Catheter-directed thrombolysis of deep vein thrombosis: literature review and practice considerations
Introduction
Venous thromboembolism (VTE) is a spectrum of disease including deep vein thrombosis (DVT) and pulmonary embolism (PE). VTE is a major public health concern, estimated to effect 1 to 2 per 1,000 people in the US each year (1-3). PE is responsible for almost all VTE related deaths. Over 80% of PE are the result of DVT in the leg or pelvic veins. These clots dislodge and travel through the venous system to the heart and into the pulmonary arteries. Estimates suggest that at least 100,000 Americans die of VTE each year, with 10–30% of patients dying within 30 days of diagnosis, and 20–25% of PE cases presenting as sudden death (1-3).
DVT represents about 2/3 of VTE cases, while the remaining third predominantly present with PE. Although DVT is classically associated with hospitalized patients, about two thirds of cases actually occur in outpatients (1). Risk factors include genetic thrombophilia, age and hypercoagulable states such as malignancy, and transient factors such as medications, immobilization, hospitalization, travel and trauma (1,2). In many cases, these factors interact and may have a multiplicative effect and can increase the rate of mortality. Although much work has been done to identify and calculate risks of DVT, a large number (up to 20%) of DVT cases are considered idiopathic, with no clear risk factor identified. As such, DVT is particularly difficult to predict and prevent.
While estimates of associated healthcare costs vary greatly, direct costs from VTE in the US are astronomical, and may be as high as $10 billion annually (1). There are many long-term consequences and indirect costs of VTE as well. About one third of patients with VTE will have a recurrence within 10 years (1,3). In addition, one third to one half of DVT patients will develop chronic venous insufficiency and post-thrombotic syndrome (PTS) (1,2,4). PTS often has a detrimental effect on quality of life. More than 50% of patients are of working age, making long term disability particularly costly (5). A recent CDC study found that patients with DVT had 80% higher risk of work-related disability than those without DVT (6).
The PTS and iliofemoral DVT (IFDVT)
Even after DVT is identified and treated with anticoagulation, permanent damage to the involved venous system often occurs due to inflammation that leads to valvular scarring and venous wall thickening. The damage becomes clinically evident when it affects valvular function, which promotes venous reflux and chronic venous hypertension (congestion). The PTS is a chronic debilitating clinical entity characterized by a spectrum of disease severity from chronic leg swelling and pain, to skin changes, claudication, and in severe cases ulceration. PTS symptoms can be confused with or exacerbated by comorbid conditions, which can include congestive heart failure, lymphedema, obesity, diabetes, and peripheral vascular disease (7).
The Villalta grading scale has been devised to standardize the scoring of PTS. Factors including pain, edema, induration, changes in skin color, and venous ectasia are scored from 0 to 3 (a higher score indicates a greater degree of severity). A total score above 5 predicts the presence of PTS (8). PTS is the principle determinant of disability and quality of life following DVT (5). One study found that quality of life in PTS is poorer than in many other chronic conditions such as diabetes and arthritis, and is in some cases as debilitating as angina, congestive heart failure, and cancer (5).
PTS is a particularly common outcome following IFDVT which is defined as involvement of the iliac veins or common femoral vein with or without concomitant distal DVT. This increased rate of PTS may be due to the inability to form collateral veins around the obstructed segment. IFDVT contrasts from distal (infrainguinal) DVT, where alternative venous drainage pathways in the leg can shunt blood past an occlusion in the form of collateralized vessels.
Prior IFDVT has been shown to lead to chronic venous claudication symptoms and to marked impairments in quality of life (9). One study found that IFDVT was the strongest predictor of PTS, more-so than recurrent ipsilateral DVT (8). IFDVT was also the strongest predictor of recurrent DVT. As will be discussed below, many innovative treatments for DVT aimed at reducing the risk of PTS are focused selectively on treating the subset of DVT patients with IFDVT.
The anticoagulation paradigm
The standard medical treatment for VTE is a 3 to 6 months course of oral anticoagulation therapy. Oral anticoagulation does not dissolve the thrombus; however, it prevents further thrombus propagation and reduces risk of recurrent VTE. As anticoagulation does not remove the clot, it does not prevent the sequela of post-thrombotic changes such as valvular damage and venous insufficiency, and the incidence of PTS after 6 months of anticoagulation therapy remains between 40% and 60% (5,10).
Even after treatment with anticoagulation, patients remain predisposed to recurrent DVT, and have a much higher risk of recurrent DVT compared to new onset DVT (3). The high recurrence rate is likely due to residual thrombus following treatment (11,12). Recent human and animal studies have identified previously-unrecognized biochemical components of thrombi that likely contribute to treatment resistance and recurrence (13,14). Novel platforms for 3D cellular culture are being utilized to study aspects of thrombosis and thrombolysis in vivo (15).
Recurrence after treatment is particularly likely in patients with malignancy, and in patients with “unprovoked” DVT (i.e., without precipitating risk factors). Some data suggests a 50-time higher relative risk for developing DVT following an unprovoked DVT compared to patients without previous DVT (16). Certain patients may require indefinite continuation of anticoagulation, or so-called “long term” anticoagulation, when the risk of recurrent DVT is very high. This regimen varies significantly in clinical practice and requires complex medical decision making to assess and balance the risks and benefits of anticoagulation versus VTE.
It is estimated that the risk for major bleeding with anticoagulation is 1–4% per year, and the risk for intracranial bleeding is 0.25–1.5% (17,18). Major bleeding includes bleeds that are intracranial, require hospitalization, necessitate transfusion, or result in a drop in hemoglobin more than 2 g/L, according to the HAS-BLED scoring system for determining the risk of bleeding on anticoagulation. There are several scoring systems available to determine a patient’s risk for bleeding, including the RIETE score, which was specifically developed for use in VTE patients (18).
Currently the role of inferior vena cava (IVC) filters in the management of venous thrombosis remains controversial and in constant development due to the risk of significant complications and unclear long-term advantages. Furthermore, low retrieval rates with irregular AC can lead to poor outcomes with high rates of IVC thrombosis. However, in select patients it is reasonable to place an IVC filter, understanding that it does not prevent or treat DVT but reduces the risk of pulmonary embolism. IVC filters can be useful in some patients with recurrent VTE despite anticoagulation, contraindications to anticoagulation, or complications from anticoagulation (19).
The use of elastic compression stockings for prevention of PTS remains controversial. Current guidelines from the American Academy of Chest Physicians recommend compression stockings for 2 years following DVT for prevention of PTS. However, the evidence for these recommendations is relatively limited and has been called into question by a subsequent multicenter, randomized, placebo-controlled trial involving 803 patients (SOX trial). The SOX trial found no evidence of benefit for prevention of PTS, quality of life, and recurrent DVT (20). As such, compression stockings are likely of little benefit in routine use for PTS prevention, but may be a reasonable option as a trial for certain patients, particularly with symptomatic edema or PTS (21).
Thrombectomy and thrombolysis
Surgical thrombectomy is used in cases of VTE involving limb or life threatening emergencies such as massive pulmonary embolism, and is not routinely used for lower extremity DVT treatment. However, multiple studies have demonstrated the benefits of thrombectomy for preventing long-term sequelae of DVT. Patients randomized to thrombectomy plus anticoagulation were found to have improved venous patency on follow-up and significantly fewer symptoms of PTS than patients who received anticoagulation alone. Furthermore, the benefits persisted up to 10 years following surgery (22,23).
An alternative to thrombectomy is systemic thrombolysis, which involves intravenous injection of an agent that lyses (dissolves) clots. Typical agents are analogs of tissue plasminogen activator (tPA). Thrombolysis offers distinct advantages over anticoagulation in that it actively breaks down clot. By contrast, anticoagulation merely prevents the growth of pre-existing clot and protects against new thrombosis. As such, intravenous tPA alone is more effective than heparin at restoring venous patency after DVT (24,25). Importantly, thrombolysis was found to reduce PTS by at least one third compared to anticoagulation alone (26).
The major disadvantage of systemic thrombolysis is the increased risk of serious bleeding complications, with intracranial hemorrhage carrying the highest mortality. Intravenous tPA carries a 3–6% risk of intracranial hemorrhage (27,28). Because of this risk, IV tPA is contraindicated for treatment of the vast majority of DVT’s. A 2014 Cochrane review of 17 studies encompassing a total of 1,103 patients, found that patients who received thrombolytics for a DVT experienced significantly more bleeding complications than with anticoagulation, although the absolute difference was small (10% vs. 8%). Most bleeding events in the review occurred in earlier studies that administered intravenous (systemic) thrombolytics (26).
The catheter-directed approach
Catheter-based endovascular techniques have revolutionized therapeutic options for DVT by altering the risk-benefit ratio of intervention. Various methods now exist including catheter directed thrombolysis (CDT), pharmacomechanical CDT (PCDT), and percutaneous mechanical thrombectomy (PMT). CDT involves percutaneous introduction of a catheter into the venous system with subsequent fluoroscopic guidance to the target vessel and prolonged infusion of a thrombolytic agent such as tPA directly into the thrombus. The catheter is left in place and the infusion usually continues for at least 24 hours.
CDT reduces the total dose of thrombolytic required and minimizes systemic drug exposure (thereby reducing the risk of systemic bleeding), while optimizing exposure of the lytic agent to the clot. With systemic thrombolysis, by comparison, drug is delivered only to the exposed surfaces at the periphery of the thrombus. Often, collaterals form around the occlusion which serves as a hemodynamic bypass and further limits tPA delivery to the thrombus.
Because CDT directly bathes the thrombus with lytic agent, it requires relatively low doses of tPA (about 0.01 mg/kg/h), usually ranging between 0.5 and 1 mg/h (12). Conversely, systemically delivered tPA (in the setting of acute ischemic stroke) usually involves a single dose of 50–100 mg (0.9 mg/kg) infused over an hour. The absolute risk of intracranial bleeding following CDT remains unknown, but it seems to be quite rare (26). A pooled analysis of 19 studies found a range of reported rates of 0–1% for intracranial bleeding following CDT (29), whereas the rate is approximately 3–6% with IV (systemic) tPA (27,28), and from 0.25–1.5% with standard anticoagulation (17,18).
Multiple studies have demonstrated that CDT is effective at restoring venous patency and reducing symptoms in the setting of acute DVT (26,30). As was demonstrated with thrombectomy and systemic thrombolysis, CDT is also particularly useful in the prevention of PTS and more effective than anticoagulation alone. In fact, PTS risk is directly correlated with the amount of thrombus remaining at the end of CDT treatment. If 90% or more of the thrombus is removed, patients appear to have negligible risk for PTS (31). CDT also reduces risk of recurrent DVT. Like PTS, a relationship between residual post-procedure thrombus load and risk of DVT recurrence has been demonstrated in several studies (32,33).
CDT has also been shown to improve quality of life in IFDVT patients compared to those who receive anticoagulation alone (31). These long-term benefits have the potential to reduce hospital re-admissions, cost of treatment for recurrent DVT, and worker disability associated with PTS. As such, while CDT is relatively costly, it may still prove to be a cost-effective adjunct to traditional anticoagulation (34). Risk reduction appears greatest if the procedure is done in the acute or early subacute phases before the clot undergoes chronic transformation and valvular damage ensues, usually within the first 2–3 weeks following onset of DVT symptoms (12,29).
Although CDT is also effective for restoring venous patency after acute popliteal and infra-inguinal DVT, long-term patency rates are lower than with IFDVT. These patients also have a lower risk of developing PTS compared to IFDVT patients (29). Consequently, the risk-benefit ratio for CDT is different with DVT involving the infrainguinal vessels, and these patients may be better suited for traditional treatment with anticoagulation and compression stockings for prevention of PTS. CDT may be a reasonable option for infrainguinal DVT if symptoms are acute and very severe, particularly in the setting of limb ischemia (cerulean dolens).
The main complication of CDT is bleeding. Significant bleeds are usually confined to the site of venous puncture, and intracranial bleeding is rare (26). Attentive clinical observation during CDT is necessary and should limit most potentially significant sequelae of venous access site bleeds (29). Although there were initial theoretical concerns of CDT increasing the risk of PE due to clot disruption, this has not been demonstrated in the current literature, and concomitant placement of an IVC filter is generally considered unnecessary (26,29). It is possible that the circulating thrombolytic agent may have a therapeutic effect for PE that balances the potential risk of mechanical clot disruption during CDT.
Although observational data is robust, to date there have been only a few randomized clinical trials of CDT. All studies involve patients with IFDVT and compare CDT plus anticoagulation to anticoagulation alone. In an early study from 2002, a relatively small cohort of 35 patients were randomized to the aforementioned groups to assess short-term outcomes by performing ultrasound after 6 months for clot burden and evidence of venous reflux (35). After thrombolysis, patency rates were significantly higher (72% vs. 12%, P<0.001) and venous reflux was significantly lower (11% vs. 41%, P=0.04).
In 2012, a much larger randomized landmark prospective trial (CaVenT) carried out by Enden et al. in Norway involving 209 patients’ assessed long-term outcomes after CDT, with PTS as a primary outcome (36). After 2 years, CDT had an absolute risk reduction for PTS of 14.4% (41.1% vs. 55.6% incidence, P=0.047). At 5-year follow-up, the absolute risk reduction had grown to 28% (43% vs. 71%, P<0.001), equating to a number needed to treat of four (37). Five CDT patients (3%) experienced major bleeding as an immediate complication. No CDT patients experienced PE and there were no intracranial bleeds. Since publication, several authors have illuminated factors in the design of the CaVenT trial that may have led to suboptimal outcomes. Enden et al. have also corroborated these comments. For instance, it was criticized that patients were not stratified optimally, as patients with more extensive DVT and pelvic involvement and concomitant PTS were allocated to CDT groups. In addition, an older drug-only form of CDT technique was chosen, whereas most current approaches favor a combination of CDT with adjunctive mechanical clot removal/disruption (29,37-40).
The ATTRACT trial (41) is a promising multicenter NIH-funded study which is the largest to date and will further define the risks and long-term benefits of CDT. The study is nearing completion and has completed its intake of patients with publication expected in early 2017. The trial includes 692 patients randomized to anticoagulation alone or to CDT plus anticoagulation. PTS incidence at 2 years is a primary outcome. The trial will also stratify patients based on anatomical location of clot and more clearly define which patients benefit from CDT. Safety, cost-effectiveness, and quality of life are secondary outcomes. Of note, ATTRACT will utilize so-called PCDT which involves additional usage of PMT at the time of catheter placement.
PCDT is a growing technology with multiple types of innovate devices now available to the interventionalist. The Trellis (Covidien, CA, USA) is a modified rotational device that uses an oscillating sinusoidal wire with ports for tPA administration between two balloons that isolate the target vascular segment. The Trellis device is no longer available in the USA. The AngioJet (Possis, MN, USA) has two modes. The first is the “pulse-spray” mode where thrombolytic can be sprayed directly into the thrombus. The second is the thrombectomy mode which uses a high-pressure saline jet followed by aspiration of the thrombus (so-called rheolytic thrombectomy). The EKOS device (EKOS, WA, USA) emits high frequency, low energy ultrasound waves to assist lysis, thereby potentially minimizing vascular damage (see Figure 1).
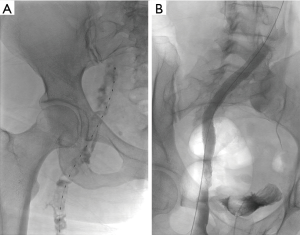
PMT devices can also be used without a thrombolytic agent (42). This may be useful in a patient with a contraindication to lytic therapy. Rotational devices (Amplatz, Microvena, MN, USA) were the first on the market and use a high velocity rotating helix to macerate the thrombus. The AngioVac Cannula (AngioDynamics, Latham, NY, USA) is a vacuum-assisted thrombectomy device and although primarily designed for large vessel (such as IVC, pulmonary artery, and femoral vein) thrombus removal, it is potentially also an attractive option. Extracorporeal filtration of thrombus from venous blood occurs while filtered blood is re-infused at an alternative anatomical site (43).
Evidence suggests that PMT as a standalone therapy (i.e., without thrombolysis) has lower long-term patency rates and higher risk of PE compared to CDT (44). On the other hand, early trials and observational data suggest that PCDT (MT plus CDT) has similar efficacy to CDT but with the potential to reduce treatment time, shorten hospital stays, and reduce total thrombolytic dose (45-47). Randomized prospective clinical trials of PCDT are lacking, however (48). A recently published Cochrane review found no randomized clinical trials on PCDT that met inclusion criteria for the study (49). It remains unknown whether PCDT increases risk for PE in the short-term, and whether temporary IVC filter placement is indicated (29). Future studies like ATTRACT will shed light on the long-term outcomes, safety, and cost-effectiveness of PCDT.
CDT guidelines and practice considerations
Treatment of DVT poses a variety of challenges for physicians. Disease is subcategorized based on the location, chronicity, and burden of thrombus. Individualization of patient care is becoming standard, and clinical risks and rewards have become nuanced with the emergence of outcomes data on various patient subgroups. In addition, therapeutic options have become increasingly more diverse and sophisticated over time. As a result, balancing this information to make the best decision for the patient involves application of an increasingly complicated formula.
In the case of CDT, this involves balancing the risks and costs of intervention with the benefits of reducing PTS and recurrent DVT. Because of the long-term benefits of CDT, it is likely to be most beneficial in younger patients with long life expectancy, low comorbidity, and with a severity of thrombus that puts them at the highest risk for recurrence and/or PTS, such as those with IFDVT.
Three medical societies offer guidelines for the use of CDT in the setting of DVT. All guidelines recommend CDT only as an adjunct to anticoagulation rather than stand-alone therapy.
The American College of Chest Physicians (CHEST) acknowledges most of the likely benefits of CDT but states that the risk and benefits are still uncertain due to limited evidence. As such, CHEST recommends anticoagulation therapy alone over CDT for patients with acute proximal DVT of the leg (50). It qualifies this recommendation by stating that patients who attach a high value to prevention of PTS and lower value to the costs of the procedure and risk of bleeding are likely to choose CDT over anticoagulation.
The American Heart Association (AHA) recommends CDT for first-line treatment of carefully selected patients with acute IFDVT (<21 days within onset of symptoms), limb-threatening compromise, and/or rapid thrombus extension or symptomatic progression despite anticoagulation (51).
The Society of Interventional Radiology (SIR) recommends CDT for: (I) acute IFDVT in ambulatory patients with low bleeding risk and long life expectancy; (II) highly symptomatic subacute and chronic IFDVT; (III) acute or subacute IVC thrombosis; (IV) limb-threatening conditions such as phlegmasia cerulea dolens. SIR states that CDT may also be indicated for a subset of patients with acute femoropopliteal DVT, although the threshold for treatment should be higher than with IFDVT. Absolute contraindications include active internal bleeding and recent (<3 months) stroke, neurosurgery or intracranial trauma. Relative contraindications include recent CPR, GI bleed, major surgery or trauma, intracranial tumor, thrombocytopenia, uncontrolled hypertension (SBP >180 mmHg), and suspicion of infected thrombus (29).
CDT should be performed by skilled practitioners with training in endovascular techniques. Common venous access sites include the popliteal, jugular, femoral, and tibial veins. Ultrasound guidance during percutaneous access is recommended to preserve the integrity of the access site and minimize bleeding risk. Using fluoroscopic guidance, the entire thrombus-containing venous segment should be targeted with a multi-side-hole catheter. Slow continuous infusion of a thrombolytic agent should then occur under close clinical monitoring.
Typically, patient monitoring should occur in an ICU or step-down unit where frequent assessment can occur by a trained nursing staff. The SIR recommends concomitant administration of unfractionated heparin during treatment, although it remains unclear whether therapeutic Xa levels are necessary. Serial monitoring of hematocrit levels and coagulation labs every 6 to 8 hours is recommended. Follow-up venograms are obtained every 8 to 24 hours to assess for residual thrombus and allow for repositioning of the catheter if necessary (29).
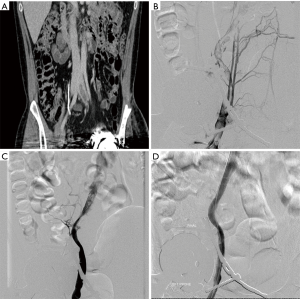
Adjunctive PMT techniques, balloon angioplasty, and stenting are useful in some patients and should be done at the discretion of the internationalist. Stenting may be particularly useful in patients with venous stenosis or with anatomic risk factors for clot formation such as in May-Thurner syndrome (12,29,44,52,53) (see Figure 2). Randomized trials are needed to evaluate the safety and efficacy of these procedures in the setting of first-line therapy for proximal DVT. Newer CDT catheters such as the EKOS device (EKOS, WA, USA) utilize ultrasound technology to disaggregate fibrin strands and facilitate thrombolysis. One study showed a 40% reduction in treatment time and 50–70% reduction in thrombolytic dosing using ultrasound-accelerated thrombolysis (54).
Conclusions
Catheter-directed thrombolysis (CDT) involves percutaneous placement of a catheter into a thrombosed vein with subsequent prolonged infusion of a thrombolytic agent directly into the targeted segment of clot. CDT has been shown to improve quality of life following DVT by preventing valvular damage and reducing long-term sequela of PTS. CDT also reduces risk of recurrent DVT more than anticoagulation alone. In carefully selected patients, CDT may prove to be a cost-effective adjunct to traditional anticoagulation. Patients with acute IFDVT and long life expectancy are particularly likely to benefit. As such, the SIR and AHA recommend CDT as first-line adjunctive therapy for acute IFDVT.
The main complication of CDT is bleeding, of which the majority is confined to the venous access site. Close clinical monitoring is necessary during CDT treatment to minimize risk to the patient. Intracranial bleeding is rare (<1%) but is a potentially lethal complication. There is no evidence to suggest increased risk of PE following CDT when compared to anticoagulation alone. Prospective randomized trials are limited and are needed to further validate CDT’s utility and assess its rate of complications.
PCDT involves adjunctive PMT combined with CDT, which has the potential to reduce treatment time and associated costs of therapy. PCDT may also reduce bleeding risk in some situations by lowering the required dosage of thrombolytic. Many novel PCDT devices have been developed and await validation with clinical trials. ATTRACT is one such trial that is nearing publication and should serve to further validate the efficacy and safety of PCDT. Studies that compare the outcomes of available CDT and PCDT techniques will be useful in guiding clinical practice. Future studies should also examine the cost-effectiveness of CDT for PTS prevention, particularly with respect to quality-adjusted life years.
Acknowledgements
Funding: R Oklu gratefully acknowledges funding from the National Institutes of Health (EB021148, CA172738, EB024403, HL137193) and the Mayo Clinic.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Beckman MG, Hooper WC, Critchley SE, et al. Venous thromboembolism: a public health concern. Am J Prev Med 2010;38:S495-501. [Crossref] [PubMed]
- Office of the Surgeon G, National Heart L, Blood I. Publications and Reports of the Surgeon General. The Surgeon General's Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism. Rockville (MD): Office of the Surgeon General (US), 2008.
- White RH. The epidemiology of venous thromboembolism. Circulation 2003;107:I4-8. [Crossref] [PubMed]
- Prandoni P, Lensing AW, Prins MH, et al. Below-knee elastic compression stockings to prevent the post-thrombotic syndrome: a randomized, controlled trial. Ann Intern Med 2004;141:249-56. [Crossref] [PubMed]
- Kahn SR, Shbaklo H, Lamping DL, et al. Determinants of health-related quality of life during the 2 years following deep vein thrombosis. J Thromb Haemost 2008;6:1105-12. [Crossref] [PubMed]
- Braekkan SK, Grosse SD, Okoroh EM, et al. Venous thromboembolism and subsequent permanent work-related disability. J Thromb Haemost 2016;14:1978-87. [Crossref] [PubMed]
- Vedantham S, Kahn SR, Goldhaber SZ, et al. Endovascular therapy for advanced post-thrombotic syndrome: Proceedings from a multidisciplinary consensus panel. Vasc Med 2016;21:400-7. [Crossref] [PubMed]
- Kahn SR, Shrier I, Julian JA, et al. Determinants and time course of the postthrombotic syndrome after acute deep venous thrombosis. Ann Intern Med 2008;149:698-707. [Crossref] [PubMed]
- Delis KT, Bountouroglou D, Mansfield AO. Venous claudication in iliofemoral thrombosis: long-term effects on venous hemodynamics, clinical status, and quality of life. Ann Surg 2004;239:118-26. [Crossref] [PubMed]
- Henke PK, Comerota AJ. An update on etiology, prevention, and therapy of postthrombotic syndrome. J Vasc Surg 2011;53:500-9. [Crossref] [PubMed]
- Galioto NJ, Danley DL, Van Maanen RJ. Recurrent venous thromboembolism. Am Fam Physician 2011;83:293-300. [PubMed]
- Oklu R, Wicky S. Catheter-directed thrombolysis of deep venous thrombosis. Semin Thromb Hemost 2013;39:446-51. [Crossref] [PubMed]
- Oklu R, Albadawi H, Jones JE, et al. Reduced hind limb ischemia-reperfusion injury in Toll-like receptor-4 mutant mice is associated with decreased neutrophil extracellular traps. J Vasc Surg 2013;58:1627-36. [Crossref] [PubMed]
- Oklu R, Albadawi H, Watkins MT, et al. Detection of extracellular genomic DNA scaffold in human thrombus: implications for the use of deoxyribonuclease enzymes in thrombolysis. J Vasc Interv Radiol 2012;23:712-8. [Crossref] [PubMed]
- Zhang YS, Davoudi F, Walch P, et al. Bioprinted thrombosis-on-a-chip. Lab Chip 2016;16:4097-105. [Crossref] [PubMed]
- Anderson FA Jr, Wheeler HB, Goldberg RJ, et al. A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study. Arch Intern Med 1991;151:933-8. [Crossref] [PubMed]
- Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010;138:1093-100. [Crossref] [PubMed]
- Ruiz-Gimenez N, Suarez C, Gonzalez R, et al. Predictive variables for major bleeding events in patients presenting with documented acute venous thromboembolism. Findings from the RIETE Registry. Thromb Haemost 2008;100:26-31. [PubMed]
- Kaufman JA, Kinney TB, Streiff MB, et al. Guidelines for the use of retrievable and convertible vena cava filters: report from the Society of Interventional Radiology multidisciplinary consensus conference. J Vasc Interv Radiol 2006;17:449-59. [Crossref] [PubMed]
- Kahn SR, Shapiro S, Ducruet T, et al. Graduated compression stockings to treat acute leg pain associated with proximal DVT. A randomised controlled trial. Thromb Haemost 2014;112:1137-41. [Crossref] [PubMed]
- Kahn SR, Galanaud JP, Vedantham S, et al. Guidance for the prevention and treatment of the post-thrombotic syndrome. J Thromb Thrombolysis 2016;41:144-53. [Crossref] [PubMed]
- Plate G, Akesson H, Einarsson E, et al. Long-term results of venous thrombectomy combined with a temporary arterio-venous fistula. Eur J Vasc Surg 1990;4:483-9. [Crossref] [PubMed]
- Plate G, Einarsson E, Ohlin P, et al. Thrombectomy with temporary arteriovenous fistula: the treatment of choice in acute iliofemoral venous thrombosis. J Vasc Surg 1984;1:867-76. [Crossref] [PubMed]
- Goldhaber SZ, Meyerovitz MF, Green D, et al. Randomized controlled trial of tissue plasminogen activator in proximal deep venous thrombosis. Am J Med 1990;88:235-40. [Crossref] [PubMed]
- Turpie AG, Levine MN, Hirsh J, et al. Tissue plasminogen activator (rt-PA) vs heparin in deep vein thrombosis. Results of a randomized trial. Chest 1990;97:172s-5s. [PubMed]
- Watson L, Broderick C, Armon MP. Thrombolysis for acute deep vein thrombosis. Cochrane Database Syst Rev 2014.CD002783. [PubMed]
- Albers GW, Bates VE, Clark WM, et al. Intravenous tissue-type plasminogen activator for treatment of acute stroke: the Standard Treatment with Alteplase to Reverse Stroke (STARS) study. JAMA 2000;283:1145-50. [Crossref] [PubMed]
- Miller DJ, Simpson JR, Silver B. Safety of thrombolysis in acute ischemic stroke: a review of complications, risk factors, and newer technologies. Neurohospitalist 2011;1:138-47. [Crossref] [PubMed]
- Vedantham S, Sista AK, Klein SJ, et al. Quality improvement guidelines for the treatment of lower-extremity deep vein thrombosis with use of endovascular thrombus removal. J Vasc Interv Radiol 2014;25:1317-25. [Crossref] [PubMed]
- Mewissen MW, Seabrook GR, Meissner MH, et al. Catheter-directed thrombolysis for lower extremity deep venous thrombosis: report of a national multicenter registry. Radiology 1999;211:39-49. [Crossref] [PubMed]
- Comerota AJ, Throm RC, Mathias SD, et al. Catheter-directed thrombolysis for iliofemoral deep venous thrombosis improves health-related quality of life. J Vasc Surg 2000;32:130-7. [Crossref] [PubMed]
- Aziz F, Comerota AJ. Quantity of residual thrombus after successful catheter-directed thrombolysis for iliofemoral deep venous thrombosis correlates with recurrence. Eur J Vasc Endovasc Surg 2012;44:210-3. [Crossref] [PubMed]
- Baekgaard N, Broholm R, Just S, et al. Long-term results using catheter-directed thrombolysis in 103 lower limbs with acute iliofemoral venous thrombosis. Eur J Vasc Endovasc Surg 2010;39:112-7. [Crossref] [PubMed]
- Enden T, Resch S, White C, et al. Cost-effectiveness of additional catheter-directed thrombolysis for deep vein thrombosis. J Thromb Haemost 2013;11:1032-42. [Crossref] [PubMed]
- Elsharawy M, Elzayat E. Early results of thrombolysis vs anticoagulation in iliofemoral venous thrombosis. A randomised clinical trial. Eur J Vasc Endovasc Surg 2002;24:209-14. [Crossref] [PubMed]
- Enden T, Klow NE, Sandvik L, et al. Catheter-directed thrombolysis vs. anticoagulant therapy alone in deep vein thrombosis: results of an open randomized, controlled trial reporting on short-term patency. J Thromb Haemost 2009;7:1268-75. [Crossref] [PubMed]
- Enden T, Haig Y, Klow NE, et al. Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomised controlled trial. Lancet 2012;379:31-8. [Crossref] [PubMed]
- Baekgaard N. Benefit of catheter-directed thrombolysis for acute iliofemoral DVT: myth or reality? Eur J Vasc Endovasc Surg 2014;48:361-2. [Crossref] [PubMed]
- Hofmann LV, Kuo WT. Catheter-directed thrombolysis for acute DVT. Lancet 2012;379:3-4. [Crossref] [PubMed]
- Sista AK, Vedantham S, Kaufman JA, et al. Endovascular Interventions for Acute and Chronic Lower Extremity Deep Venous Disease: State of the Art. Radiology 2015;276:31-53. [Crossref] [PubMed]
- Vedantham S, Goldhaber SZ, Kahn SR, et al. Rationale and design of the ATTRACT Study: a multicenter randomized trial to evaluate pharmacomechanical catheter-directed thrombolysis for the prevention of postthrombotic syndrome in patients with proximal deep vein thrombosis. Am Heart J 2013;165:523-30.e3. [Crossref] [PubMed]
- Oklu R, Ghasemi-Rad M, Irani Z, et al. Aspiration thrombectomy using the penumbra catheter. J Vasc Interv Radiol 2015;26:454-5. [Crossref] [PubMed]
- Smith SJ, Behrens G, Sewall LE, et al. Vacuum-assisted thrombectomy device (AngioVac) in the management of symptomatic iliocaval thrombosis. J Vasc Interv Radiol 2014;25:425-30. [Crossref] [PubMed]
- Vedantham S, Piazza G, Sista AK, et al. Guidance for the use of thrombolytic therapy for the treatment of venous thromboembolism. J Thromb Thrombolysis 2016;41:68-80. [Crossref] [PubMed]
- Kim HS, Patra A, Paxton BE, et al. Catheter-directed thrombolysis with percutaneous rheolytic thrombectomy versus thrombolysis alone in upper and lower extremity deep vein thrombosis. Cardiovasc Intervent Radiol 2006;29:1003-7. [Crossref] [PubMed]
- Lin PH, Zhou W, Dardik A, et al. Catheter-direct thrombolysis versus pharmacomechanical thrombectomy for treatment of symptomatic lower extremity deep venous thrombosis. Am J Surg 2006;192:782-8. [Crossref] [PubMed]
- Tichelaar VY, Brodin EE, Vik A, et al. A Retrospective Comparison of Ultrasound-Assisted Catheter-Directed Thrombolysis and Catheter-Directed Thrombolysis Alone for Treatment of Proximal Deep Vein Thrombosis. Cardiovasc Intervent Radiol 2016;39:1115-21. [Crossref] [PubMed]
- Karthikesalingam A, Young EL, Hinchliffe RJ, et al. A systematic review of percutaneous mechanical thrombectomy in the treatment of deep venous thrombosis. Eur J Vasc Endovasc Surg 2011;41:554-65. [Crossref] [PubMed]
- Robertson L, McBride O, Burdess A. Pharmacomechanical thrombectomy for iliofemoral deep vein thrombosis. Cochrane Database Syst Rev 2016;11:CD011536. [PubMed]
- Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141:e419S-96S.
- Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation 2011;123:1788-830. [Crossref] [PubMed]
- Ganguli S, Kalva S, Oklu R, et al. Efficacy of lower-extremity venous thrombolysis in the setting of congenital absence or atresia of the inferior vena cava. Cardiovasc Intervent Radiol 2012;35:1053-8. [Crossref] [PubMed]
- Wicky S, Pinto EG, Oklu R. Catheter-directed thrombolysis of arterial thrombosis. Semin Thromb Hemost 2013;39:441-5. [Crossref] [PubMed]
- Parikh S, Motarjeme A, McNamara T, et al. Ultrasound-accelerated thrombolysis for the treatment of deep vein thrombosis: initial clinical experience. J Vasc Interv Radiol 2008;19:521-8. [Crossref] [PubMed]


