Anti-fouling strategies for central venous catheters
Introduction
Blood contacting medical devices such as central venous catheters (CVCs) all encounter the common complication of increased infectious risk and thrombus formation. Catheters penetrate the natural skin barrier and provide a continuous nidus for external organisms to enter. The introduction of a foreign object through these layers also brings organisms into the central circulation upon placement. Thrombus forms on devices consisting of platelet aggregates and cross-linked fibrin following a complex process of cellular and protein-cell interactions. Systemic agents are frequently used for the prevention of thrombus formation as well as subsequent treatment if a thrombus has formed. These come with their own risks and complications. Studying the etiology of platelet aggregation, cell signaling, adhesion and thrombosis around artificial devices can provide novel and targeted methods to make a better experience and outcome for patients when intervention is necessary. This paper will focus on CVCs, an ubiquitous class of tools in the healthcare team’s arsenal not only used for treatment but also data collection. CVCs provide many benefits including easy access to the blood stream to deliver medications and nutritions and to provide continuous access for dialysis and laboratory testing. Even though complications from catheters are low, the quantity of catheters used makes complications such as infection and occlusion a common experience. As such, preventing these complications can prevent patient harm, improve the patient experience, reduce cost, and improve patient outcomes. We will explore a variety of catheter anti-fouling strategies with a focus on infection and thrombotic occlusion as these often require repeated intervention.
CVCs
The first central venous catheterization occurred in 1929 by Werner Forssmann and was performed with a ureteric catheter by way of the antecubital vein. Fluoroscopy was used to guide the catheter into the right ventricle. Since that time, CVCs have become an essential tool for a variety of purposes and are generally considered safe. As with all medical procedures, catheters do come with risks of complications which may lead to catheter failure or catheter replacement. Infections and occlusions are the two main culprits with occlusion occurring in 14–36% of patients within 1–2 years of placement (1).
Infection
Catheter-related bloodstream infections (CRBSI) are a problem in the United States and abroad with 250,000 cases in the United States alone (2). The International Nosocomial Infection Control Consortium (INICC) estimates a rate significantly higher in developing countries (3). The CRBSI rate in England in 2012 was 4.58 per 1,000 CVC days (4). Meta-analysis study conducted at Johns Hopkins University showed that bloodstream infections were the third largest cause of nosocomial infections with a mortality rate ranging from 12–25% (5,6).
CRBSIs are expensive. Some estimates have placed a CRBSI event between $4,888 to $11,591 (4). This would equate to a conservative estimate of one billion dollars per year in the USA and would place a significant burden on any healthcare institution. A cost-effective method of prevention should be employed to reduce the number and severity of complications. In 2016, analysis of PICUs in England showed that a cost-effective method for addressing this problem was to use antibiotic coated CVCs specifically in settings where the risk of CRBSI was low (4). Guidelines naturally recommend antibiotic CVCs in high risk patients (4,7).
Risk factors for CRBSIs include the type of underlying disease and its severity, immunodeficiency, catheter type and material, time course of catheter use, and whether the catheter is tunneled or not. Local risk factors include hygiene around the insertion site, type of dressing, moisture at the entry/exit site, and S. aureus nasal colonization status.
The general causative organism includes S. aureus, P. aeruginosa and candida as the most common three with S. aureus as the predominant pathogen (8). Less common pathogens are coagulase negative staphylococci, E. coli and K. pneumonia are also associated with CRBSI. Very little is known about viral or parasitic causes of catheter related infection or the normal skin flora of these types of organisms.
Infection—pathogenesis
The etiology of CRBSIs is likely due to a combination of two factors, the ability of microorganisms to migrate through the defect created by the CVC entry site and direct inoculation during placement of the catheter (9-11). Short term CVCs, considered of less than 10 days’ duration, are most commonly infected by cutaneous organisms that traverse on the external surface of the catheter. Long term catheters, greater than 10 days, appear to have a different fouling mechanism involving the luminal surfaces of the catheter. As such, short term catheters tend to benefit from prevention methods that are directed at the external surface while long term catheters benefit from an endoluminal infection prevention approach (6).
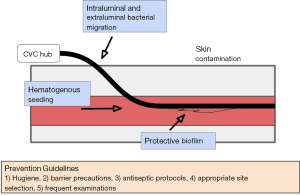
Biofilm related infections are extremely difficult to eradicate due to high tolerance of the biofilm to antibiotics and resistance to the host immune systems (12,13). There is no efficient method to detect biofilm formation and thus no methods for early intervention. Management often entails catheter removal and replacement, subjecting the patient to another procedure and its associated risks. Figure 1 summarizes the entry points of infectious causes of catheter fouling.
Infection—prevention
Sterile technique and frequent monitoring: guidelines have been established for effective placement and routine care of CVCs which include hand hygiene regimens, barrier precautions, antiseptic protocols during placement with use of agents like chlorhexidine, directed site selection strategies as well as daily examinations (13,14). Removal and replacement of a CVC is also recommended for prevention and treatment. Sterile, transparent, and semi-permeable dressings are recommended with frequent dressing changes (often weekly) especially if the dressing or area is soiled or damaged. Preventing line entry points from submersion in water is recommended, no soaking. Additionally, vigorous activity involving the area the catheter traverses can cause damage to the line or displace it and so avoiding this if possible.
One simple solution to preventing catheter fouling by infection is direct anti-infective agent impregnation into catheter materials. Materials can be bonded in a variety of ways to a catheter including on the inner surface, the exterior surface and within the catheter material itself. A Cochrane systematic review concluded that antibiotic impregnated catheters are effective but should be used in the correct patients as not all patients benefit (15). Minocycline and rifampin demonstrate improved rates of infection when impregnated in catheter materials (16-18). Silver has also been used as a coating agent internally and externally, which has not been shown to be beneficial (11). Heparin also has been used as a coating agent to prevent infectious fouling (16). Chlorhexidine and sulfadiazine are also associated with a decreased incidence of catheter colonization and CRBSI when compared to uncoated catheters (19,20). The underlying material used in catheters can make a large difference in the incidence of CRBSI. Polytetrafluoroethylene and polyurethane catheters are associated with decreased rates of CRBSI when compared to polyvinyl chloride and polyethylene (21).
Infection—outcomes
INICC multidimensional infection control approach has been a large success in curbing CRBSIs by up to 50% or more as well as CRBSI associated deaths by 58% (22-27). These steps include creating a central line kit consolidating useful items, thorough education about precautions and complications, end outcome surveillance, continuous process surveillance, feedback on outcomes as well as feedback on infection prevention practices (26).
Occlusion
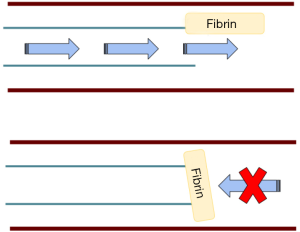
Occlusions occur often involving 14–36% of patients within 1 to 2 years of placement of the catheter (1). Aspiration and flushing are the two major actions of a catheter and complete occlusion is when neither action is possible. It is likely more common for incomplete occlusion to occur in isolation where flushing of the line is possible but aspiration is not. This is most often due to fibrin sheath formation that causes a one-way valve effect as shown in Figure 2. Occlusion can occur, in general, from mechanical obstructions (kinked tubing, tight sutures, external pressure, vessel wall opposition), precipitated infusates, fibrin sheaths or thrombosis. Some data estimate that up to 66% of patients who have long term CVCs will have a thrombotic event and are associated with long term vascular complications (28-33). Fibrin sheaths can occur as early as 24 hours after placement of a CVC (34). Fibrin sheaths usually do not cause any symptoms or long-term consequences, however, there is a small risk of embolization which could have more serious effects.
Occlusion—thrombosis
Virchow’s triad is a classic cornerstone of basic thrombosis risk factors in which three major factors control the development of a thrombus: (I) stasis; (II) endothelial injury; (III) altered coagulability. Clinical risk factors that affect the triad and are indicators for thrombosis include recent surgery, trauma, prior thrombosis, increasing age, pregnancy or puerperium, and oral contraceptives. Hypercoagulable states include cancer, heart failure, obesity, myeloproliferative disorders, nephrotic syndrome, and familial causes such as factor V Leiden, prothrombin mutations and protein C or S deficiency.
Thrombosis—pathogenesis
Healthy endothelium is drastically different than artificial surfaces as it has endogenous mechanisms to actively resist thrombosis while artificial surfaces often do not. The endothelium is also able to actively regulate flow. Injury to the endothelium triggers a complicated cell signaling cascade that promotes thrombosis. These features include but are not limited to electrostatic forces of surface molecules and active chemical release including platelet and coagulation protein inhibitors and molecules to activate fibrinolysis (35). The subendothelial layer contains von Willebrand factor (vWF), collagen, laminin, thrombospondin and vitronectin creating a highly thrombogenic environment. Basic artificial surfaces have little to no directed activity against adherent proteins or cells, no remodeling capabilities, and no active mechanism to enhance or inhibit cell signaling. Thus, a process of protein adsorption, cellular adhesion, thrombin generation, and activation of complement occurs at the surface which eventually creates thrombotic occlusion. Figure 3 illustrates the basic steps of protein adsorption, cellular adhesion, aggregation, and stabilization.
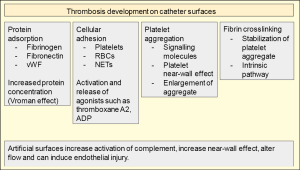
Surface adsorption of proteins by electrostatic interactions and physical conformational changes of these proteins are likely the first step in device thrombosis. Adsorbed proteins can form a layer 2–10 nm in thickness and enhance the concentration of these proteins 1,000 times higher than in plasma. Adsorption is reversible and can change the local concentrations of adsorbed proteins. This is known as the Vroman effect (39). Hydrophilicity is a key determinant of protein adsorption. Hydrophobic surfaces adsorb proteins more readily. Given that protein structure determines function, adsorption to an artificial surface can alter protein structure and subsequently biologic activity. This feature of surface-protein interaction may be a ripe target for preventing complications (40).
A key component in thrombosis is fibrinogen and it is likely one of the first components to adsorb to artificial surfaces. Fibronectin and vWF will subsequently work with fibrinogen to mediate platelet adhesion. The complement system and coagulation pathway proteins will be readily activated in this highly concentrated environment facilitating thrombosis.
Cell adhesion to the surface via the deposited protein layer is the next step to thrombosis. Fibrinogen is the major factor in platelet aggregation on artificial surfaces (41). As platelets aggregate to the adsorbed fibrinogen they release thromboxane A2, ADP and other agonists which activate further aggregation. Fibrinogen will also attach to leukocytes and create an inflammatory state that increases platelet aggregation via multiple signaling molecules such as platelet activating factor and tumor necrosis factor (TNF). Red blood cell (RBC) adhesion is passive but once adhered can release ADP which stimulates platelets (41).
Once the artificial surface is covered with proteins that promote cell adhesion and platelets have adhered to the surface proteins, further aggregation and cross-linking can occur to enlarge and stabilize the early aggregate. The two major systems that influence the late portion of the cascade involve coagulation proteins leading to thrombin generation and the complement system.
Coagulation proteins also adhere to the artificial surface promoting activation of the cascade and stability in platelet aggregation by fibrin crosslinking. Increased clotting activation is thought to be mediated through the intrinsic coagulation pathway (42). Clotting time when tested in vitro can be decreased 3-fold solely by the presence of a catheter (37,38). This may be mediated through the intrinsic pathway, which has been shown by inhibiting this effect with corn trypsin inhibitor, a potent and specific inhibitor of FXIIa.
In other blood contacting systems, for example dialysis machines, after blood contacts the artificial surfaces the complement system is activated (43). This also occurs with catheters (44). The complement cascade consists of three pathways: classical, alternative and lectin. The two pathways most likely to be activated by artificial surfaces are the classical pathway and the alternative pathway. This occurs by Kallikrein cleaving FXIIa and by C3 and C5 deposition onto the surfaces which are then activated and promote leukocyte attraction and adhesion.
More underlying mechanisms of thrombus stability are being explored which can be later used for targeting. Thrombus contains complicated arrays of histone/DNA complexes when in the acute phase (45). Local inflammation developed around thrombus creates neutrophil extracellular traps (NETs), an array of DNA and proteins with the intent of antimicrobial activity. These can then provide a scaffold with altered flow for platelet adhesion and further propagation of the thrombus (36).
Thrombosis—physical properties
A typical design for a CVC can be seen in Figure 4. Naturally, catheter surface structure impacts the flow dynamics of blood which may significantly affect rates of thrombosis. Catheter structure involves a smooth surface made of a variety of materials. These materials are continuously under development and the manufacturing complexities may influence the outcomes of translational research. At the end of a catheter are eyelets which are holes in the catheter that allow exchange of fluids and are known areas of complications (46).

Physical proximity of platelets with developing thrombus and local flow dynamics directly affect the formation of thrombi. A platelet must be near the surface of other platelets for a sufficient period to form the appropriate electrostatic and chemical bonds. The number and concentration of platelets near a vessel wall and catheter surface will dramatically change the occurrence of platelet-platelet and platelet-surface interactions. Platelet motion and distribution within vessels are strongly affected by the motion of adjacent RBCs and surrounding shear stressors. The shear rate describes the flow of fluid between two parallel plates which is used to describe the flow of blood and plasma within vessel walls. A platelet “near-wall excess” is a well-known phenomenon that describes a multi-micron-wide fluid layer adjacent to vessel walls where there is an increased density of platelets (47). An increased concentration of platelets at the periphery increases the interactions that platelets have with the catheter surface as well as a developing thrombus.
Thrombosis—prevention
The obvious first step to preventing thrombosis of catheter devices would be to target protein adsorption to catheter surfaces. Studies into modifying the surface structure and biomaterial have not yet been able to remove the need for systemic anticoagulant agents. Failure rates of ventricular assist devices have been reported to be as high as 6% when due to thrombosis (48).
Inhibiting cell adsorption is another likely target for prevention. Inhibiting protein and cell adsorption is often attempted by modifying the electrostatic and hydrophobic/hydrophilic properties of the material. Physical properties of systems such as entropy can be leveraged to prevent adsorption and adhesion. For instance, water molecules evenly distributed on a hydrophilic surface will decrease the local entropy and may be readily displaced by proteins or cells. Surface structure modification can readily affect these physical properties.
Hydrophilic polymers
Polyethylene glycol (PEG), also known as polyethylene oxide (PEO), is highly hydrophilic and creates a water-solvated structure forming a surface that has highly mobile chains that physically prevents protein and cellular adsorption (49). While in vitro studies showed some benefit, no substantial in vivo studies have (49).
Zwitterionic materials such as phosphorylcholines improve on the level of hydrophilicity demonstrated by PEO by offering both positively and negatively charged components. Phosphorylcholines coatings have multiple advantages including better stability than PEG. They are inherently non-thrombogenic. Additionally, phosphorylcholines can be used as a storage container for other biologically active molecules and released over time (50). An important feature of zwitterionic groups is to maintain the ability to physically exclude fouling materials such as proteins, a method of steric hindrance. In phosphorylcholines the head groups are electrically neutral at physiologic pH. Available clinical studies revolve around coronary stents with acceptable but not stellar outcomes (51,52) which means further development may be needed to gain full advantage. Animal studies unfortunately have been less convincing (53).
Surface coating
Pyrolytic carbon coating is used in a variety of vascular settings including heart valves. Valves are coated via a process called chemical vapor deposition. Pyrolytic carbon offers good biocompatibility and demonstrates decreased platelet adhesion when compared to other materials, although there are surface micro contours that allow for more platelet adhesion than once thought (54). When compared to uncoated artificial devices when used in humans, however, no significant benefit in patency rates has been shown (55-57).
Albumin, a prominent protein in natural systems, has been used as a coating on catheters with in vitro studies demonstrating benefit. Albumin has been shown to resist platelet and leukocyte adhesion (58,59). Elastin is a vital component of the vessel wall, possibly the best biocompatible thrombo-preventive surface known. Thus, elastin inspired coatings have been pursued. Overcoming the difficulties of developing elastin-like coatings (60,61) multiple components have been built into the surface of catheters such as polyethylene terephthalate. These products have demonstrated promising in vitro results with anti-platelet activity (62). Animal studies also show promise (63). Elastin-like polypeptides also offer other benefits including the ability to store and deliver drugs over time as well as the ability to self-assemble (64).
Heparin is a ubiquitous systemic agent for preventing thrombosis. Naturally, heparin has been coated onto multiple devices (65). Unfortunately, studies on coronary artery stents with heparin coating have not demonstrated benefits significantly above bare metal stents (66,67). One possible explanation for the lack of benefit is in the binding properties used and so other methods of binding heparin to device surfaces are being investigated (68-70). Additionally, direct thrombin inhibitors such as argatroban and bivalirudin could offer benefits (71-73) but no significant studies on efficacy are available.
Sirolimus, initially an antifungal medication, has been used as a coating on heparinized stents and demonstrated an improved restenosis rate when compared to other stents when tested in animals (74). The study also noted good biocompatibility with no change to the inflammatory response indicating a possible role for sirolimus and future development.
Targeting NETs
One potential avenue that has not been studied is addressing the aggregation effects of NETs on platelets. Immunothrombosis, a proposed method of fighting and containing infection (75) may play a role in catheter related thrombosis due to an inflammatory response incited by foreign materials or microorganism invasion. The extracellular DNA identified in acute thrombi may be a target for DNA splitting enzymes and may lead to improved prevention (45). Pretreatment with nucleases has been shown to reduce NET levels which could lead to reduced thrombogenicity (76). Further evaluation of the development of NETs near sites of foreign devices may help to explore this possibility.
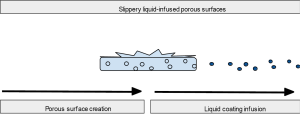
Slippery liquid-infused porous surfaces (SLIPS)
SLIPS are a new and unique design exploiting roughened surfaces to create variable topography that improves contact with a coating. Figure 5 illustrates this relationship between porous material and liquid surface. This coating ideally would be chemically inert, inciting no inflammatory response and preventing protein and cellular adhesion for improved anti-fouling. Solid structures with these nanoscale substructures on the surface combined with high density but liquid repelling coatings can protect against a wide variety of substances, whether they are viscous, thin liquids or full of cells (77-81). SLIPS have additional value in that they are self-healing and require less maintenance with improved stability for long term applications. Application of SLIPS has been performed on the enamel of rabbits and demonstrated greatly reduced dental plaque (82).
Micropatterning
Micropatterning of surfaces is a unique strategy that utilizes the well demonstrated effects that surface topology can have on cellular response (83). In another demonstration of the power of biomimicry, micropatterned surfaces can be developed that mimic the natural antifouling surfaces present on aquatic animals and other life forms (84). One such surface, present on lotus leaves, uses uniform conical cells creating a super hydrophobic surface that causes liquid beading to clean the surface from adhered contaminants (85). Another surface involves mimicking shark skin by creating microgrooves that create a low-drag and self-cleaning surface. After creation of an artificial structure it was shown to reduce zoospore settlement by approximately 85% (86) which may have implications for antithrombotic effects as well.
Thrombosis—treatment
Treatment for thrombosed catheters generally includes thrombolytic therapy or removal/replacement of the catheter. Complete thrombosis of a catheter can occur with thrombosis of the surrounding veins causing deep venous thrombosis and necessitating further treatment such as systemic anticoagulation or catheter directed thrombolytics (1,87-90). Pulmonary embolism is the most feared complication with catheter thrombosis. Embolic events after catheter removal occur due to dislodging the thrombus material when manipulating the catheter. There are no supported guidelines for when additional catheters should be placed and individual assessments must occur on a patient to patient basis (87).
Discussion
The role of CVCs in the outpatient and hospital setting is substantial and often without severe complications. Despite this, the volume of catheters used makes even rare complications not an uncommon experience. A variety of strategies for antifouling methods have been attempted with variable results including catheter impregnation techniques with antibiotics, various polymer materials with antimicrobial and antiplatelet properties, taking advantage of electrostatic forces to create hydrophilic slippery surfaces as well as coating inert materials with active proteins. These are summarized in Table 1. Exciting new avenues of research in SLIPS and omniphobic surfaces offer the promise of improved biocompatibility and reducing thrombotic complications. Continued research into the molecular structure of thrombus and the inflammatory response may offer additional methods of preventing platelet aggregation and stabilization such as targeting extracellular DNA seen in NETs. Many of these antifouling methods have not been directly attempted in CVCs. Additional studies and development of competing catheter systems need to be started to confirm and improve on the techniques described. Easier and faster detection of catheter related complications via biosensors could lead to novel techniques of prevention expanding upon the techniques described. Further studies and funding in these areas will have a benefit to patients and health-systems alike.

Full table
Acknowledgements
Funding: R Oklu gratefully acknowledges funding from the National Institutes of Health (EB021148, CA172738, EB024403, HL137193) and the Mayo Clinic.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Baskin JL, Pui CH, Reiss U, et al. Management of occlusion and thrombosis associated with long-term indwelling central venous catheters. Lancet 2009;374:159-69. [Crossref] [PubMed]
- O'Grady NP, Alexander M, Dellinger EP, et al. Guidelines for the prevention of intravascular catheter-related infections. Centers for disease control and prevention. MMWR Recomm Rep 2002;51:1-29. [PubMed]
- Rosenthal VD, Maki DG, Mehta Y, et al. International nosocomial infection control consortiu (INICC) report, data summary of 43 countries for 2007-2012. Device-associated module. Am J Infect Control 2014;42:942-56. [Crossref] [PubMed]
- Harron K, Mok Q, Hughes D, et al. Generalisability and cost-impact of antibiotic-impregnated central venous catheters for reducing risk of bloodstream infection in paediatric intensive care units in England. PLoS One 2016;11:e0151348. [Crossref] [PubMed]
- Maki DG, Kluger DM, Crnich CJ. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc 2006;81:1159-71. [Crossref] [PubMed]
- Gahlot R, Nigam C, Kumar V, et al. Catheter-related bloodstream infections. Int J Crit Illn Inj Sci 2014;4:162-7. [Crossref] [PubMed]
- Harron K, Ramachandra G, Mok Q, et al. Consistency between guidelines and reported practice for reducing the risk of catheter-related infection in British paediatric intensive care units. Intensive Care Med 2011;37:1641-7. [Crossref] [PubMed]
- Parameswaran R, Sherchan JB, Varma DM, et al. Intravascular catheter-related infections in an Indian tertiary care hospital. J Infect Dev Ctries 2011;5:452-8. [Crossref] [PubMed]
- Raad I, Hanna H, Maki D. Intravascular catheter-related infections: advances in diagnosis, prevention, and management. Lancet Infect Dis 2007;7:645-57. [Crossref] [PubMed]
- Crnich CJ, Maki DG. The promise of novel technology for the prevention of intravascular device–related bloodstream infection. i. pathogenesis and short-term devices. Clin Infect Dis 2002;34:1232-42. [Crossref] [PubMed]
- Chen YM, Dai AP, Shi Y, et al. Effectiveness of silver-impregnated central venous catheters for preventing catheter-related blood stream infections: A meta-analysis. Int J Infect Dis 2014;29:279-86. [Crossref] [PubMed]
- Chauhan A, Ghigo JM, Beloin C. Study of in vivo catheter biofilm infections using pediatric central venous catheter implanted in rat. Nat Protoc 2016;11:525-41. [Crossref] [PubMed]
- Percival SL, Suleman L, Vuotto C, et al. Healthcare-associated infections, medical devices and biofilms: risk, tolerance and control. J Med Microbiol. 2015;64:323-34. [Crossref] [PubMed]
- Miller SE, Maragakis LL. Central line-associated bloodstream infection prevention. Curr Opin Infect Dis 2012;25:412-22. [Crossref] [PubMed]
- Lai NM, Chaiyakunapruk N, Lai NA, et al. Catheter impregnation, coating or bonding for reducing central venous catheter-related infections in adults. Cochrane Database Syst Rev 2016;3:CD007878. [PubMed]
- Raad I, Mohamed JA, Reitzel RA, et al. Improved antibiotic-impregnated catheters with extended-spectrum activity against resistant bacteria and fungi. Antimicrob Agents Chemother 2012;56:935-41. [Crossref] [PubMed]
- Jamal MA, Rosenblatt JS, Hachem RY, et al. Prevention of biofilm colonization by gram-negative bacteria on minocycline-rifampin-impregnated catheters sequentially coated with chlorhexidine. Antimicrob Agents Chemother 2014;58:1179-82. [Crossref] [PubMed]
- Casey AL, Mermel LA, Nightingale P, et al. Antimicrobial central venous catheters in adults: a systematic review and meta-analysis. Lancet Infect Dis 2008;8:763-76. [Crossref] [PubMed]
- Veenstra DL, Saint S, Saha S, et al. Efficacy of antiseptic-impregnated central venous catheters in preventing catheter-related bloodstream infection: a meta-analysis. JAMA 1999;281:261-7. [Crossref] [PubMed]
- Ramritu P, Halton K, Collignon P, et al. A systematic review comparing the relative effectiveness of antimicrobial-coated catheters in intensive care units. Am J Infect Control 2008;36:104-17. [Crossref] [PubMed]
- Mehall JR, Saltzman DA, Jackson RJ, et al. Catheter materials affect the incidence of late blood-borne catheter infection. Surg Infect (Larchmt) 2001;2:225-9; discussion 229-30. [Crossref] [PubMed]
- Rosenthal VD, Ramachandran B, Villamil-Gómez W, et al. Impact of a multidimensional infection control strategy on central line-associated bloodstream infection rates in pediatric intensive care units of five developing countries: findings of the International Nosocomial Infection Control Consortium (INICC). Infection 2012;40:415-23. [Crossref] [PubMed]
- Rosenthal VD, Jarvis WR, Jamulitrat S, et al. Socioeconomic impact on device-associated infections in pediatric intensive care units of 16 limited-resource countries. Pediatr Crit Care Med 2012;13:399-406. [Crossref] [PubMed]
- Jaggi N, Rodrigues C, Rosenthal VD, et al. Impact of an International nosocomial infection control consortium multidimensional approach on central line-associated bloodstream infection rates in adult intensive care units in eight cities in India. Int J Infect Dis 2013;17:e1218-24. [Crossref] [PubMed]
- Higuera F, Rosenthal VD, Duarte P, et al. The effect of process control on the incidence of central venous catheter-associated bloodstream infections and mortality in intensive care units in Mexico. Crit Care Med 2005;33:2022-7. [Crossref] [PubMed]
- Rosenthal VD, Dueñas L, Sobreyra-Oropeza M, et al. Findings of the international nosocomial infection control consortium (INICC), part III: effectiveness of a multidimensional infection control approach to reduce central line-associated bloodstream infections in the neonatal intensive care units of 4 developing countries. Infect Control Hosp Epidemiol 2013;34:229-37. [Crossref] [PubMed]
- Rosenthal VD, Maki DG, Rodrigues C, et al. Impact of international nosocomial infection control consortium (INICC) strategy on central line–associated bloodstream infection rates in the intensive care units of 15 developing countries. Infect Control Hosp Epidemiol 2010;31:1264-72. [Crossref] [PubMed]
- Rooden CJ, Tesselaar ME, Osanto S, et al. Deep vein thrombosis associated with central venous catheters - a review. J Thromb Haemost 2005;3:2409-19. [Crossref] [PubMed]
- Verso M, Agnelli G. Venous thromboembolism associated with long-term use of central venous catheters in cancer patients. J Clin Oncol 2003;21:3665-75. [Crossref] [PubMed]
- Journeycake JM, Buchanan GR. Thrombotic complications of central venous catheters in children. Curr Opin Hematol 2003;10:369-74. [Crossref] [PubMed]
- Glaser DW, Medeiros D, Rollins N, et al. Catheter-related thrombosis in children with cancer. J Pediatr 2001;138:255-9. [Crossref] [PubMed]
- Boersma RS, Jie KS, Verbon A, et al. Thrombotic and infectious complications of central venous catheters in patients with hematological malignancies. Ann Oncol 2008;19:433-42. [Crossref] [PubMed]
- Balestreri L, De Cicco M, Matovic M, et al. Central venous catheter-related thrombosis in clinically asymptomatic oncologic patients: a phlebographic study. Eur J Radiol 1995;20:108-11. [Crossref] [PubMed]
- Hoshal VL, Ause RG, Hoskins PA. Fibrin sleeve formation on indwelling subclavian central venous catheters. Arch Surg 1971;102:353-8. [Crossref] [PubMed]
- Palta S, Saroa R, Palta A. Overview of the coagulation system. Indian J Anaesth 2014;58:515-23. [Crossref] [PubMed]
- Fuchs TA, Brill A, Duerschmied D, et al. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A 2010;107:15880-5. [Crossref] [PubMed]
- Jaffer IH, Fredenburgh JC, Hirsh J, et al. Medical device-induced thrombosis: What causes it and how can we prevent it? J Thromb Haemost 2015;13:S72-81. [Crossref] [PubMed]
- Yau JW, Stafford AR, Liao P, et al. Mechanism of catheter thrombosis: Comparison of the antithrombotic activities of fondaparinux, enoxaparin, and heparin in vitro and in vivo. Blood 2011;118:6667-74. [Crossref] [PubMed]
- Jung SY, Lim SM, Albertorio F, et al. The Vroman effect: a molecular level description of fibrinogen displacement. J Am Chem Soc 2003;125:12782-6. [Crossref] [PubMed]
- Hlady V, Buijs J. Protein adsorption on solid surfaces. Curr Opin Biotechnol 1996;7:72-7. [Crossref] [PubMed]
- Savage B, Ruggeri ZM. Selective recognition of adhesive sites in surface-bound fibrinogen by glycoprotein IIb-IIIa on nonactivated platelets. J Biol Chem 1991;266:11227-33. [PubMed]
- Yau JW, Liao P, Fredenburgh JC, et al. Selective depletion of factor XI or factor XII with antisense oligonucleotides attenuates catheter thrombosis in rabbits. Blood 2014;123:2102-7. [Crossref] [PubMed]
- Johnson RJ. Complement activation during extracorporeal therapy: biochemistry, cell biology and clinical relevance. Nephrol Dial Transplant 1994;9:36-45. [PubMed]
- Kazatchkine MD, Haeffner-Cavaillon N. Mechanisms and consequences of complement activation during hemodialysis. Boston: Springer, 1989:19-26.
- Oklu R, Albadawi H, Watkins MT, et al. Detection of extracellular genomic DNA scaffold in human thrombus: Implications for the use of deoxyribonuclease enzymes in thrombolysis. J Vasc Interv Radiol 2012;23:712-8. [Crossref] [PubMed]
- Jacobsen SM, Stickler DJ, Mobley HL, et al. Complicated catheter-associated urinary tract infections due to Escherichia coli and Proteus mirabilis. Clin Microbiol Rev 2008;21:26-59. [Crossref] [PubMed]
- Tilles AW, Eckstein EC. The near-wall excess of platelet-sized particles in blood flow: Its dependence on hematocrit and wall shear rate. Microvasc Res 1987;33:211-23. [Crossref] [PubMed]
- Bluestein D, Girdhar G, Einav S, et al. Device thrombogenicity emulation: A novel methodology for optimizing the thromboresistance of cardiovascular devices. J Biomech 2013;46:338-44. [Crossref] [PubMed]
- Lee JH, Lee HB, Andrade JD. Blood compatibility of polyethylene oxide surfaces. Prog Polym Sci 1995;20:1043-79. [Crossref]
- Lewis AL, Stratford PW. Phosphorylcholine-coated stents. J Long Term Eff Med Implants 2002;12:231-50. [Crossref] [PubMed]
- Habara S, Mitsudo K, Kadota K, et al. Serial clinical and angiographic follow-up after phosphorylcholine-coated stent implantation. Int Heart J 2011;52:88-91. [Crossref] [PubMed]
- Galli M, Bartorelli A, Bedogni F, et al. Italian BiodivYsio open registry (BiodivYsio PC-coated stent): study of clinical outcomes of the implant of a PC-coated coronary stent. J Invasive Cardiol 2000;12:452-8. [PubMed]
- Kuiper KK, Robinson KA, Chronos NA, et al. Phosphorylcholine-coated metallic stents in rabbit iliac and porcine coronary arteries. Scand Cardiovasc J 1998;32:261-8. [Crossref] [PubMed]
- Goodman SL, Tweden KS, Albrecht RM. Three-Dimensional Morphology and Platelet Adhesion on Pyrolytic Carbon Heart Valve Materials. Cells Mater 1995;5:15-30.
- Sick PB, Gelbrich G, Kalnins U, et al. Comparison of early and late results of a Carbofilm-coated stent versus a pure high-grade stainless steel stent (the Carbostent-Trial). Am J Cardiol 2004;93:1351-6. [Crossref] [PubMed]
- Sick PB, Brosteanu O, Ulrich M, et al. Prospective randomized comparison of early and late results of a carbonized stent versus a high-grade stainless steel stent of identical design: The PREVENT trial. Am Heart J 2005;149:681-8. [Crossref] [PubMed]
- Kim YH, Lee CW, Hong MK, et al. Randomized comparison of carbon ion-implanted stent versus bare metal stent in coronary artery disease: The Asian Pacific Multicenter Arthos Stent Study (PASS) trial. Am Heart J 2005;149:336-41. [Crossref] [PubMed]
- Park K, Mosher DF, Cooper SL. Acute surface-induced thrombosis in the canine ex vivo model: Importance of protein composition of the initial monolayer and platelet activation. J Biomed Mater Res 1986;20:589-612. [Crossref] [PubMed]
- Kim SW, Lee RG, Oster H, et al. Platelet adhesion to polymer surfaces. Trans Am Soc Artif Intern Organs 1974;20 B:449-55.
- McPherson DT, Morrow C, Minehan DS, et al. Production and Purification of a Recombinant Elastomeric Polypeptide, G-(VPGVG)19-VPGV, from Escherichia coli. Biotechnol Prog 1992;8:347-52. [Crossref] [PubMed]
- Trabbic-Carlson K, Setton LA, Chilkoti A. Swelling and mechanical behaviors of chemically cross-linked hydrogels of elastin-like polypeptides. Biomacromolecules 2003;4:572-80. [Crossref] [PubMed]
- Woodhouse KA, Klement P, Chen V, et al. Investigation of recombinant human elastin polypeptides as non-thrombogenic coatings. Biomaterials 2004;25:4543-53. [Crossref] [PubMed]
- Jordan SW, Haller CA, Sallach RE, et al. The effect of a recombinant elastin-mimetic coating of an ePTFE prosthesis on acute thrombogenicity in a baboon arteriovenous shunt. Biomaterials 2007;28:1191-7. [Crossref] [PubMed]
- Rodríguez-Cabello JC, Arias FJ, Rodrigo MA, et al. Elastin-like polypeptides in drug delivery. Adv Drug Deliv Rev 2016;97:85-100. [Crossref] [PubMed]
- Biran R, Pond D. Heparin coatings for improving blood compatibility of medical devices. Adv Drug Deliv Rev 2017;112:12-23. [Crossref] [PubMed]
- Wöhrle J, Al-Khayer E, Grötzinger U, et al. Comparison of the heparin coated vs the uncoated Jostent--No influence on restenosis or clinical outcome. Eur Heart J 2001;22:1808-16. [Crossref] [PubMed]
- Vrolix MC, Legrand VM, Reiber JH, et al. Heparin-coated Wiktor stents in human coronary arteries (MENTOR Trial). Mentor trial investigators. Am J Cardiol 2000;86:385-9. [Crossref] [PubMed]
- Gao W, Lin T, Li T. Sodium alginate/heparin composites on PVC surfaces inhibit the thrombosis and platelet adhesion: Applications in cardiac surgery. Int J Clin Exp Med 2013;6:259-68. [PubMed]
- Deng J, Liu X, Ma L, et al. Heparin-mimicking multilayer coating on polymeric membrane via LbL assembly of cyclodextrin-based supramolecules. ACS Appl Mater Interfaces 2014;6:21603-14. [Crossref] [PubMed]
- Liu T, Liu Y, Chen Y, et al. Immobilization of heparin/poly-(L)-lysine nanoparticles on dopamine-coated surface to create a heparin density gradient for selective direction of platelet and vascular cells behavior. Acta Biomater 2014;10:1940-54. [Crossref] [PubMed]
- Lu L, Li QL, Maitz MF, et al. Immobilization of the direct thrombin inhibitor-bivalirudin on 316L stainless steel via polydopamine and the resulting effects on hemocompatibility in vitro. J Biomed Mater Res A 2012;100:2421-30. [PubMed]
- Wyers MC, Phaneuf MD, Rzucidlo EM, et al. In vivo assessment of a novel Dacron surface with covalently bound recombinant hirudin. Cardiovasc Pathol 1999;8:153-9. [Crossref] [PubMed]
- Nakayama Y, Yamaoka S, Yamanami M, et al. Water-soluble argatroban for antithrombogenic surface coating of tissue-engineered cardiovascular tissues. J Biomed Mater Res B Appl Biomater 2011;99:420-30. [Crossref] [PubMed]
- Bae IH, Lim KS, Park DS, et al. Sirolimus coating on heparinized stents prevents restenosis and thrombosis. J Biomater Appl 2017;31:1337-45. [Crossref] [PubMed]
- Kimball AS, Obi AT, Diaz JA, et al. The emerging role of NETs in venous thrombosis and immunothrombosis. Front Immunol 2016;7:236. [Crossref] [PubMed]
- Oklu R, Albadawi H, Jones JE, et al. Reduced hind limb ischemia-reperfusion injury in Toll-like receptor-4 mutant mice is associated with decreased neutrophil extracellular traps. J Vasc Surg 2013;58:1627-36. [Crossref] [PubMed]
- Sunny S, Vogel N, Howell C, et al. Lubricant-infused nanoparticulate coatings assembled by layer-by-layer deposition. Adv Funct Mater 2014;24:6658-67. [Crossref]
- Vogel N, Belisle RA, Hatton B, et al. Transparency and damage tolerance of patternable omniphobic lubricated surfaces based on inverse colloidal monolayers. Nat Commun 2013;4:2167. [Crossref] [PubMed]
- Epstein AK, Wong TS, Belisle RA, et al. Liquid-infused structured surfaces with exceptional anti-biofouling performance. Proc Natl Acad Sci U S A 2012;109:13182-7. [Crossref] [PubMed]
- Wong TS, Kang SH, Tang SK, et al. Bioinspired self-repairing slippery surfaces with pressure-stable omniphobicity. Nature 2011;477:443-7. [Crossref] [PubMed]
- Leslie DC, Waterhouse A, Berthet JB, et al. A bioinspired omniphobic surface coating on medical devices prevents thrombosis and biofouling. Nat Biotechnol. Nat Biotechnol 2014;32:1134-40. [Crossref] [PubMed]
- Yin J, Mei ML, Li Q, et al. Self-cleaning and antibiofouling enamel surface by slippery liquid-infused technique. Sci Rep 2016;6:25924. [Crossref] [PubMed]
- Harrison RG. On the stereotropism of embryonic cells. Science 1911;34:279-81. [Crossref] [PubMed]
- Kirschner CM, Brennan AB. Bio-inspired antifouling strategies. Annu Rev Mater Res 2012;42:211-29. [Crossref]
- Nishimoto S, Bhushan B. Bioinspired self-cleaning surfaces with superhydrophobicity, superoleophobicity, and superhydrophilicity. RSC Adv 2013;3:671-90. [Crossref]
- Carman ML, Estes TG, Feinberg AW, et al. Engineered antifouling microtopographies--correlating wettability with cell attachment. Biofouling 2006;22:11-21. [Crossref] [PubMed]
- Wall C, Moore J, Thachil J. Catheter-related thrombosis: a practical approach. J Intensive Care Soc 2016;17:160-7. [Crossref] [PubMed]
- Wicky S, Pinto EG, Oklu R. Catheter-directed thrombolysis of arterial thrombosis. Semin Thromb Hemost 2013;39:441-5. [Crossref] [PubMed]
- Ganguli S, Kalva S, Oklu R, et al. Efficacy of lower-extremity venous thrombolysis in the setting of congenital absence or atresia of the inferior vena cava. Cardiovasc Intervent Radiol 2012;35:1053-8. [Crossref] [PubMed]
- Oklu R, Wicky S. Catheter-directed thrombolysis of deep venous thrombosis. Semin Thromb Hemost 2013;39:446-51. [Crossref] [PubMed]