above the stent were due to inadequate coverage of the original NIVL lesion at the junction of the iliac vein and inferior vena cava (IVC). Lesions detected below the stent in NIVL limbs were due to: a previously missed distal NIVL stenosis; a previously overlooked retro-inguinal stenosis or a de novo lesion of uncertain etiology. In post-thrombotic limbs, extra-stent lesions were stenosis or trabeculae in the inflow/outflow segments adjoining the stent that were new or missed during the original procedure.
Intra-stent lesions were similar for NIVL and post-thrombotic disease. Three distinct types were found: a soft ISR lesion; a hard more fibrous ISR lesion, or external compression of the stent from inadequate ballooning or recoil of previously dilated stenosis.
Overall, re-intervention in the setting of stent occlusion or non-occlusive malfunction significantly improved presenting symptoms and provided durable relief (Figure 6).
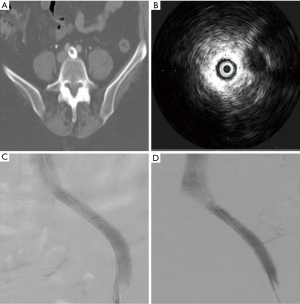
Discussion
The indications for re-intervention in malfunctioning iliac stents are very simple: (I) patients returning to clinic with recurrent symptoms; (II) occlusion/malfunction identified on routine surveillance.
Statistically, re-intervention for occluded iliac vein stents should be an infrequently performed procedure. To minimize the need for re-intervention, it is most important to perform initial stent placement appropriately. Given that contrast venography is poorly sensitive to iliac vein obstruction, IVUS must be used to evaluate the vein area as well as for the presence of transmural fibrosis with luminal webs and membranes. IVUS guided stenting also assists in identifying the appropriate landing zone for the stents.
Stent surveillance is also a key component of determining when re-intervention is required. Too often, patients do not have appropriate follow-up intervals allowing for early identification of in stent restenosis, external stent compression or recurrent thrombosis. In patients who have had stents placed, regular venous duplex is recommended with a subsequent clinic visit. Initially, patients should have an ultrasound every 3 months for the first year, then every 6 months for the second year; and if patients remain stable and asymptomatic, yearly follow-up is then recommended.
Re-intervention should occur when patients become symptomatic, even without obvious diagnostic ultrasound changes. Additionally, when decreased stent diameter is identified on ultrasound, evaluation of the stent with special attention to the proximal and distal landing zones should occur with IVUS. The intervention itself will vary based upon the pathophysiology but the key concepts remain the same: (I) clear as much thrombus as possible; (II) ensure that the stent has appropriate proximal and distal landing zones.
Extension of the stent proximally or distally may be necessary to cover previously missed “skip lesions”. Even though the total length of the stented area and extension of the stent underneath the inguinal ligament are risk factors for stent occlusion and development of in-stent restenosis, the absolute number are low and stent extension should not be abandoned in treatment of recurrent disease. Stent occlusions will be seen more frequently if the venous lesions are incompletely covered. In the setting of re-intervention, more care must be taken to evaluate the proximal and distal landing zones with IVUS in addition to a thorough evaluation of the outflow tract. External stent compression can be very easy to miss without the use of IVUS.
Once re-intervention is required, patients must then again be placed on a rigorous follow-up schedule. Initially, a monthly ultrasound with a subsequent clinic visit could be obtained for 2–3 months with conversion to every 3 months and later every 6 months, and eventually back to yearly follow-up once stability has been documented. Yearly follow-up for patients with iliac stents is recommended as ISR has been identified in cases as far as 13 years out.
Also, patients should be evaluated to determine if they are appropriate candidates for anticoagulation. There are numerous regimens used and if the proceduralist does not feel comfortable making that determination referral to the primary care provider or a hematologist may be necessary.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Rajachandran M, Schainfeld RM. Diagnosis and treatment of may-Thurner syndrome. Vasc Dis Manag 2014;11:265-73.
- Neglén P, Hollis KC, Olivier J, et al. Stenting of the venous outflow in chronic venous disease: long-term stent-related outcome, clinical, and hemodynamic result. J Vasc Surg 2007;46:979-90. [Crossref] [PubMed]
- Khan S. “Acquired” May-Thurner Syndrome. NCVH 2015. Available online: https://www.ncvh.org/pdf/2015%20NCVH/5-29-Fri/PDFs/1556_Sohail%20Khan.pdf
- Raju S, Neglen P. High prevalence of nonthrombotic iliac vein lesions in chronic venous disease: a permissive role in pathogenicity. J Vasc Surg 2006;44:136-43; discussion 144. [Crossref] [PubMed]
- May R, Thurner J. The cause of the predominantly sinistral occurrence of thrombosis of the pelvic veins. Angiology 1957;8:419-27. [Crossref] [PubMed]
- O'Sullivan GJ, Semba CP, Bittner CA, et al. Endovascular management of iliac vein compression (May-Thurner) syndrome. J Vasc Interv Radiol 2000;11:823-36. [Crossref] [PubMed]
- Foley TR, Waldo SW, Armstrong EJ. Iliofemoral Deep Vein Thrombosis. 2015. Available online: http://www.acc.org/latest-in-cardiology/articles/2015/11/23/13/39/iliofemoral-deep-vein-thrombosis
- Eberhardt RT, Raffetto JD. Chronic venous insufficiency. Circulation 2014;130:333-46. [Crossref] [PubMed]
- Fan CM. Venous pathophysiology. Semin Intervent Radiol 2005;22:157-61. [Crossref] [PubMed]
- Rhodes JM, Gloviczki P, Canton LG, et al. Factors affecting clinical outcome following endoscopic perforator vein ablation. Am J Surg 1998;176:162-7. [Crossref] [PubMed]
- Da Silva A, Navarro MF, Batalheiro J. The importance of chronic venous insufficiency. Various preliminary data on its medico-social consequences. Phlebologie 1992;45:439-43. [PubMed]
- Shull KC, Nicolaides AN. Significance of popliteal reflux in relation to ambulatory venous pressure and ulceration. Arch Surg 1979;114:1304-6. [Crossref] [PubMed]
- Johnson BF, Manzo RA, Bergelin RO, et al. Relationship between changes in the deep venous system and the development of the postthrombotic syndrome after an acute episode of lower limb deep vein thrombosis: a one- to six-year follow-up. J Vasc Surg 1995;21:307-12; discussion 313. [Crossref] [PubMed]
- Mewissen MW, Seabrook GR, Meissner MH, et al. Catheter-directed thrombolysis for lower extremity deep venous thrombosis: report of a national multicenter registry. Radiology 1999;211:39-49. [Crossref] [PubMed]
- Bækgaard N, Broholm R, Just S. Indications for stenting during thrombolysis. Phlebology 2013;28 Suppl 1:112-6. [Crossref] [PubMed]
- Raju S, Tackett P Jr, Neglen P. Reinterventions for nonocclusive iliofemoral venous stent malfunctions. J Vasc Surg 2009;49:511-8. [Crossref] [PubMed]