Statins as a preventative therapy for venous thromboembolism
Introduction
Acute venous thromboembolism (VTE) presents as deep vein thrombosis (DVT) and pulmonary embolism (PE). These are widely prevalent diseases causing over a quarter of a million deaths and affecting nearly 1 million people annually (1). In Europe, one study estimated approximately one third of a million VTE related mortalities occur every year (2). VTE clearly has a significant impact on healthcare costs, patient morbidity and patient mortality.
Acute VTE presents as an important time for mortality prevention but the sequelae of thromboembolism can be devastating as well. Chronic thrombosis presents different challenges than acute VTE because of the often-systemic nature of treatment. This includes managing the tradeoff between bleeding risk and clot stabilization, often by systemic anticoagulation. Post-thrombotic syndrome (PTS) is a feared long-term complication of DVT that carries a high morbidity (3-5) and can affect somewhere between 23–60% of patients (6). Conventional care for acute VTE involves initial treatment with heparin to prevent clot extension and then supportive care. Subsequently, long term anticoagulation with warfarin to prevent re-thrombosis, embolic events, and PTS is implemented. With regards to PE, lytic therapy can also be implemented to promote rapid reduction in clot burden; however, this comes at the risk of potentially life-threatening bleeding. Innovative treatments involve mechanical thrombectomy and other interventional procedures such as catheter directed thrombolytic therapy which may lower the risk of bleeding as a lower dose of lytic can be utilized. Additionally, various new direct oral anticoagulant agents (DOACs) such as rivaroxaban and apixaban have been developed that provide various benefits including less frequent monitoring for safe use (7). Unfortunately, anticoagulation increases risks for minor and major bleeding. In 2003, intracranial bleeds of 1.15% per year and a mortality rate with major bleeds of 13% was reported (8). More recent meta-analysis in 2012 showed a major bleed rate on warfarin of 4.7% while a cohort study published in 2016 revealed a combined rate of bleeding with dabigatran or warfarin of 4.6% (9,10). New therapy with DOACs has also improved bleeding rates over warfarin, potentially decreased by half (RR of 0.55) (11). Still, effective treatment for VTE is complicated and in need of additional therapies.
The JUPITER study was a randomized controlled trial in 2009 that linked rosuvastatin use with decreased VTE events in patients with elevated C-reactive protein (CRP), put statins on the map for possible adjunctive therapy (12). Specifically, the study looked at 17,802 patients with normal levels of low density lipoprotein cholesterol (LDL-C) levels and elevated CRP levels and found that patients who used rosuvastatin had significantly decreased rates of DVT.
The mechanism of action of statins and its prevention of thrombosis has not been well elucidated but multiple mechanisms have been proposed. Decreasing tissue factor (TF) (13,14), plasminogen activator inhibitor-1 (PAI-1) (14,15), and increased tissue plasminogen activator (tPA) expression, decreased platelet aggregation and increased thrombomodulin expression (14-16) are included among these propositions. A clear benefit for statins in the context of current therapy is a low risk profile that doesn’t increase bleeding making it available for use in conjunction with current standard of care.
PE
About 60–100 thousand Americans die of VTE in a year (17). Unfortunately, death occurs in 1 out of every 4 patients with PE before ever reaching help while 1 in 3 of the other patients will have a recurrence in the subsequent 10 years (17). Shock secondary to severe pulmonary hypertension or right heart failure are terrible prognosticators for mortality in patients with massive PE. Treatment rapidly becomes gentle IV fluids if hypotensive with respect to right ventricular strain, boosting cardiac inotropy, respiratory support and anticoagulation with consideration of mechanical thrombectomy or thrombolytics. Warfarin, unfractionated heparin, and low molecular weight heparin have been consistently used for treatment of thrombosis and emboli. Newer anticoagulants include direct factor Xa inhibitors (rivaroxaban and apixaban for example) and direct thrombin inhibitors (dabigatran). These therapies may eventually replace older therapies as techniques for reversal and additional trials become readily available. Aspirin has been shown to be effective in preventing arterial thrombosis but has been found lacking when tested with venous disease (17). Thrombolysis is a tool reserved for patients with immediate life-threatening hypotension because of the potential for inducing catastrophic intracranial hemorrhage. The PEITHO study demonstrated significantly increased risk of intracranial bleeding with thrombolytic therapy when compared to controls (18). Available data suggests that patients over the age of 75 years should only be treated when the benefits clearly outweigh the increased risk of intracranial bleeding, a non-trivial assessment (17).
Long term adverse events after acute PE include recurrence, chronic thromboembolic pulmonary hypertension (CTEPH) and arterial thrombotic events. Recurrent VTE is the most common among these adverse events occurring at a rate of 22% within the first 5 years, worse in patients with unprovoked events (19). There are multiple risk factors, but interestingly elevated D-dimer levels after stopping anticoagulation is related and relevant for statin therapy (19). CTEPH is much less common and concentrated within the first two years after an event, possibly a target time frame for intensive therapy (19). Preventing PE recurrence may help to prevent CTEPH as recurrence is a risk factor for its development (19). Arterial thrombotic events are likely related to atherosclerotic risk factors of which statins have a clear role.
Inflammation and DVT
Genetic factors related to hypercoagulable states should also be addressed as they may significantly contribute to VTE events (20). Genetic factor XI variants are associated with increased first time VTE (21). Risk factors are not always associated with VTE in isolation and further understanding of their interactions can lead to better targeted treatments.
The valve pocket sinus is an area prone to thrombosis likely related to multiple factors including hypoxia and altered flow, illustrated in Figure 1. Endothelial cells in this area may be activated by hypoxia or an inflammatory stimulus that triggers the thrombosis cascade (22). It is not clear exactly how this cascade is triggered, however it has been proposed that P-selectin, E-selectin and von Willebrand factor (vWF) on the endothelium induce circulating leukocytes, microvesicles (small membrane vesicles released from activated cells) and platelets to express TF which then continues onward (22).
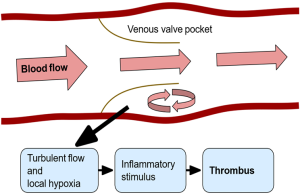
Multiple biomarkers have been implicated in increasing the risk of thrombosis: interleukin 6 (IL-6), interleukin 8 (IL-8), P-selectin, monocyte chemotactic protein 1 (MCP-1), tumor necrosis factor alpha (TNF-alpha) and CRP. IL-6, IL-8 and CRP were shown to be elevated on the first day of admission for patients with DVT (23). Specifically for recurrent DVT, IL-6, IL-8 and MCP-1 were increased after the patient experienced an initial thrombotic event (24). In a primate model of DVT, TNF-alpha was shown to be elevated (25) while inhibition of TNF-alpha lead to a reduction in thrombosis in another rat model (26). Many studies have been performed attempting to elucidate the effects of VTE on cells and signaling as well as post thrombotic syndrome, venous inflammation and thrombus resolution (26-29).
There is some evidence that CRP might be involved at the onset of VTE. CRP interacts with coagulation activation, platelets and blocking fibrinolytics (30). As such, it could be a good target to reduce to prevent thrombosis. Further evidence is needed, however, to establish which comes first, thrombosis or inflammation (30). Not all studies agree on active biomarkers in thrombosis: IL-6, MCP-1 and hs-CRP were also shown to have no correlation with thrombosis (31,32).
Statins and inflammation
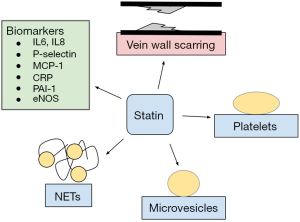
Statin use has been associated with numerous biomarkers which may give a clue to how statins affect the inflammatory process. Statins have been associated with reduction in CRP, IL-6, IL-8, MCP-1 and PAI-1 as well as increased levels of endothelial nitric oxide synthase (eNOS), thrombomodulin, and apolipoprotein A1 (33-39). Basic science studies show that statins have an active anti-inflammatory effect with associated decreases in biomarkers that affect multiple cell lines including hepatocytes, human aortic endothelial cells, and human umbilical vein endothelial cells (33,35,37,38).
CRP is a complicated inflammatory marker that has shown mixed responses in studies with statin use. The majority of studies show statin use correlates with a decreased level of CRP (33-38,40) while others show no effect at all (41). The randomized double-blind pravastatin inflammation and CRP evaluation (PRINCE) study in 2001 with 2,884 participants showed that pravastatin significantly reduced CRP levels in an LDL-independent manner (42). High sensitivity CRP (hsCRP) is often used as a marker of inflammation and is associated with atherosclerosis as well as venous thrombosis. It is also correlated with higher levels of factor VIII. Despite this, hsCRP does not appear to be a driving factor of thrombus formation (43). It is also not clear the ordering of VTE and inflammation despite an association between the two. A small study with 26 patients who had venous thrombosis found that a 3-day administration of atorvastatin reduced inflammation by reduced IL-6, IL-8 and soluble P-selectin, and increased IL-10 (an anti-inflammatory molecule) while not causing an effect on hsCRP. The study indicated that statin anti-inflammatory action likely drives in part the reduction in venous thrombosis despite no change to hsCRP (41).
In an analysis of the multi-ethnic study of atherosclerosis (MESA) of over 6,000 patients, statin use was associated with lower levels of D-dimer, CRP and factor VIII; higher fibrinogen and PAI-1 levels were also observed (40). Despite this, decreased factor VIII levels are likely not the cause of statins’ effect on VTE (40).
It is possible that statins’ effects are due to effects on lipid levels and blood coagulation parameters rather than an anti-inflammatory mechanism. Murine models support statin’s effect on thrombus burden and vein wall scarring directly rather than indirectly through lipid modulation (44). This study in 2015 on 282 mice showed reductions in thrombus PAI-1, TF, neutrophils, myeloperoxidase (MPO), neutrophil extracellular traps (NETs) and macrophages (44). In another murine based study, rosuvastatin reduced DVT in ApoE gene deleted mice with hyperlipidemia through decreased neutrophil migration and decreased plasma levels of PAI-1 and molecules related to IL-6 (45).
Direct antiplatelet effects may also contribute to the mechanism of reducing venous thrombosis. Blood from statin-treated mice significantly reduced platelet aggregation supporting some mechanism of direct action against platelets (44). Statins may have antiplatelet effects by interfering with redox signaling (46). Statins may exert this effect by NADPH oxidase downregulation (47). Inhibition of NOX2 activation causes downstream effects via platelet thromboxane A2 (TxA2) and 8-iso-PGF2alpha formation (47). In vitro studies support this idea with mechanisms that include TxA2 (43), however, the Statins Reduce Thrombophilia trial (START) investigated the effect of rosuvastatin on TxA2-mediated platelet activation and found that it is unlikely that this is the etiology (43). Another mechanism may involve peroxisome proliferator activated receptors that are involved in inflammatory responses (47). A randomized controlled trial with 50 patients compared rosuvastatin versus no intervention on the effects of thromboxane-mediated platelet aggregation ex vivo. Rosuvastatin did not change platelet reactivity under these conditions (48). The atorvastatin and thrombogenicity of carotid atherosclerotic plaque (ATROCAP) study showed statins reduce TF activity as well as macrophage infiltration of vessels which decreases cell-mediated thrombin generation (42). Daily atorvastatin or rosuvastatin has significantly reduced venous stasis thrombus burden by 25% (44). Statins also reduced DVT-induced vein wall scarring, a precursor to PTS (44). Statins inhibit the mevalonate pathway, particularly the isoprenylation of signaling proteins which may downregulate coagulation and platelet function (49).
Another promising avenue of research in the underpinnings of statin anti-inflammatory mechanism lies in TF and its expression on microvesicles. Proposed mechanisms for antithrombotic activity include decreased expression of TF, procoagulant microparticles and alteration of clotting factor such as factor V, thrombin and TF pathway inhibitors (50). Simvastatin has been shown to reduce peripheral blood TF expression and TF-positive microvesicles in hyperlipidemic monkeys (22). This suggests that statin’s anticoagulant activity may be mediated in part by inhibition of monocyte TF expression (22). Experimental studies show promise in microparticles with TF as a clue to hypercoagulability in cancer and that statins have a role in lowering the number of circulating TF positive microparticles (50). Rho-associated coiled-coil-containing protein kinase (ROCK), a protein involved in membrane trafficking, has been shown to decrease with statin use (50). The MicroSTAT trial observed a significant reduction in circulating TF bearing microparticles with administration of rosuvastatin; however this is unclear if it is a clinically significant reduction (50). Prostate cancer causes a pro-thrombotic state by releasing TF bearing cholesterol rich prostasomes and statins have been shown to decrease the levels of circulating TF-bearing prostasomes (51).
Some studies have looked at how statins may affect clot structure and ease of lysis. It has been shown that idiopathic VTE can be characterized by lower plasma fibrin clot permeability, thicker fibers and prolonged clot lysis time making this a potential target for treatment (52). Studies that demonstrate rapid alterations in fibrin clot structure/function with statin use have not had consistent outcomes (43). vWF and D-dimer may be affected by statin therapy but the studies are poor (43). Interestingly, statins protect against cigarette smoke extract induced fibrinolytic malfunction (53). Atorvastatin, in a small study of 28 first time VTE patients, has been shown to increase clot permeability and susceptibility to lysis when given for three days following VTE (54) (Table 1).

Full table
Statins and VTE
Multiple studies have concluded that statin use is associated with decreased rates of VTE (12,56-61). The JUPITER trial, with a broad selection of patients, showed that patients who took rosuvastatin daily versus those who took placebo were associated with a significant decrease in VTE events. Statins may provide an odds ratio (OR) of 0.73 as a reduction of VTE (62), a decrease in proximal leg DVT (63) and a reduced incidence of first occurrences (64). Nearly 1 million postmenopausal women with 23,505 cases of VTE were analyzed in a study that found that current use of statins was associated with decreased risk of VTE with an OR of 0.83 (65). This retrospective cohort study of 127,822 subjects included 1,375 VTE cases and demonstrated a correlation with simvastatin use and the risk of first ever idiopathic VTE (66). A recent Cochrane review evaluated the evidence of statins for primary prevention of VTE and drew the conclusion that while the available evidence suggests that rosuvastatin was associated with reduced incidence, the number of randomly controlled trials was lacking and the evidence was not of enough quantity to make clinical changes (67). Other analyses have drawn similar conclusions (42) (Figure 2).
Other studies have looked at statin use in selected subgroups of patients which may also help to elucidate the mechanism of action. Post-menopausal women, patients with solid organ tumors, as well as patients with recurrent DVT have been targeted (56-58,60,61). Statin and ASA use was associated with decreased peripherally inserted central catheter (PICC) related DVT in one case control study with 909 hospitalized patients (68). Cancer patients in general, due to the increased risk of thrombotic events, may benefit from statin use (69). This retrospective study of 1,746 patients with ovarian cancer, however, found no association with statin use and risk for VTE possibly due to alternative mechanisms of thrombosis induced by the cancer or by insufficient doses (70). The PROSPER trial studied pravastatin effects on the risk of coronary and cerebrovascular disease in elderly individuals but additional studies showed no association with statins and reduced VTE events (71). Additionally, no blood markers could be found that was predictive of VTE (71). Nephrotic syndrome is a known risk factor for VTE but no routine preventive measures are taken. This study analyzed if statin use could be used to lower the risk of VTE in these patients and found that there was a statistically significant preventive effect (72). Statins may be used to attenuate the increased risk of VTE from hormone therapy but this finding needs further confirmation (65). Given the increased risk of thrombosis related to surgical procedures, preoperative use of statins in non-cardiac surgical settings has been looked at. A retrospective study involving 7,777 patients showed a decrease in major complications with an OR of 0.41 (73). One study with a little over 700 patients showed no associated between statin therapy and VTE in ICU patients through a reanalysis of prospective cohort study patients (74). The Breast Cancer in Northern Israel Study is a case-control study that enrolled 3,585 patients with breast cancer who experienced 261 VTE events. After multivariate analysis, statins were not found to provide reduced risk of VTE (75).
Differences among statin types has not been well studied, but one case control study did suggest that differences may exist by showing that pravastatin had a greater effect of reducing DVT events than simvastatin (60). A meta-analysis of data up to 2012 aggregated almost 150 thousand patients when comparing statins with no statin treatments as well as high dose versus standard dose statin therapy. It concluded that while a positive protective effect against VTE exists, it is mild and low dose therapy was not significantly different than high dose therapy (76). In contradiction to this, hazard ratios for VTE events for high dose statin therapy has been reported at 0.25 and low dose at 0.38 (77). Combined statin and antiplatelet therapy may provide a synergistic effect (77) (Table 2).

Full table
Statins and recurrent VTE
Statins have also been shown to be beneficial in preventing recurrent VTE, possibly by reducing thrombus burden after the initial event while continuing to provide anti-inflammatory effects. No randomized prospective trials have been completed looking at statins effects on recurrent VTE but multiple studies suggest this effect. Statins were shown to be a protective factor when examining patients for residual thrombotic occlusion on follow up (78). Statin use was associated with reduced VTE recurrence in a retrospective case-control study involving 27,862 patients in Denmark (79). The largest effect was observed for recurrent DVT. A large study on elderly patients in Canada with a history of VTE (25,681 patient) showed that statin use was associated with decreased risk of recurrent VTE by approximately half (80). Another study with 2,798 patients with a prior VTE showed statin use was associated with a 26% lower risk of recurrent VT even when subdivided to eliminate patients with cardiovascular disease (81). When statin treatment was assessed relative to vitamin K antagonist use, statins continued to show benefit regardless of VKA use (82). Secondary analysis of the EINSTEIN DVT/PE study found statin use suggested a reduced risk of recurrent VTE but could not be conclusive (83).
In this study, age may play a role in statins effect on preventing VTE recurrence: 44,330 patients with VTE were studied in Denmark and found that statin use was overall associated with a significantly lower risk of recurrent VTE compared with no statin use (84). Surprisingly, younger patients made up the majority of the lower risk group while elderly individuals, greater than 80 years of age, had an opposite correlation with statin use associated with increased VTE events (84).
Not all studies found supported statins as a preventive method for recurrence. Multivariate analysis in 432 patients after discontinuing anticoagulation did not find an association with statin use and decreased VTE (85).
Statins and post thrombotic syndrome
Despite standard anticoagulation therapy, 20–100% of patients over a 10-year period after DVT will develop PTS (6). Additional treatment options are clearly needed. One basic science study in 2015 looked at the effect of statins on decreasing thrombus burden and decreasing vein wall injury in mice. These two features were targeted as they are known mediators of post thrombotic syndrome. This study showed that with daily atorvastatin or rosuvastatin treatment, venous thrombus burden was reduced by 25% without changing lipid levels, coagulation parameters or blood cell counts promoting statins as the causative effect (44). Given this finding, statins may influence PTS incidence.
An additional study with 234 patients randomized to heparin or heparin and statin therapy after DVT found that there was a significant decrease in post thrombotic syndrome in the rosuvastatin group (38.3% vs. 48.5%) (86).
Discussion
Statin therapy almost certainly has an active anti-inflammatory component that contributes to decreased thrombus formation. The precise mechanism of action is not quite clear but multiple biomarkers have been shown to be affected by statin therapy as well as associated with thrombus formation and resolution. The focus of basic research has been on inflammatory markers and signaling using IL-6, IL-8, P-selectin, MCP-1, TNF-alpha and CRP. IL-6, a known regulator of acute phase response activation, has been frequently studied. Additional mechanisms of action for statin therapy that are enticing explanations for reduction in VTE include reductions in TF, particularly embedded on microvesicles, as well as targeted action against platelet activity, NETs, or thrombus binding strength. It is important to continue to evaluate the underlying principles of thrombus and embolus formation not only to verify statin action mechanisms but to understand the relationship other therapies might have with statin therapy and new developments.
Current evidence seems to indicate that general use of statins for primary prophylaxis of VTE is not well supported. The findings from the meta-analysis conducted by Rahimi et al. which included 29 trials and 150,000 patients are significant (76). The authors concluded that statins likely affect VTE events but the effect has not been shown to be as high as previously thought. The meta-analysis used a variety of randomized controlled trials evaluating statin use against controls but evaluating other primary outcomes such as cholesterol levels or coronary vascular disease as end points. VTE event data was gathered for safety purposes but yielded additional research benefit. The promises of statins as substantial in preventing VTE events appears to have not panned out. This may be too early to conclude, however, given the multiple studies prior and the still modest effect seen in the meta-analysis of potentially up to 1/5 reduction. Statin adherence may have played a role in the outcome as statins are well known to cause myopathy (85). Ten percent of patients on stains report statin-induced myopathy and up to one third of these decide to discontinue statins due to this (55,87). One study showed that up to half of patients stop treatment within 6 months but found that the risk of VTE was 19% lower in the most adherent group compared to the least adherent group (66). For now, it is likely that statins provide a benefit in reducing VTE risk but the effect is likely more modest than previously hoped and insufficient data is available to evaluate the risks and benefits for primary preventative therapy.
Secondary VTE prevention may be a better target for statin therapy for multiple reasons. The patient base is narrowed and more easily defined. Higher rates of thrombosis are seen in the setting of a history of prior thrombosis and may provide a more accurately measurable relationship as well as improved patient care. Retrospective analysis of patients in Denmark up to 2009 showed a significantly decreased risk of recurrent VTE (84). In another study a reduction of 28% was seen for recurrent VTE with a stronger effect for high potency statins (79). A significant effort should be made to evaluate these outcomes in a randomized controlled trial that can bring clinical change if results are positive.
Statins have evidence to suggest that their use can safely decrease the incidence and recurrence of VTE. Multiple statins including pravastatin, simvastatin, atorvastatin, rosuvastatin and more have been studied often without inter-statin comparisons. A few studies have been done but not to a sufficient level to make strong conclusions. Simvastatin may be more effective than atorvastatin while pravastatin may be more effective than simvastatin when evaluating for preventing DVT (59,77). Simvastatin may be more effective than pravastatin in postmenopausal women (56). Directed effort to elucidate types and potency of statins during trials should take place to clarify these outcomes.
Post thrombotic syndrome is a dreaded complication of VTE which has a limited set of treatments. Adding statin therapy as another mechanism for prevention of developing post thrombotic syndrome would be a boon for patient outcomes. There are few studies specifically evaluating post thrombotic syndrome incidence after VTE treated with statins but the published data was an encouraging finding for continued evaluation. Clearly more directed studies toward post thrombotic syndrome are needed to confirm these outcomes, evaluate intervention strategies and clarify the pathophysiology and mechanism of action.
In summary, statins have a growing body of evidence that is yet indeterminate on the true effects on VTE. Earlier studies promised great outcomes but more recent studies and analysis of randomized controlled trials have slowed enthusiasm. Potential still exists for targeted therapy in recurrent VTE and complications of thrombosis, potentially as adjunctive therapy with anticoagulation or as prevention of post thrombotic syndrome. Multiple theories have been proposed and partially evaluated for the mechanism of action of statins and the pathophysiology of thrombosis including a variety of biomarkers, antiplatelet and anti-thrombotic effects, reduction of microvesicles, etc. Statins have been well studied in terms of cardiovascular risk and safety profile and offer no significant interaction with standard of care for acute and chronic VTE. Unfortunately, more clinical studies need to be done and replicated, particularly prospective trials evaluating complications such as recurrence as well as synergistic effects on current treatment methodologies. This will lead to confidence in the proposed outcomes and generalizability. Many clinical questions need to be answered including outcomes across types of statins, dosing regimens, length of therapy if statins are to be used for another clinical indication.
Acknowledgements
Funding: R Oklu gratefully acknowledges funding from the National Institutes of Health (EB021148, CA172738, EB024403, HL137193) and the Mayo Clinic.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Raskob GE, Silverstein R, Bratzler DW, et al. Surveillance for DVT and Pulmonary Embolism. Am J Prev Med 2010;38:S502-9. [Crossref] [PubMed]
- Cohen AT, Agnelli G, Anderson FA, et al. Venous thromboembolism (VTE) in Europe. The number of VTE events and associated morbidity and mortality. Thromb Haemost 2007;98:756-64. [PubMed]
- Ackroyd JS, Browse NL. The investigation and surgery of the post-thrombotic syndrome. J Cardiovasc Surg (Torino) 1986;27:5-16. [PubMed]
- Hopkins NF, Wolfe JH. ABC of vascular diseases. Deep venous insufficiency and occlusion. BMJ 1992;304:107-10. [Crossref] [PubMed]
- Negus D. The post-thrombotic syndrome. Ann R Coll Surg Engl 1970;47:92-105. [PubMed]
- Ashrani AA, Heit JA. Incidence and cost burden of post-thrombotic syndrome. J Thromb Thrombolysis 2009;28:465-76. [Crossref] [PubMed]
- Lee LH. DOACs - advances and limitations in real world. Thromb J 2016;14:17. [Crossref] [PubMed]
- Linkins LA, Choi PT, Douketis JD. Clinical impact of bleeding in patients taking oral anticoagulant therapy for venous thromboembolism: a meta-analysis. Ann Intern Med 2003;139:893-900. [Crossref] [PubMed]
- Warkentin AE, Donadini MP, Spencer FA, et al. Bleeding risk in randomized controlled trials comparing warfarin and aspirin: A systematic review and meta-analysis. J Thromb Haemost 2012;10:512-20. [Crossref] [PubMed]
- Wang SV, Franklin JM, Glynn RJ, et al. Prediction of rates of thromboembolic and major bleeding outcomes with dabigatran or warfarin among patients with atrial fibrillation: new initiator cohort study. BMJ 2016;353:i2607. [Crossref] [PubMed]
- Kamath SD, Mcmahon BJ. Update on Anticoagulation: What the Interventional Radiologist Needs to Know. Semin Intervent Radiol 2016;33:122-31. [Crossref] [PubMed]
- Glynn RJ, Danielson E, Fonseca FAH, et al. A randomized trial of rosuvastatin in the prevention of venous thromboembolism. N Engl J Med 2009;360:1851-61. [Crossref] [PubMed]
- Undas A, Brozek J, Musial J. Anti-inflammatory and antithrombotic effects of statins in the management of coronary artery disease. Clin Lab 2002;48:287-96. [PubMed]
- Arslan F, Pasterkamp G, de Kleijn DP. Unraveling pleiotropic effects of statins: bit by bit, a slow case with perspective. Circ Res 2008;103:334-6. [Crossref] [PubMed]
- Nishino M, Hoshida S, Kato H, et al. Preprocedural statin administration can reduce thrombotic reaction after stent implantation. Circ J 2008;72:232-7. [Crossref] [PubMed]
- Perez A, Bartholomew JR. Interpreting the JUPITER trial: statins can prevent VTE, but more study is needed. Cleve Clin J Med 2010;77:191-4. [Crossref] [PubMed]
- Harikrishnan P, Palaniswamy C, Aronow WS. Update on pharmacologic therapy for pulmonary embolism. J Cardiovasc Pharmacol Ther 2014;19:159-69. [Crossref] [PubMed]
- Meyer G, Planquette B, Sanchez O. Fibrinolysis for Acute Care of Pulmonary Embolism in the Intermediate Risk Patient. Curr Atheroscler Rep 2015;17:68. [Crossref] [PubMed]
- den Exter PL, van der Hulle T, Lankeit M, et al. Long-term clinical course of acute pulmonary embolism. Blood Rev 2013;27:185-92. [Crossref] [PubMed]
- Crous-Bou M, Harrington LB, Kabrhel C. Environmental and Genetic Risk Factors Associated with Venous Thromboembolism. Semin Thromb Hemost 2016;42:808-20. [Crossref] [PubMed]
- Harrington LB, Wiggins KL, Sitlani CM, et al. The association of F11 genetic variants with the risk of incident venous thrombosis among women, by statin use. Thromb Haemost 2016;115:682-4. [Crossref] [PubMed]
- Mackman N. New insights into the mechanisms of venous thrombosis. J Clin Invest 2012;122:2331-6. [Crossref] [PubMed]
- Roumen-Klappe EM, Den Heijer M, Van Uum SH, et al. Wollersheim H. Inflammatory response in the acute phase of DVT. J Vasc Surg 2002;35:701-6. [Crossref] [PubMed]
- van Aken BE, den Heijer M, Bos GM, et al. Recurrent venous thrombosis and markers of inflammation. Thromb Haemost 2000;83:536-9. [PubMed]
- Wakefield TW, Greenfield LJ, Rolfe MW, et al. Inflammatory and procoagulant mediator interactions in an experimental baboon model of venous thrombosis. Thromb Haemost 1993;69:164-72. [PubMed]
- Wakefield TW, Strieter RM, Downing LJ, et al. P-selectin and TNF inhibition reduce venous thrombosis inflammation. J Surg Res 1996;64:26-31. [Crossref] [PubMed]
- Wojcik BM, Wrobleski SK, Hawley AE, et al. Interleukin-6: a potential target for post-thrombotic syndrome. Ann Vasc Surg 2011;25:229-39. [Crossref] [PubMed]
- Meier TR, Myers DD, Wrobleski SK, et al. Prophylactic P-selectin inhibition with PSI-421 promotes resolution of venous thrombosis without anticoagulation. Thromb Haemost 2008;99:343-51. [PubMed]
- Henke PK, Wakefield TW, Kadell AM, et al. Interleukin-8 administration enhances venous thrombosis resolution in a rat model. J Surg Res 2001;99:84-91. [Crossref] [PubMed]
- Lippi G, Favaloro EJ, Montagnana M, et al. C-reactive protein and venous thromboembolism: causal or casual association? Clin Chem Lab Med 2010;48:1693-701. [Crossref] [PubMed]
- Vormittag R, Hsieh K, Kaider A, et al. Interleukin-6 and interleukin-6 promoter polymorphism (-174) G > C in patients with spontaneous venous thromboembolism. Thromb Haemost 2006;95:802-6. [PubMed]
- Vormittag R, Vukovich T, Schönauer V, et al. Basal high-sensitivity-C-reactive protein levels in patients with spontaneous venous thromboembolism. Thromb Haemost 2005;93:488-93. [PubMed]
- Rezaie-Majd A, Maca T, Bucek RA, et al. Simvastatin reduces expression of cytokines interleukin-6, interleukin-8, and monocyte chemoattractant protein-1 in circulating monocytes from hypercholesterolemic patients. Arterioscler Thromb Vasc Biol 2002;22:1194-9. [Crossref] [PubMed]
- Albert MA, Danielson E, Rifai N, et al. Effect of statin therapy on C-reactive protein levels. JAMA 2001;286:64-70. [Crossref] [PubMed]
- Arnaud C, Burger F, Steffens S, et al. Statins reduce interleukin-6-induced C-reactive protein in human hepatocytes: new evidence for direct antiinflammatory effects of statins. Arterioscler Thromb Vasc Biol 2005;25:1231-6. [Crossref] [PubMed]
- Di Garbo V, Bono M, Di Raimondo D, et al. Non lipid, dose-dependent effects of pravastatin treatment on hemostatic system and inflammatory response. Eur J Clin Pharmacol 2000;56:277-84. [Crossref] [PubMed]
- Jougasaki M, Ichiki T, Takenoshita Y, et al. Statins suppress interleukin-6-induced monocyte chemo-attractant protein-1 by inhibiting Janus kinase/signal transducers and activators of transcription pathways in human vascular endothelial cells. Br J Pharmacol 2010;159:1294-303. [Crossref] [PubMed]
- Morikawa S, Takabe W, Mataki C, et al. The effect of statins on mRNA levels of genes related to inflammation, coagulation, and vascular constriction in HUVEC. Human umbilical vein endothelial cells. J Atheroscler Thromb 2002;9:178-83. [Crossref] [PubMed]
- Shi J, Wang J, Zheng H, et al. Statins increase thrombomodulin expression and function in human endothelial cells by a nitric oxide-dependent mechanism and counteract tumor necrosis factor alpha-induced thrombomodulin downregulation. Blood Coagul Fibrinolysis 2003;14:575-85. [Crossref] [PubMed]
- Besseling J, Hutten BA, Meijers JC, et al. Statin therapy and levels of hemostatic factors in a healthy population: the Multi-Ethnic Study of Atherosclerosis. J Thromb Haemost 2013;11:1078-84. [Crossref] [PubMed]
- Żółciński M, Cieśla-Dul M, Potaczek DP, et al. Atorvastatin favorably modulates proinflammatory cytokine profile in patients following deep vein thrombosis. Thromb Res 2013;132:e31-5. [Crossref] [PubMed]
- Poredos P, Jezovnik MK. Dyslipidemia, statins, and venous thromboembolism. Semin Thromb Hemost 2011;37:897-902. [Crossref] [PubMed]
- Lijfering WM, Biedermann JS, Kruip MJ, et al. Can we prevent venous thrombosis with statins: an epidemiologic review into mechanism and clinical utility. Expert Rev Hematol 2016;9:1023-30. [Crossref] [PubMed]
- Kessinger CW, Kim JW, Henke PK, et al. Statins improve the resolution of established murine venous thrombosis: reductions in thrombus burden and vein wall scarring. PLoS One 2015;10:e0116621. [Crossref] [PubMed]
- Patterson KA, Zhang X, Wrobleski SK, et al. Rosuvastatin reduced DVT in ApoE gene deleted mice with hyperlipidemia through non-lipid lowering effects. Thromb Res 2013;131:268-76. [Crossref] [PubMed]
- Violi F, Pignatelli P. Statins as regulators of redox signaling in platelets. Antioxid Redox Signal 2014;20:1300-12. [Crossref] [PubMed]
- Violi F, Carnevale R, Pastori D, et al. Antioxidant and antiplatelet effects of atorvastatin by Nox2 inhibition. Trends Cardiovasc Med 2014;24:142-8. [Crossref] [PubMed]
- Biedermann JS, Cannegieter SC, Roest M, et al. Platelet reactivity in patients with venous thrombosis who use rosuvastatin: a randomized controlled clinical trial. J Thromb Haemost 2016;14:1404-9. [Crossref] [PubMed]
- Palomäki A. Statins and thrombogenesis. Duodecim 2011;127:2131-8. [PubMed]
- Zwicker JI. Unconventional approaches to the prevention of cancer associated thrombosis. Thromb Res 2014;133 Suppl 2:S44-8. [Crossref] [PubMed]
- Aberg M, Johnell M, Wickström M, et al. Simvastatin reduces the production of prothrombotic prostasomes in human prostate cancer cells. Thromb Haemost 2008;100:655-62. [PubMed]
- Undas A, Zawilska K, Ciesla-Dul M, et al. Altered fibrin clot structure/function in patients with idiopathic venous thromboembolism and in their relatives. Blood 2009;114:4272-8. [Crossref] [PubMed]
- Hu XY, Ma YH, Wang C, et al. Effects of simvastatin on cigarette smoke extract induced tissue-type plasminogen activator and plasminogen activator inhibitor-1 expression in human umbilical vein endothelial cells. Chin Med J (Engl) 2009;122:2380-5. [PubMed]
- Zolcinski M, Ciesla-Dul M, Undas A. Effects of atorvastatin on plasma fibrin clot properties in apparently healthy individuals and patients with previous venous thromboembolism. Thromb Haemost 2012;107:1180-2. [Crossref] [PubMed]
- Rosenbaum D, Dallongeville J, Sabouret P, et al. Discontinuation of statin therapy due to muscular side effects: a survey in real life. Nutr Metab Cardiovasc Dis 2013;23:871-5. [Crossref] [PubMed]
- Doggen CJ, Lemaitre RN, Smith NL, et al. HMG CoA reductase inhibitors and the risk of venous thrombosis among postmenopausal women. J Thromb Haemost 2004;2:700-1. [Crossref] [PubMed]
- Khemasuwan D, Divietro ML, Tangdhanakanond K, et al. Statins decrease the occurrence of venous thromboembolism in patients with cancer. Am J Med 2010;123:60-5. [Crossref] [PubMed]
- Lacut K, Oger E, Le Gal G, et al. Statins but not fibrates are associated with a reduced risk of venous thromboembolism: a hospital-based case-control study. Fundam Clin Pharmacol 2004;18:477-82. [Crossref] [PubMed]
- Momi S, Impagnatiello F, Guzzetta M, et al. NCX 6560, a nitric oxide-releasing derivative of atorvastatin, inhibits cholesterol biosynthesis and shows anti-inflammatory and anti-thrombotic properties. Eur J Pharmacol 2007;570:115-24. [Crossref] [PubMed]
- Ramcharan AS, Van Stralen KJ, Snoep JD, et al. HMG-CoA reductase inhibitors, other lipid-lowering medication, antiplatelet therapy, and the risk of venous thrombosis. J Thromb Haemost 2009;7:514-20. [Crossref] [PubMed]
- Ray JG, Mamdani M, Tsuyuki RT, et al. Use of statins and the subsequent development of deep vein thrombosis. Arch Intern Med 2001;161:1405-10. [Crossref] [PubMed]
- Ashrani AA, Barsoum MK, Crusan DJ, et al. Is lipid lowering therapy an independent risk factor for venous thromboembolism? A population-based case-control study. Thromb Res 2015;135:1110-6. [Crossref] [PubMed]
- Lim W, Meade M, Lauzier F, et al. Failure of anticoagulant thromboprophylaxis: risk factors in medical-surgical critically ill patients. Crit Care Med 2015;43:401-10. [Crossref] [PubMed]
- Lassila R, Jula A, Pitkäniemi J, et al. The association of statin use with reduced incidence of venous thromboembolism: a population-based cohort study. BMJ Open 2014;4:e005862. [Crossref] [PubMed]
- Fournier JP, Duijnhoven RG, Renoux C, et al. Concurrent use of statins and hormone therapy and risk of venous thromboembolism in postmenopausal women: a population-based case-control study. Menopause 2014;21:1023-6. [Crossref] [PubMed]
- Rabinowich L, Steinvil A, Leshem-Rubinow E, et al. Adherence to statins is associated with reduced incidence of idiopathic venous thromboembolism: real-life data from a large healthcare maintenance organisation. Heart 2012;98:1817-21. [Crossref] [PubMed]
- Li L, Sun T, Zhang P, et al. Statins for primary prevention of venous thromboembolism. Cochrane Database Syst Rev 2011.CD008203. [PubMed]
- Chopra V, Fallouh N, McGuirk H, et al. Patterns, risk factors and treatment associated with PICC-DVT in hospitalized adults: A nested case-control study. Thromb Res 2015;135:829-34. [Crossref] [PubMed]
- Lötsch F, Königsbrügge O, Posch F, et al. Statins are associated with low risk of venous thromboembolism in patients with cancer: a prospective and observational cohort study. Thromb Res 2014;134:1008-13. [Crossref] [PubMed]
- Shai A, Rennert HS, Rennert G, et al. Statins, aspirin and risk of thromboembolic events in ovarian cancer patients. Gynecol Oncol 2014;133:304-8. [Crossref] [PubMed]
- Freeman DJ, Robertson M, Brown EA, et al. Incident venous thromboembolic events in the Prospective Study of Pravastatin in the Elderly at Risk (PROSPER). BMC Geriatr 2011;11:8. [Crossref] [PubMed]
- Resh M, Mahmoodi BK, Navis GJ, et al. Statin use in patients with nephrotic syndrome is associated with a lower risk of venous thromboembolism. Thromb Res 2011;127:395-9. [Crossref] [PubMed]
- Iannuzzi JC, Rickles AS, Kelly KN, et al. Perioperative pleiotropic statin effects in general surgery. Surgery 2014;155:398-407. [Crossref] [PubMed]
- Al Harbi SA, Khedr M, Al-Dorzi HM, et al. The association between statin therapy during intensive care unit stay and the incidence of venous thromboembolism: a propensity score-adjusted analysis. BMC Pharmacol Toxicol 2013;14:57. [Crossref] [PubMed]
- Shai A, Rennert HS, Lavie O, et al. Statins, aspirin and risk of venous thromboembolic events in breast cancer patients. J Thromb Thrombolysis 2014;38:32-8. [Crossref] [PubMed]
- Rahimi K, Bhala N, Kamphuisen P, et al. Effect of statins on venous thromboembolic events: a meta-analysis of published and unpublished evidence from randomised controlled trials. PLoS Med 2012;9:e1001310. [Crossref] [PubMed]
- Khemasuwan D, Chae YK, Gupta S, et al. Dose-related effect of statins in venous thrombosis risk reduction. Am J Med 2011;124:852-9. [Crossref] [PubMed]
- Hirmerova J, Seidlerova J, Filipovsky J. Risk factors for residual thrombotic occlusion after proximal DVT of the legs. Int Angiol 2010;29:317-22. [PubMed]
- Schmidt M, Cannegieter SC, Johannesdottir SA, et al. Statin use and venous thromboembolism recurrence: a combined nationwide cohort and nested case-control study. J Thromb Haemost 2014;12:1207-15. [Crossref] [PubMed]
- Tagalakis V, Eberg M, Kahn S, et al. Use of statins and reduced risk of recurrence of VTE in an older population. A population-based cohort study. Thromb Haemost 2016;115:1220-8. [Crossref] [PubMed]
- Smith NL, Harrington LB, Blondon M, et al. The association of statin therapy with the risk of recurrent venous thrombosis. J Thromb Haemost 2016;14:1384-92. [Crossref] [PubMed]
- Biere-Rafi S, Hutten BA, Squizzato A, et al. Statin treatment and the risk of recurrent pulmonary embolism. Eur Heart J 2013;34:1800-6. [Crossref] [PubMed]
- Wells PS, Gebel M, Prins MH, et al. Influence of statin use on the incidence of recurrent venous thromboembolism and major bleeding in patients receiving rivaroxaban or standard anticoagulant therapy. Thromb J 2014;12:26. [Crossref] [PubMed]
- Nguyen CD, Andersson C, Jensen TB, et al. Statin treatment and risk of recurrent venous thromboembolism: a nationwide cohort study. BMJ Open 2013;3:e003135. [Crossref] [PubMed]
- Delluc A, Tromeur C, Le Moigne E, et al. Lipid lowering drugs and the risk of recurrent venous thromboembolism. Thromb Res 2012;130:859-63. [Crossref] [PubMed]
- San Norberto EM, Gastambide MV, Taylor JH, et al. Effects of rosuvastatin as an adjuvant treatment for deep vein thrombosis. Vasa 2016;45:133-40. [Crossref] [PubMed]
- Whayne TF Jr. Statin myopathy: significant problem with minimal awareness by clinicians and no emphasis by clinical investigators. Angiology 2011;62:415-21. [Crossref] [PubMed]


