Risk factors for stent graft thrombosis after transjugular intrahepatic portosystemic shunt creation
Introduction
Transjugular intrahepatic portosystemic shunt (TIPS) creation is a minimally-invasive procedure developed by Josef Rösch to decompress the hepatic portal system to manage complications of portal hypertension (1,2). Initial shunts were created using bare metal stents with relatively high rates of shunt malfunction (3). The Viatorr stent graft (Gore Medical, Flagstaff, AZ, USA) was specifically designed for TIPS creation and has led to significantly improved shunt patency and function (4,5). Despite advancements made in technique and stent technology, thrombosis causing stenosis or occlusion is still a complication that can lead to TIPS malfunction (6,7). While improved patency of the Viatorr stent graft has been shown in prior studies, relatively less is known regarding risk factors for stent graft thrombosis after TIPS creation. In this study, we aim to assess factors predictive of TIPS thrombosis based on a large single institution experience using the Viatorr stent graft.
Methods
Patients
The study was approved by our local Institutional Review Board (IRB), and consent waiver was obtained. Subjects who underwent successful primary TIPS creation from June 2003 to January 2016 were considered for the study (n=328). Electronic medical records were retrospectively reviewed for demographics, medical history, pre- and post-TIPS laboratory values and radiologic examinations. Only patients in whom the Viatorr stent graft was used for TIPS creation were included in the analysis (n=249). Patients without follow-up data regarding TIPS patency were excluded (n=75). After applying inclusion and exclusion criteria, 174 subjects were analyzed.
Definitions
The primary outcome of the study was TIPS thrombosis with shunt occlusion on either angiography, Doppler ultrasound, or contrast-enhanced computed tomography (CT) scan. Primary patency was defined as uninterrupted patency, or censored at the time of the first intervention for thrombosis. Secondary patency included the time from intervention for thrombosis to a subsequent diagnosis of shunt occlusion. The last imaging study documenting a patent shunt was used to define patency intervals. The most recent available laboratory values prior to the TIPS procedure were used to record pre-TIPS lab data. Post-TIPS lab data represented laboratory values up to 30 days after TIPS creation. The model for end-stage liver disease (MELD) and MELD-Na scores were calculated (8). In addition, the albumin-bilirubin (ALBI) score and grade were also calculated (9). Patients with “obesity” listed in their problem list or for whom the calculated BMI was ≥30 were considered obese. For refractory ascites, clinical success was defined as decreased frequency of paracentesis/thoracentesis, decreased dosing of diuretic medications, better control of ascites on current diuretic medications, or subjective improvement per patient on follow-up office visits within 6 months of TIPS creation. For variceal bleeding, clinical success was defined as having no recurrence of gastrointestinal bleeding at any point after TIPS creation. Pre- and post-TIPS hepatic encephalopathy (HE) were classified as not present (grade 0) if the patient was not on medications to treat HE symptoms (lactulose, rifaximin or neomycin) and there was no record of HE symptoms on file, medically-controlled (grade 1) if the patient was on any of the above medications and no HE symptoms were reported, and uncontrolled (grade 2) if the patient was on any HE medications and there was a record of persistent HE symptoms on file. Any increase in grade of HE on post-TIPS evaluation relative to the pre-TIPS evaluation was considered worsened HE.
Statistical analysis
Numerical measures were reported as mean ± standard deviation (SD), and categorical variables were summarized as frequency (percentage). Comparisons of mean values and frequencies between two categories were done using the independent samples t-test, and the χ2 test, respectively. To evaluate risk factors for stent graft thrombosis, univariate competing risk Cox proportional hazard models were constructed for each exposure variable. Factors with statistically significant associations were then selected to construct a multivariate model. The univariate models were constructed both with and without considering the exposure variable as a time-varying covariate to assess the proportional hazard assumption. Factors with statistically significant time interactions were considered time-varying variables, and also entered in the multivariate model as a time-varying factor. Liver transplantation after TIPS creation was considered a competing risk factor in all models. No significant interaction between exposure variables was detected. Cochran-Armitage test for trend was used to evaluate trends of categorical measures. All statistical analysis was performed using Stata IC 14.2 for Mac, and P values <0.05 were considered statistically significant.
Results
Out of the total study cohort (n=328), 23 cases (7.0%) underwent liver transplantation, with a median time to transplant of 11.5 (range, 0.4–81.4) months. A total of 167 patients (50.9%) died during the follow-up period. Overall median survival after TIPS creation was 26.9 months [95% confidence interval (CI), 19.2–37.1]. Comparing patients included in the analysis (n=174) with those excluded due to lack of follow-up (n=75), those with no follow-up were older (P=0.022), had lower clinical success rates (P<0.001), worse liver function (P=0.006 for MELD-Na) and decreased survival (P<0.001) (Table 1).
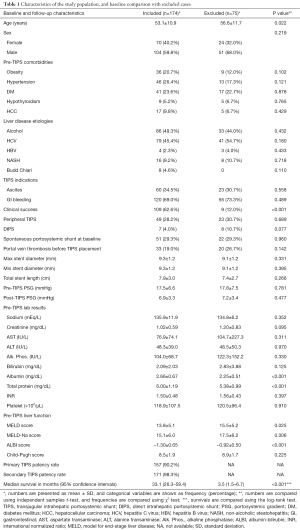
Full table
Mean follow-up time in the study cohort was 24.60±30.20 (range, 0.03–139.17) months. Primary TIPS thrombosis occurred in 17 cases (9.8%), with an annual calculated incidence of thrombosis of 38.7 per 1,000 person/year (95% CI, 19.3–77.3). After TIPS revision for thrombosis in 12 cases, repeat thrombosis occurred in three cases during a median of 21.2 (range, 0–33.3) months for a total 5-year secondary patency rate of 95.7%. One-, 2- and 5-year primary patency rates were 94.1%, 91.7%, and 78.2%, respectively (Figure 1).
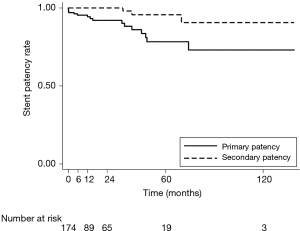
Seventeen cases in the analyzed cohort underwent liver transplantation within a median of 13.4 (range, 0.4–81.4) months, in whom no TIPS thrombosis was observed prior to transplantation. Seventy-seven patients (44.3%) in the analyzed cohort died during the follow-up period. Excluding transplanted patients, 1-, 2- and 5-year survival rates in those with TIPS thrombosis compared to those without TIPS thrombosis were 63.6%, 56.9% and 39.4%, and 70.1%, 59.9% and 37.7%, respectively (P=0.675). New or increased HE was observed in 80 patients (50.6%) after TIPS creation, with no significant difference between those with TIPS thrombosis and those without TIPS thrombosis (51.0% vs. 46.7%, respectively; P=0.747).
Twenty-seven cases underwent TIPS revision during a median of 7.3 months after initial TIPS creation. Twelve revisions were performed for TIPS thrombosis during a median of 14.6 months after initial TIPS creation. Pre-revision portosystemic pressure gradient (PSG) measurements were available in 20 cases, including in 6 cases with TIPS thrombosis. The median change in PSG value after initial TIPS creation and prior to revision was +3 (range, −5 to +26) mmHg (P=0.115) in all patients, and +6.5 (range, −1 to +26) mmHg (P=0.219) in patients with TIPS thrombosis.
In univariate analysis, patient age [sub-hazard ratio (sHR): 1.12; P=0.001], BMI <30 (sHR: 15.48; P=0.006), portal vein thrombus at the time of TIPS creation (sHR: 2.86; P=0.034) and higher post-TIPS portosystemic pressure gradients (sHR: 1.14; P=0.003) were significant predictors of TIPS thrombosis (Table 2). TIPS thrombosis was observed more frequently in cases presenting as clinical failure compared to cases where the procedure was clinically successful [11/65 (16.9%) vs. 6/109 (5.5%), P=0.014]. No significant association was found between observed baseline laboratory variables and the risk of TIPS thrombosis (Table 2). In multivariate analysis, advanced age, BMI <30 and increased post-TIPS portosystemic gradients remained significant independent predictors of TIPS thrombosis (Table 3). There was a significant increase in incidence of TIPS thrombosis at follow-up through increasing tertiles of post-TIPS portosystemic pressure gradients [4.3%, 6.4% and 17.7% for tertile 1 (<5 mmHg), 2 (5–8 mmHg) and 3 (>8 mmHg), respectively; P value for increasing trend =0.017] (Figure 2). In fact, each 1 mmHg increase in the post-TIPS portosystemic pressure gradient led to an adjusted 14% higher hazard of primary shunt thrombosis at follow-up.
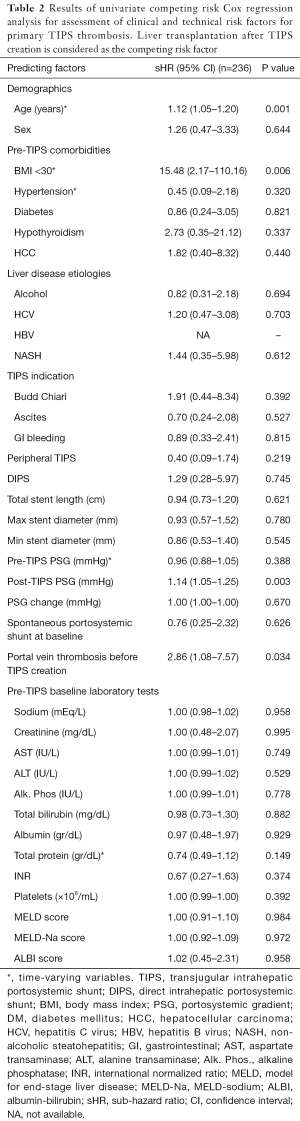
Full table
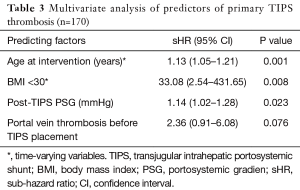
Full table

The 17 cases with TIPS thrombosis were individually analyzed for possible predisposing technical or anatomic factors. Factors assessed included lack of shunt extension to the inferior vena cava (IVC), filling defects/residual thrombus on completion angiography, portal vein diameter <10 mm, hypercoagulable states, and need for shunt extension using bare metal stents (Table 4).
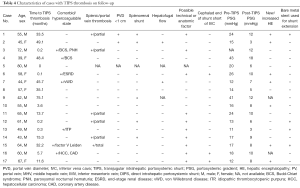
Full table
Discussion
The Viatorr stent graft has enabled significantly improved long-term TIPS patency compared to bare metal stents. Despite several reports documenting the improved patency of TIPS with stent grafts, few studies have specifically analyzed predictors of TIPS thrombosis with stent grafts. One study on long-term shunt patency published in 2004 found that nonalcoholic liver disease and increasing platelet counts independently predicted development of shunt insufficiency, but this study used bare-metal stents and not the Viatorr stent-graft (10). Many of the more recent studies on TIPS patency and malfunction focus not on predictors of thrombosis or stenosis, but on sensitivity of imaging modalities in detecting shunt stenosis and methods of recanalization once shunt dysfunction is discovered (11,12).
The respective 1- and 2-year patency rates of 94.1% and 91.7% shown in our cohort were consistent with other studies on stent-graft patency after TIPS creation (13,14). In this study, we further show that patient age, low BMI, and the post-TIPS portosystemic gradient were independent predictors of TIPS thrombosis with stent grafts.
Older age has been shown to carry a higher risk of venous thrombosis (15). Although the biological mechanisms behind contribution of age to thrombosis is poorly understood, increased coagulability of blood with age is suggested as a possible mechanism (16). A lower risk of stent thrombosis in patients with higher BMI has been observed and is controversial. An “obesity paradox” has been described after percutaneous coronary interventions, with decreased risk of mortality, major adverse cardiac events and in-stent thrombosis in obese people (17-19), although another study did not confirm such a finding (20). More aggressive screening and therapeutic interventions for cardiovascular and metabolic risk factors in obese people and higher prevalence of comorbid conditions in studied populations have been suggested as the potential contributors of this observation (19).
Perhaps the most significant independent predictor of TIPS thrombosis from our study was the incremental association between increasing portosystemic pressure gradients after TIPS creation and the risk of shunt thrombosis. As stated above, those with a portosystemic gradient <5, 5–8, and >8 mmHg had a risk of thrombosis of 4.3%, 6.4% and 17.7%, respectively. Additionally, according to our multivariate survival model, each 1 mmHg increase in the post-TIPS portosystemic pressure gradient led to a 14% higher adjusted hazard of shunt thrombosis on follow-up. Portosystemic pressure gradient reduction after TIPS creation has been well documented to significantly decrease risk of recurrent variceal bleeding (21,22). For patients with refractory ascites, a more aggressive decrease in the portosystemic gradient may increase risk of HE (23). The observation in our study of increased TIPS thrombosis with higher post-TIPS PSG very well may represent technical or anatomic factors suggesting inefficient shunting, such as residual portal vein thrombus, use of bare metal stents for shunt extension into portal vein, or a small portal vein. Indeed, we noted possible technical or anatomic factors resulting in inefficient shunting in at least 10 of 17 cases reviewed with TIPS thrombosis. Given the incremental association between the post-TIPS portosystemic gradient and TIPS thrombosis, our data suggest optimization of shunt flow at the time of TIPS creation would be important to help maintain shunt patency.
The risk of portal vein thrombosis at the time of TIPS creation for subsequent TIPS thrombosis has been discussed in a recent review (24). Although TIPS creation has been shown to decrease progression of portal vein thrombosis, and also to be superior to endoscopic band ligation and non-selective beta blockers when portal vein thrombosis presents with gastrointestinal (GI) bleeding (24), it is also associated with a higher risk of shunt thrombosis. In our study, pre-TIPS portal vein thrombosis (PVT) was a predictor of shunt thrombosis on univariate analysis. While PVT lost significance in the multivariate-adjusted model (P=0.076), this may be due to loss of statistical power with multivariate-adjusting (calculated power for pre-TIPS PVT in multivariate model: 0.25). Alternatively, it is possible that a part of the predictability of pre-TIPS PVT is explained by other factors.
Although we evaluated a relatively large cohort, our study has limitations. The retrospective nature of the study introduces selection biases. Patients included for analysis had better liver function at baseline compared to excluded patients who were lost to follow-up, although baseline liver function was not a predictor of TIPS thrombosis in our study. TIPS thrombosis events and survival of those lost to follow-up were also unknown. Furthermore, a potential lag time between TIPS thrombosis and detection may overestimate thrombosis-free intervals. In addition, post-TIPS PSG values have been reported to change with time (25). Data regarding time-dependent post-TIPS PSG measurements were not available for our patients, and risk stratification based on PSG levels was based on measurements immediately after TIPS creation.
In conclusion, this study demonstrates that TIPS creation with stent grafts has a relatively low rate of shunt thrombosis. Factors predicting shunt thrombosis include older age, low BMI, and a higher post-TIPS portosystemic gradient suggesting a higher resistance to flow through the shunt that may be partially technical in nature. Understanding factors leading to TIPS thrombosis may improve procedural metrics and follow-up surveillance strategies.
Acknowledgements
None.
Footnote
Conflicts of Interest: K Farsad provides consulting services to Cook Medical. JA Kaufman provides research support and consulting services to Cook Medical. The other authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by Oregon Health and Science University Institutional Review Board (No. 17696).
References
- Rosch J, Hanafee WN, Snow H. Transjugular portal venography and radiologic portacaval shunt: an experimental study. Radiology 1969;92:1112-4. [Crossref] [PubMed]
- Saad WE. The history and future of transjugular intrahepatic portosystemic shunt: food for thought. Semin Intervent Radiol 2014;31:258-61. [Crossref] [PubMed]
- Cejna M. Should stent-grafts replace bare stents for primary transjugular intrahepatic portosystemic shunts? Semin Intervent Radiol 2005;22:287-99. [Crossref] [PubMed]
- Clark TW. Management of shunt dysfunction in the era of TIPS endografts. Tech Vasc Interv Radiol 2008;11:212-6. [Crossref] [PubMed]
- Qi X, Tian Y, Zhang W, et al. Covered versus bare stents for transjugular intrahepatic portosystemic shunt: an updated meta-analysis of randomized controlled trials. Therap Adv Gastroenterol 2017;10:32-41. [Crossref] [PubMed]
- Cura M, Cura A, Suri R, et al. Causes of TIPS dysfunction. AJR Am J Roentgenol 2008;191:1751-7. [Crossref] [PubMed]
- Ripamonti R, Ferral H, Alonzo M, et al. Transjugular intrahepatic portosystemic shunt-related complications and practical solutions. Semin Intervent Radiol 2006;23:165-76. [Crossref] [PubMed]
- Organ Procurement and Transplantation Network. Policies. 2017:106. Available online: https://optn.transplant.hrsa.gov/media/1200/optn_policies.pdf
- Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol 2015;33:550-8. [Crossref] [PubMed]
- ter Borg PC, Hollemans M, Van Buuren HR, et al. Transjugular intrahepatic portosystemic shunts: long-term patency and clinical results in a patient cohort observed for 3-9 years. Radiology 2004;231:537-45. [Crossref] [PubMed]
- Miraglia R, Maruzzelli L, Luca A. Recanalization of occlusive transjugular intrahepatic portosystemic shunts inaccessible to the standard transvenous approach. Diagn Interv Radiol 2013;19:61-5. [PubMed]
- Owen JM, Gaba RC. Transjugular Intrahepatic Portosystemic Shunt Dysfunction: Concordance of Clinical Findings, Doppler Ultrasound Examination, and Shunt Venography. J Clin Imaging Sci 2016;6:29. [Crossref] [PubMed]
- Rossle M, Siegerstetter V, Euringer W, et al. The use of a polytetrafluoroethylene-covered stent graft for transjugular intrahepatic portosystemic shunt (TIPS): Long-term follow-up of 100 patients. Acta Radiol 2006;47:660-6. [Crossref] [PubMed]
- Rossi P, Salvatori FM, Fanelli F, et al. Polytetrafluoroethylene-covered nitinol stent-graft for transjugular intrahepatic portosystemic shunt creation: 3-year experience. Radiology 2004;231:820-30. [Crossref] [PubMed]
- Van Gent JM, Calvo RY, Zander AL, et al. Risk factors for deep vein thrombosis and pulmonary embolism after traumatic injury: A competing risks analysis. J Trauma Acute Care Surg 2017;83:1154-60. [Crossref] [PubMed]
- Crous-Bou M, Harrington LB, Kabrhel C. Environmental and Genetic Risk Factors Associated with Venous Thromboembolism. Semin Thromb Hemost 2016;42:808-20. [Crossref] [PubMed]
- Lancefield T, Clark DJ, Andrianopoulos N, et al. Is there an obesity paradox after percutaneous coronary intervention in the contemporary era? An analysis from a multicenter Australian registry. JACC Cardiovasc Interv 2010;3:660-8. [Crossref] [PubMed]
- Park DW, Kim YH, Yun SC, et al. Association of body mass index with major cardiovascular events and with mortality after percutaneous coronary intervention. Circ Cardiovasc Interv 2013;6:146-53. [Crossref] [PubMed]
- Schmiegelow M, Torp-Pedersen C, Gislason GH, et al. Relation of body mass index to risk of stent thrombosis after percutaneous coronary intervention. Am J Cardiol 2012;110:1592-7. [Crossref] [PubMed]
- Akin I, Tolg R, Hochadel M, et al. No evidence of "obesity paradox" after treatment with drug-eluting stents in a routine clinical practice: results from the prospective multicenter German DES.DE (German Drug-Eluting Stent) Registry. JACC Cardiovasc Interv 2012;5:162-9. [Crossref] [PubMed]
- D'Amico G, Garcia-Pagan JC, Luca A, et al. Hepatic vein pressure gradient reduction and prevention of variceal bleeding in cirrhosis: a systematic review. Gastroenterology 2006;131:1611-24. [Crossref] [PubMed]
- Suk KT. Hepatic venous pressure gradient: clinical use in chronic liver disease. Clin Mol Hepatol 2014;20:6-14. [Crossref] [PubMed]
- Madoff DC, Wallace MJ, Ahrar K, et al. TIPS-related hepatic encephalopathy: management options with novel endovascular techniques. Radiographics 2004;24:21-36; discussion 36-7. [Crossref] [PubMed]
- Trebicka J. Emergency TIPS in a Child-Pugh B patient: When does the window of opportunity open and close? J Hepatol 2017;66:442-50. [Crossref] [PubMed]
- Silva-Junior G, Turon F, Baiges A, et al. Timing Affects Measurement of Portal Pressure Gradient After Placement of Transjugular Intrahepatic Portosystemic Shunts in Patients With Portal Hypertension. Gastroenterology 2017;152:1358-65. [Crossref] [PubMed]


