Deep venous thrombosis in pregnancy: incidence, pathogenesis and endovascular management
Introduction
Pregnancy and the puerperium are well-established risk factors for venous thromboembolism (VTE), a disease that includes pulmonary embolism (PE) and deep venous thrombosis (DVT). Approximately 30% of apparently isolated episodes of PE are associated with silent DVT and in patients presenting with symptoms of DVT, the incidence of silent PE ranges from 40–50% (1). VTE is both more common and more complex to diagnose in those patients who are pregnant than in those who are not. PE is the leading cause of maternal death in the developed world (1-5). Delayed diagnosis, delayed or inadequate treatment and inadequate thromboprophylaxis account for many of these deaths. In this review, we will focus on DVT during pregnancy, summarizing its risk factors, pathogenesis, complications, diagnostic criteria and tools, prophylaxis, medical and endovascular management.
Epidemiology
Women are up to 5 times more likely to develop DVT during pregnancy than when not pregnant (1-6). The hypercoagulable state of pregnancy likely evolved to protect women from excessive bleeding during miscarriage and childbirth. In fact, in developing nations, hemorrhage is the leading cause of maternal death. However, in the United States and other developed nations, the leading cause of maternal death is embolic disease (1-7). The frequency of thrombosis is similar in all three trimesters, and is also increased in the first 6 weeks of the post-partum (2,4,6). In addition to the mortality and immediate morbidity, there is also long term morbidity associated with the postthrombotic syndrome (PTS). The majority of women, who suffer from DVT during pregnancy, develop sequelae that range from edema and skin changes to recurrent thrombosis and ulceration (6).
Certain conditions have been associated with the highest risk of pregnancy related DVT. These include inherited or acquired thrombophilias, a previous history of thrombosis, antiphospholipid syndrome, lupus, heart disease and sickle cell disease (4). When these are present, the need for prophylactic anticoagulation should be addressed (4). Other independent risk factors are age 35 and older, null parity, multiple gestations, obesity and immobility, these increase the risk 1.5–2 fold (4,7). Two studies, James et al. and Jacobsen et al., also found an association between gestational diabetes and thrombosis (4,7). Although data is limited, assisted reproduction is also considered a risk factor (7). In the puerperium, post-partum infection increases the risk of thrombosis by 4-fold and cesarean delivery increases the risk 2-fold (4). Jacobsen et al. and Lindqvist et al., found a higher prevalence of venous thrombosis in the postnatal period among patients with preeclampsia (7-9).
Pathogenesis of DVT during pregnancy
Pregnancy is a prothrombotic state; it has all components of Virchow’s triad: venous stasis, endothelial damage and hypercoagulability.
Venous stasis results from a hormonally induced decrease in venous tone and obstruction of venous flow by the enlarging uterus. A reduction of venous flow velocity of approximately 50% occurs in the legs by weeks 25–29 of gestation. This lasts until approximately 6 weeks postpartum, at which time normal venous velocities return (10,11). Among pregnant and postpartum women, the left lower extremity is the most common site of DVT (82%). Anatomic reasons (compression of the left common iliac vein by the right common iliac artery which is accentuated by the enlarging uterus) have been postulated (6).
Endothelial damage in pelvic veins can occur at the time of delivery or from venous hypertension (2). Pelvic vein thrombosis, which is uncommon outside of pregnancy, accounts for 6–11% of DVT during pregnancy and the puerperium (6).
During normal pregnancy, a hypercoagulable state is initiated. This is the most important risk factor contributing to thrombosis during pregnancy. Fibrin generation is increased, fibrinolytic activity is decreased, levels of coagulation factors II, VII, VIII and X are all increased (2,12). There is a progressive fall in protein S levels and acquired resistance to activated protein C (2,12). All of these changes reflect the physiological preparation for the hemostatic challenge of delivery. This hemostatic activation is demonstrated by increased markers of hemostatic activation, such as prothrombin fragment F1+2 and D-dimer (1,12).
Risk assessment and prophylaxis for DVT/VTE
Despite the increased risk for thrombosis during pregnancy and the postpartum period, most women do not require anticoagulation. In most cases the risks of anticoagulation outweigh its benefits. The risk of maternal bleeding complications with heparin and low molecular weight heparin (LMWH) is reported to be as high as 2% (13). Thromboprophylaxis during pregnancy is problematic because it involves long term parenteral LMWH or unfractionated heparin (UFH). Both are expensive, inconvenient and painful to administer and are associated with risks of bleeding, osteoporosis and heparin induced thrombocytopenia (HIT); although these complications, particularly HIT are very uncommon with LMWH (14,15). Given its benefits compared to UFH, LMWH are the preferred agent for prophylaxis and treatment of DVT during pregnancy (14,15). A disadvantage of LMWH over UFH is its longer half-life, which may be a problem at the time of delivery. UFH is preferred in patients with renal insufficiency, as LMWH is primarily excreted by the kidneys and may accumulate in those with severe renal dysfunction (15). Warfarin crosses the placenta and is teratogenic, it is a US FDA category D drug. Warfarin is associated with a 14–56% reported risk of miscarriage during the first trimester and carries up to 30% risk for congenital anomalies (16-19) when taken during the critical period of organogenesis (4th–8th week after conception). Placental transfer of warfarin later in pregnancy can result in fetal bleeding (20) or still birth (16-19). Long term sequelae include a 14% reported risk of adverse neurologic outcome and a 4% reported risk of low intelligence quotient (IQ) (21). Data for the use of fondaparinux, a selective factor Xa inhibitor, during pregnancy is limited. Although studies using models did not show passage through the placental barrier (20), Dempfle et al. found it crossed through the placenta in five women who took it for 1–101 days because of heparin allergy (22). Anti-factor Xa levels in the umbilical cord plasma of the newborns was found to be one tenth the concentration of maternal plasma. The clinical significance of this is unknown, but no adverse effects were noted on the newborns (22). At present time there is insufficient data to support the routine use of fondaparinux for prophylaxis of VTE during pregnancy. It is reserved for those cases of severe cutaneous allergy to heparin or HIT. Small case series and case reports have shown it to be safe (22-24) but it is important to recognize that most of these involve exposure during second and third trimesters. Other factor Xa inhibitors (e.g., dabigatran, rivaroxaban, apixaban, edoxaban) are likely to cross the placenta and their human reproductive risks are unknown (15).
Determining which patients should receive thromboprophylaxis has always been a challenge. Its rational administration depends on identifying those women who have an increased risk of thrombosis and accurately quantifying this risk. The threshold for recommending post-partum prophylaxis is lower than for antepartum prophylaxis due to the shorter length of required treatment (up to 6 weeks), the higher average risk of DVT in the postpartum and the safety of warfarin during this time, even if the mother is breastfeeding (not excreted in breast milk) (14). The relatively equal distribution of DVT throughout all trimesters suggests that when antepartum prophylaxis is warranted, it should be initiated early in the first trimester (2,4,6,8).
During pregnancy, a history of hereditary or acquired thrombophilia or a history of previous DVT has been determined to be the most important risk factors. The risk becomes even higher if the maternal age is >35 years or if there are other additional independent risk factors such as obesity, immobility, null parity, multiple gestations (4-7) or smoking (25). In the post-partum period, hypertension (probably due to preeclampsia), immobility and recent surgery (C-section) appear to be the most important independent risk factors (4-7).
Available data suggest that women with a history of previous venous thrombosis have an increased risk of recurrence during pregnancy. Although it is estimated that the risk is within 2–10%, the absolute rates of recurrence are unknown. There have been no large clinical trials assessing the role of prophylaxis in pregnant women with prior DVT. There is an ongoing multinational randomized controlled prospective trial, the Highlow study, which is recruiting pregnant women with a previous history of venous thrombosis and an indication for thromboprophylaxis. Its aim is to determine the true risk of recurrent VTE, the optimal dose of LMWH for prophylaxis and its safety. The Netherlands, France, Ireland, Belgium and Norway are participating; they will be enrolling patients through 2019, with an expected sample size of approximately 1,000 women. The results are expected by 2020 (26).
Congenital thrombophilias are present in at least 15% of the general population and approximately 50% of gestational venous thromboses are associated with heritable thrombophilias (27). Multiple studies have looked at the relationship between hereditary thrombophilias and VTE. However, limitations in their methods have made it difficult to make accurate assessments of their risk. The highest risk has been found with homozygosity for factor V Leiden and homozygosity of the prothrombin G20210A variant (14). The more common inherited thrombophilias such as heterozygous factor V Leiden and heterozygous prothrombin G20210A variant were associated with lower risk (14). Deficiencies of endogenous anticoagulants such as antithrombin, protein C and protein S were associated with moderate risk (14). Given the background incidence of VTE during pregnancy of approximately 1/1,000 deliveries, it is clear that the absolute risk of VTE in women without a prior event remains modest for those women with the most common inherited thrombophilias. Acquired thrombophilias have been less well studied, but persistent APLAs (lupus anticoagulants or anticardiolipin antibodies) are likely associated with an increased risk of pregnancy related VTE (14). The American College of Obstetricians and Gynecologists (ACOG) recommends testing for antiphospholipid antibodies and inherited thrombophilias if there is a prior history of VTE (15).
The American College of Chest Physicians (ACCP) and ACOG recommend prophylaxis with LMWH for all pregnant patients with a previous history of venous thrombosis and documented thrombophilia, as well as for those with a history of multiple (>2) episodes of DVT (14,15). There is no consensus on what the ideal dose should be, the recommendation is for prophylactic, intermediate or adjusted dose (Table 1) (14,15). For patients with history of a single idiopathic DVT, but no thrombophilia or those with a transient risk factor that has resolved, the recommendation from both agencies is for close clinical surveillance during pregnancy and prophylaxis postpartum (14,15). For pregnant patients with a heritable or acquired thrombophilia but no prior history of venous thrombosis, the recommendation of the ACCP is not to routinely use prophylaxis antepartum, but to perform an individual risk assessment; however, postpartum anticoagulation is recommended (14). For those with antithrombin deficiency, the ACCP recommends antepartum and postpartum prophylaxis (14). The ACOG recommends prophylaxis for all women with documented thrombophilia during the antepartum and postpartum (15). LMWH should be discontinued the moment that the women are in established labor, or think they may be in labor and 24 hours prior to planned C-section (15). Switching from LMWH to UFH may be considered during the last month of pregnancy, as it has a shorter half-life (15).

Full table
Limited data is available on assisted reproduction as a risk factor. The cases reported are related to severe hyperstimulation syndrome, which happens in 0.5–6.5% of all hyperstimulations (7). This syndrome is associated with hemoconcentration and has very high levels of estradiol. Ascites and pleural effusions are common. These clinical presentations, combined with immobility and pregnancy induced hypercoagulability make these women particularly predisposed to venous thrombosis (7). These patients should be under close clinical surveillance for VTE.
More recently, several groups have suggested the use of scoring systems to aid with risk assessment. One of these groups, Dargaud et al., has postulated the use of the Lyon VTE risk score as a means of providing a rational decision process to implement safe and effective antepartum thromboprophylaxis in pregnant women at high risk of DVT (28). The Lyon score, assesses the risk of VTE during pregnancy according to three main criteria: history of previous VTE, known thrombophilia markers and contemporary risk factors dependent on the current pregnancy. In each category, a point value is assigned to each item according with the degree of risk estimated in the literature (Table 2). The use of tools, such as this score system, offers the possibility of personalized medicine which could be more effective and possibly more cost-efficient than generalized guidelines.
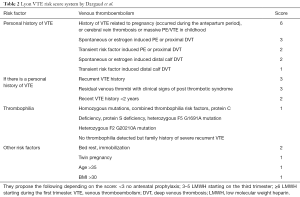
Full table
Diagnosis of DVT in the pregnant woman
The most common presenting symptoms of DVT are swelling in 88% of pregnant women and 79% of postpartum women and extremity discomfort in 79% of pregnant women and 95% of postpartum women (6). Additional symptoms include difficulty walking, in 21% of pregnant and 32% of postpartum women. Erythema was reported in 26% of both groups (6). The incidence of isolated DVT in the iliac veins is higher during pregnancy (6). Isolated iliac vein thrombosis may present with abdominal pain, back pain and/or swelling of the entire leg (1,6). These symptoms may be masked by the swelling and discomfort that accompany normal pregnancies, making the diagnosis of DVT during pregnancy more challenging.
Stasis and swelling of the legs can occur due to mechanical compression of the lymphatic vessels and veins which happens with the enlarging uterus. Therefore, edema is a less reliable sign of DVT in pregnant women. Pelvic and back pain may be misinterpreted as normal/expected discomfort or due to musculoskeletal issues, when these symptoms may be emanating from a proximal (ilio-femoral) DVT. These non-specific symptoms are often ignored until the thrombus extends distally into the femoral veins causing pain and swelling of the whole affected leg.
The D-dimer essay is positive even during uncomplicated pregnancies. This indicates increased thrombin activity and increased fibrinolysis following fibrin formation throughout pregnancy, the result of the pregnancy related hypercoagulable state (12). Thus, this test is non-specific and not reliable for the diagnosis of DVT during pregnancy.
All pregnant women with signs and symptoms suggestive of DVT should have objective testing performed expeditiously, as sudden death is not uncommon among pregnant patients with features compatible with VTE (1). Unless contraindicated, anticoagulation treatment is recommended when the clinical suspicion is high, until the diagnosis of DVT is ruled out (1). To confirm the diagnosis in this subset of patients, the use of non-invasive and non-ionizing imaging is preferable. Both, for the health of the fetus as well as the mother as ovaries are radiation sensitive. Currently, there are two such non-invasive methods, ultrasound and magnetic resonance imaging (MRI). Contrast enhanced computed tomography (CT) may be used to diagnose pelvic DVT when MRI is not available, but is not ideal, and not routinely recommended, as it is associated with fetal and maternal radiation exposure.
Routine ultrasonography for the diagnosis of DVT includes direct examination of the thrombus with gray scale imaging, compression technique and color flow Doppler. DVT is diagnosed when the veins fail to compress completely. Sometimes grey scale imaging can demonstrate the thrombus, but this may be limited by a large body habitus or by artefactual intraluminal echoes, thus this is not the primary focus for diagnosis. In obese or very edematous patients, grey scale imaging is limited and the use of color Doppler is helpful to adequately localize the vessels. Compression of the calf or plantar flexion can accentuate the veins, and further assist with adequate imaging.
Compression ultrasonography has a sensitivity of 97% and a specificity of 94% for the diagnosis of symptomatic femoro-popliteal DVT in the general population (29). Ultrasonography is without risk, inexpensive and readily available. It is the test of choice for pregnant patients with suspected DVT. However, it is less accurate for pelvic vein thrombosis, primarily because of their deep location. Furthermore, the size of the pregnant uterus in the latter half of pregnancy makes imaging of these veins even more difficult. In addition, the compression technique is obviously difficult to perform in the pelvis and much more so in the pregnant pelvis.
A study by Torkzad et al. found, in women between 23 and 37 weeks of gestation, that ultrasound revealed 42% of pelvic and abdominal DVT whereas MRI 98.5% (30). Ultrasound should continue to be the primary method of diagnosis of DVT, but if the ultrasound is negative and clinical suspicion is still present, one should not hesitate to order an MRI. MRI is also useful in cases where determining the true extent of a DVT into the pelvis/abdomen will influence management. Imaging protocols without gadolinium are preferred. Pulse sequences such as 2D time of flight with arterial flow suppression and T1 weighted gradient echo with fat saturation are used (30). On the T1 weighted images, high signal intensity within a vein represents methemoglobin in the thrombus, indicating an acute thrombus. Enlargement of the vein and perivascular inflammation are also signs of acuity (30).
Clinical outcomes and management
Once diagnosed, DVT must be treated not only to prevent PE, but also to prevent PTS. Moderate to severe PTS is a debilitating chronic outcome of proximal DVT. It has been suggested that PTS is due to incomplete recanalization and/or permanent damage to the venous valves resulting in valvular reflux (31). Its pathophysiology is not well understood, but it manifests clinically as leg heaviness, fatigue, aching and edema (32). Severe PTS may result in venous ulcers (32). PTS may occur in as many as 60% of patients after acute DVT involving the iliac and/or femoral vein segments (33). In a study by Chang et al., looking at long term outcomes in pregnancy related DVT, they found that 42% of women with lower extremity DVT developed PTS, which was severe in 7% (34).
Medical management is the first line of therapy for DVT. As with prophylaxis, LMWH is the drug of choice for therapy, at full therapeutic or adjusted dose (Table 1) (14,15). Here, we will focus on inferior vena cava (IVC) filter placement and pharmacomechanical catheter directed thrombolysis (PCDT).
Radiation exposure
The pregnant patient presents a complex management challenge. Both IVC filter placement and catheter directed thrombolysis (CDT) require radiation exposure, which can cause multiple effects in the developing fetus, depending on the dose of radiation and the stage of fetal development. The International Commission on Radiological Protection has recommended that “no deterministic effects of practical significance” would be expected in the developing human at doses lower than 100 mGy (35).
Radiation exposure greater than 50–100 mGy during the first 0–2 weeks of gestation or before implantation may cause demise of the embryo (36). The risk for teratogenesis occurs during the period of organogenesis between 2 and 20 weeks of gestation (36). The fetus is especially sensitive to radiation between 8 and 15 weeks, during which there is rapid neuronal development and migration (36). Radiation exposure greater than 100 mGy during this period may lead to mental retardation, microcephaly, and intrauterine growth restriction (36). At doses greater than 100 mGy, data from animal studies, atomic bomb survivors, and patients exposed to radiation for medical reasons estimate a decrease of approximately 0.025 intelligent quotient points per 1 mGy (36). Carcinogenesis arises from stochastic or nondeterministic effects. These effects result in random DNA mutations, which can occur at any radiation dose. The relative risks for childhood cancer are greater during early gestation. Relative risk for childhood cancer from diagnostic-level radiation has been estimated to be approximately 3.19 in the first trimester, 1.29 in the second trimester, and 1.30 in the third trimester (36). With a fetal dose of 50 mGy, there is an estimated twofold increase in relative risk for fatal childhood cancer compared with risk when there has been no ionizing radiation exposure (36).
If possible, during fluoroscopy guided procedures, the uterus should be positioned outside the field of view. This, so that the conceptus is exposed to scattered radiation only, resulting in minimal dose (37). Depending on the position of the conceptus within the mother, it may be possible to use a lead shield to protect the uterus from external scattered radiation (scatter emanating from the exposed tissue or imaging equipment) (37). If fetal radiation is unavoidable, exposure should be reduced by minimizing fluoroscopic time, decreasing the number of images acquired, using magnification only when necessary, employing the lowest possible frame rate, optimizing collimation, and using image hold instead of additional exposures (36). The patient should be placed as close to the receptor as possible, with the distance maximized between the source of the X-ray and the receptor (36).
A recent single center retrospective analysis of patient radiation dose during IVC filter placement, found among 230 consecutive cases reviewed a mean radiation dose of 67.55 mGy (38).
The fetal radiation dose resulting from PCDT in the first trimester can be calculated in the range of 175–245 mGy (39), which is associated with a childhood cancer risk of 1.3–2%, 6–10 times that associated with environmental/background radiation exposure (39).
A qualified medical physicist should be involved in these cases for accurate estimation of the fetal dose, by using the equipment parameters and the patient’s geometry for Monte Carlo calculations (36).
IVC filters
There is limited experience with IVC filter placement during pregnancy. Serious complications such as filter fracture, migration, failure of retrieval of temporary devices and IVC perforations have been reported (40-44). There are no specific ACCP or ACOG recommendations for IVC filter placement during pregnancy. The Royal Society of Obstetricians and Gynaecologists VTE guidelines (45) recommend to “considering the use of a temporary IVC filter in the peripartum period for patients with iliac vein thrombosis or in patients with proven DVT and who have recurrent PE despite adequate anticoagulation”.
The Society of Interventional Radiology (SIR) recognizes absolute and relative indications for IVC filter placement, there are no specific separate indications for pregnant women. The Journal of Vascular and Interventional Radiology (JVIR) recently published a systematic review of all publications of IVC filters during pregnancy, from 1981 through 2014 (46). The review, by Harris et al., included a total of 124 pregnancies, all from case reports and small case series. They found that, as suggested by the SIR guidelines, most IVC filters during pregnancy were placed for the absolute indications of failure of medical therapy for VTE despite anticoagulation and complications of anticoagulation including HIT, heparin allergy, significant bleeding or contraindication to anticoagulation due to recent neurosurgery (46). Relative indications included unstable, floating or very large DVT’s near the time of delivery and thrombus extending into the IVC (46).
The review also found that filters were placed with success in all stages of pregnancy including latent labor and that filters left in situ went on to have successful pregnancies (46). The gravid uterus was not found to prevent accurate IVC filter placement via a jugular or femoral route (46). No fatal PE occurred after filter placement in the publications reviewed (46). There was no recorded fetal morbidity or mortality (46). One maternal death was recorded in 1990. This occurred after an air embolism during the first trimester, while inserting a Greenfield filter, into the jugular vein, through a cutdown approach (47). Since percutaneous techniques with smaller sheaths and lower profile filters have become the norm, no other maternal fatalities have been reported. Reported complications of IVC filter placement included migration, fracture, inability to retrieve and occlusion of the filter with thrombus that could not be lysed. Of the 124 cases reviewed by Harris et al., 8.8% had complications directly related to the filter (46). In the general population, a 0.3% incidence of procedural complications and a 2.7% rate of filter associated caval occlusion were reported by Athanasoulis and associates, on their 26-year retrospective study of 1,765 IVC filter implantations (48).
It has often been thought that suprarenal filter placement is preferred in pregnancy and in young women who may become pregnant. Below the level of the renal veins, the IVC may be compressed by the gravid uterus. This, theoretically, could displace the filter, particularly when contracting, leading to filter migration, fracture or damage to the IVC wall (49). Harris et al. found that filters were safely placed in both the infrarenal and suprarenal positions. Of the cases reviewed, 55.6% had suprarenal filter placement and 25.8% infrarenal (46). The remainder 18.5% did not report the specific position where the filters were placed (46).
There is great variance in the commercially available IVC filters as in the filters that have been inserted into pregnant women in the published reports. There is no ideal filter to date, but the technology is rapidly progressing. The use of retrievable filters is particularly attractive because of the transient nature of increased thromboembolism that pregnancy causes and because these are young patients. This is especially important as a randomized control trial data (50) suggests that at 8 years, IVC filters placed in males and females with proximal DVT are associated with increased rate of DVT and no benefit in survival.
IVC filters can be used during pregnancy to prevent PE. However, there is currently no evidence to support their routine use in pregnant women with DVT. Until further studies are carried out, their use should be considered for the same absolute indications as in the non-pregnant population or in individuals in whom there are concerns surrounding delivery. Although there is limited long-term follow-up information, it does appear that IVC filters can be used safely, when appropriate, during pregnancy.
CDT
Concerns about thrombolytic therapy during pregnancy center on its maternal effects (major hemorrhage) and its effects on the placenta (i.e., premature labor, placental abruption, fetal demise), as transplacental passage of tissue plasminogen activator and streptokinase is minimal (51). Since there are no large studies on thrombolysis during pregnancy, there is agreement between most available guidelines that the use of thrombolytic therapy in pregnancy is best reserved for limb or life threatening maternal thromboembolism (15). PCDT is a minimally invasive technique for the treatment of acute iliofemoral DVT. As we learn more about the effectiveness and safety of this therapy, its indications could be expanded in the pregnant and postpartum population, to prevent the sequelae of moderate to severe PTS.
Anticoagulation, as monotherapy is known to lead to high rates of PTS in the general population, ranging between 25% and 46% at 2 years, and rising up to 90% in 5 years (52). It has been shown that if DVT is iliofemoral, the incidence of moderate to severe PTS significantly increases, with 44% of patients eventually developing venous claudication and 15% ultimately developing venous ulcers at 5 years (52). Results of the Acute Venous Thrombosis: Thrombus Removal with Adjunctive Catheter Directed Thrombolysis (ATTRACT) trial were presented during the March 2017 SIR Meeting. The results showed that 25.2% of patients with iliofemoral DVT treated with anticoagulation alone, went on to develop moderate to severe PTS, vs. 18.4% of patients who were treated with PCDT in combination with anticoagulation. However, it did show a 1.7% risk of bleeding for those patients treated with combination therapy vs. 0.3% for those treated with anticoagulation alone. Prior studies have shown a PCDT risk of major bleeding of 2.8% (53,54). In comparison, one international registry examined bleeding related events in 2,454 patients treated with systemic thrombolysis for PE. This registry found an increase in major bleeding (21.7% vs. 8.8%) and intracranial bleeding (3.0% vs. 0.3%) between patients who received systemic thrombolysis, compared to those who received anticoagulation alone (38). Given its high risk of bleeding complications, systemic thrombolysis is not recommended for the treatment of DVT.
Percutaneous catheter and stent innovations have led to targeted treatment improvements which have reduced the complications encountered in systemic thrombolysis. CDT attempts to minimize bleeding by placing a multi-side-hole catheter within the thrombus so that thrombolytic agents can be delivered within the thrombus where they are bound. This increases drug exposure time to the actual thrombus and limits drug exposure to that same thrombus, thus allowing for the use of smaller focused doses of thrombolytic agents. The use of adjunct mechanical methods of clot retrieval, also decreases the need for high dose or prolonged thrombolytic infusions, this is termed PCDT. In particular, the use of PCDT with tissue plasminogen activator (tPA) has shown good results with reduction of complications such as major bleeding (54). Studies have also shown that single therapy sessions of PCDT can resolve DVT in a single session without the need of a thrombolytic infusion (52,54). Commonly used adjunctive endovascular techniques include aspiration thrombectomy (use of a syringe or suction device to aspirate thrombus from the vein via a catheter, device, or sheath), balloon maceration (use of an angioplasty balloon to macerate or fragment thrombus), balloon angioplasty (inflation of a catheter-mounted balloon with the specific intent of enlarging the venous lumen), and stent placement (deployment of a metallic endoprosthesis to enlarge and maintain the venous lumen). A detailed discussion on techniques for PCDT is not the purpose of this review.
Despite the increased incidence of pregnancy related DVT, there is a paucity of available data for the use of PDCT in the pregnant and post-partum patient population. Given that these patients are generally young and healthy, they may receive maximum benefits form PCDT. A 2015 study by Bloom et al., evaluated 11 consecutive patients undergoing PCDT for pregnancy related DVT (39). Greater than 90% clot lysis was achieved in 82% of patients, 73% of patients required metal stents for residual stenosis (three patients had May-Thurner compression). There were no major complications. Complications were one self-limiting popliteal hematoma and rethrombosis in two patients that required repeat PCDT. All patients were available for follow-up at a median of 20 months and no one had developed PTS. Three of the patients studied decided to take their pregnancy to term and had successful pregnancies with iliac vein stents, on LMWH, without further VTE (39). In another 2014 study, Herrera et al., reported on 11 pregnant patients with iliofemoral DVT, who underwent catheter directed or pharmacomechanical thrombolysis (55). They all had complete or near complete resolution of thrombus with significant improvement or resolution of their clinical symptoms. Stenting was done in 8 of the 11 patients for residual stenosis. They all delivered healthy term infants. At a mean follow-up of 1.3 years, US showed normal valve function and patent veins in 10 of the 11 patients (55). The results of these two small studies are encouraging. Validation of these results is needed from larger prospective trials with longer follow-up.
It has been recommended to avoid PCDT during fetal organogenesis (first trimester) due to the risks associated with radiation exposure. If it deemed necessary, an open discussion about the option of termination of pregnancy should be had (39). During the second and third trimesters, with use of appropriate “as low as reasonably achievable” techniques to minimize radiation exposure, PCDT should be performed for threatened life or limb and can be considered for failure of conservative management. With the current available data, its routine use for prevention of PTS is not recommended. If extensive iliofemoral DVT is diagnosed late in the third trimester, placement of an IVC filter may be considered along with anticoagulation, followed by PDCT as soon as deemed safe after delivery. PDCT could be considered in the postpartum period for patients with iliofemoral DVT, as these are young and usually otherwise healthy women. A recent, prospective, large, randomized study of the general population (ATTRACT) showed that patients with iliofemoral DVT were 25% less likely to develop moderate to severe PTS when treated with PCDT in combination with anticoagulation, but it also showed a 1.7% risk of bleeding for those patients treated with combination therapy vs. 0.3% for those treated with anticoagulation alone.
Conclusions
Women are at increased risk of VTE during pregnancy and the postpartum period. Treatment and prevention of VTE in this patient population is complicated by the need to consider fetal, as well as maternal wellbeing when making management decisions. Although our knowledge of risk factors for pregnancy related VTE, the safe and effective use of anticoagulants in this patient population, as well as the use of IVC filters and catheter directed therapies continues to grow, there are still important gaps. The lack of high quality research and conclusive trial data demonstrating the safety and efficacy of treatment options for VTE during pregnancy highlights the need for prospective trials with larger numbers of patients.
All women must be provided with the opportunity to participate in shared decision making regarding their management. To make the best decisions, absolute risks and benefits of interventions, guideline recommendations and the patients’ values and preferences must all be taken into account.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Marik PE, Plante LA. Venous thromboembolic disease in pregnancy. N Engl J Med 2008;359:2025. [Crossref] [PubMed]
- Kujovich JL. Hormones and pregnancy: thromboembolic risks for women. BR J Haematol 2004;126:443. [Crossref] [PubMed]
- Heit JA, Kobbervig CE, James AH, et al. Trends in the incidence of venous thromboembolism during pregnancy and postpartum: a 30 year population based study. Ann Int Med 2005;143:697. [Crossref] [PubMed]
- James AH, Jamison MG, Brancazio LR, et al. Venous thromboembolism during pregnancy and the postpartum period: incidence, risk factors and mortality. Am J Obstet Gynecol 2006;194:1311. [Crossref] [PubMed]
- Simpson EL, Lawrenson RA, Nightingale AL, et al. Venous Thromboembolism in pregnancy and the puerperium: incidence and additional risk factors from a London perinatal database. BJOG 2001;108:56-60. [Crossref] [PubMed]
- James AH, Tapson VF, Goldberg SZ. Thrombosis during pregnancy and the postpartum period. Am J Obstet Gynecol 2005;193:216-9. [Crossref] [PubMed]
- Jacobsen AF, Skjeldestad FE, Sandest PM. Incidence and risk patterns of venous thromboembolism in pregnancy and puerperium- a register-based case-control study. Am J Obstet Gynecol 2008;198:233.e1-7. [Crossref] [PubMed]
- Lindqvist P, Dahlback B, Marsal K. Thrombotic risk during pregnancy: a population study. Obstet Gynecol 1999;94:595-9. [PubMed]
- Brenner B. Haemostatic changes in pregnancy. Thromb Res 2004;114:409-14. [Crossref] [PubMed]
- Macklon NS, Greer IA. The deep venous system in the puerperium: An ultrasound study. Br J Obstet Gynaecol 1997;104:198-200. [Crossref] [PubMed]
- Macklon NS, Greer IA, Bowman AW. An ultrasound study of gestational and postural changes in the deep venous system of the leg in pregnancy. Br J Obstet Gynaecol 1997;104:191-7. [Crossref] [PubMed]
- Eichinger S. D-dimer testing in pregnancy. Semin Vasc Med 2005;5:375-8. [Crossref] [PubMed]
- Ginsberg JS, Kowalchuck G, Hirsh J, et al. Heparin therapy during pregnancy: risks to the fetus and mother. Arch Intern Med 1989;149:2233-6. [Crossref] [PubMed]
- Bates SM, Greer IA, Middeldorp S. Venous Thromboembolism, thrombophilia, antithrombotic therapy and pregnancy: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence- based clinical practice guidelines. Chest 2012;141:e691s-736s.
- Bates SM, Middeldorp S, Rodger M. Guidance for the treatment and prevention of obstetric associated venous thromboembolism. J Thromb Thrombolysis 2016;41:92-128. [Crossref] [PubMed]
- Sadler L, McCowan L, White H, et al. Pregnancy outcomes and cardiac complications in women with mechanical, biopresthetic and hemograft valves. BJOG 2000;107:245-53. [Crossref] [PubMed]
- Nassar AH, Hobeika EM, Abd Esmad HM, et al. Pregnancy outcomes in women with prosthetic heart valves. Am J Obstet Gynecol 2004;191:1009-13. [Crossref] [PubMed]
- Blickstein D, Blickstein I. The risk of fetal loss associated with warfarin anticoagulation. Int J Gynaecol Obstet 2002;78:221-5. [Crossref] [PubMed]
- Cotrufo M, De Feo M, De Santo LS, et al. Risk of warfarin during pregnancy with mechanical valve prostheses. Obstet Gynecol 2002;99:35-40. [PubMed]
- Meschengieser SS, Fondevila CG, Santarelli MT, et al. Anticoagulation in pregnant women with mechanical heart valve prosthesis. Heart 1999;82:23-6. [Crossref] [PubMed]
- Wesseling J, Van Driel D, Heymans HS, et al. Long term effects of growth and development of school age children. Thromb Haemost 2001;85:609-13. [PubMed]
- Dempfle CE. Minor transplacental passage of fondaparinux in vivo. N Engl J Med 2004;350:1914-5. [Crossref] [PubMed]
- Mazzolai L, Hohfeld P, Spertini F, et al. Fondaparinux is a safe alternative in case of heparin intolerance during pregnancy. Blood 2006;108:1569-70. [Crossref] [PubMed]
- Elsaigh E, Tachil J, Nash MJ, et al. The use of fondaparinux in pregnancy. Br J Haematol 2015;168:762-4. [Crossref] [PubMed]
- Calhoun B, Hoover E, Seybold D, et al. Outcomes in an obstetrical population with hereditary thrombophilia and high tobacco use. J Matern Fetal Neonatal Med 2017;20:1-5. [Crossref] [PubMed]
- Bleker SM, Buchmüller A, Chauleur C, et al. Low molecular weight heparin to prevent recurrent venous thromboembolism in pregnancy: Rationale and design of the Highlow study, a randomized trial of two doses. Thromb Res 2016;144:62-8. [Crossref] [PubMed]
- De Stefano V, Rosi E, Za T, et al. Prophylaxis and treatment of venous thromboembolism in individuals with inherited thrombophilia. Semin Thromb Hemost 2006;32:767-80. [Crossref] [PubMed]
- Dargaud Y, Rugeri L, Fleury C, et al. Personalized thromboprophylaxis using a risk score for the management of pregnancies with high risk of thrombosis: a prospective clinical study. J Thromb Haemost 2017;15:897-906. [Crossref] [PubMed]
- Kearon C, Julian JA, Newman TE, et al. Noninvasive diagnosis of venous thrombosis: McMaster Diagnostic imaging practice guidelines initiative. Ann Int Med 1998;128:663-77. [Crossref] [PubMed]
- Torkzad MR, Bremme K, Hellgren M, et al. Magnetic resonance imaging and ultrasonography in diagnosis of pelvic vein thrombosis during pregnancy. Thromb Res 2010;126:107-12. [Crossref] [PubMed]
- Galanaud JP, Khan SR. Postthrombotic syndrome: a 2014 update. Curr Opin Cardiol 2014;29:514-9. [Crossref] [PubMed]
- Kahn SR, Shrier I, Julian JA, et al. Determinants and time course of the postthrombotic syndrome after acute deep venous thrombosis. Ann Intern Med 2008;149:698-707. [Crossref] [PubMed]
- Abouzahr and Wardlaw. Maternal mortality in 2000: Estimates developed by WHO, UNICEF and UNFPA. Geneva: World Health Organization, 2004.
- Chang J, Elam-Evans LD, Berg CG, et al. Pregmancy related mortality surveillance- United States, 1991-99. MMWR Surveill Summ 2003;52:1-8. [PubMed]
- The 2007 Recommendations of the International Commission on Radiological Protection. ICRP publication 103. Ann ICRP 2007;37:1-332. [PubMed]
- Tirada N, Dreizin D, Khati NJ, et al. Imaging Pregnant and Lactating Patients. Radiographics 2015;35:1751-65. [Crossref] [PubMed]
- McCollough CH, Schueler BA, Atwell TD, et al. Radiation Exposure and Pregnancy: When Should We Be Concerned? Radiographics 2007;27:909-17. [Crossref] [PubMed]
- Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet 1999;353:1386-9. [Crossref] [PubMed]
- Bloom AI, Farkas A, Kalish Y. Pharmacomechanical catheter directed thrombolysis for pregnancy related iliofemoral deep vein thrombosis. J Vasc Interv Radiol 2015;26:992-1000. [Crossref] [PubMed]
- Gupta S, Ettles DF, Robinson GJ, et al. Inferior Vena Cava Filter Use in pregnancy: preliminary experience. BJOG 2008;115:785-8. [Crossref] [PubMed]
- Cheung MC, Asch MR, Gandhi S, et al. Temporary inferior vena caval filter use in pregnancy. J Thromb Haemost 2005;3:1096-7. [Crossref] [PubMed]
- Ganguli S, Tham JC, Kolmos F, et al. Fracture and migration of a suprarenal Inferior vena cava filter in a pregnant patient. J Vasc Interv Radiol 2006;17:1707-11. [Crossref] [PubMed]
- Milford W, Chadha Y, Lust K. Use of a retrievable inferior vena cava filter in term pregnancy: case report and review of the literature. Aust N Z J Obstet Gynaecol 2009;49:331-3. [Crossref] [PubMed]
- McConvilee RM, Kennedy PT, Collins AJ, et al. Failed retrieval of an inferior vena cava filter during pregnancy because of filter tilt. Cardiovasc Intervent Radiol 2009;32:174-7. [Crossref] [PubMed]
- Royal College of Obstetricians and Gynaecologists. Thromboembolic disease in pregnancy and the puerperium: Acute management. Green-top Guideline No. 37b 2015.
- Harris SA, Velieni R, Davies AH. Inferior Vena Cava Filters in pregnancy: A systematic review. J Vasc Interv Radiol 2016;27:354-60.e8. [Crossref] [PubMed]
- Eagle CJ, Davies JM. Lethal air embolism during placement of a Kimray-Greenfield filter. J Cardiothorac Anesth 1990;4:616-20. [Crossref] [PubMed]
- Athanasoulis CA, Kaufman J, Halpern EF, et al. Inferior Vena Cava Filters: review of a 26 year single centerexperience. Radiology 2000;216:54-66. [Crossref] [PubMed]
- Kawamata K, Chiba Y, Tanaka R, et al. Experience of temporary vena cava filters inserted in the perinatal period to prevent pulmonary embolism in pregnant women with deep vein thrombosis. J Vasc Surg 2005;41:652-6. [Crossref] [PubMed]
- PREPIC Study Group. Eight-year follow-up of patients with permanent vena cava filters in the prevention of pulmonary embolism: the PREPIC (Prevention du Risque d'Embolie Pulmonaire par Interruption Cave) randomized study. Circulation 2005;112:416-22. [Crossref] [PubMed]
- Pfeifer GW. Distribution and placental transfer of 131-I streptokinase. Australas Ann Med 1970;19 Suppl 1:17-8. [Crossref] [PubMed]
- Oklu R, Wicky S. Catheter-directed thrombolysis of deep venous thrombosis. Semin Thromb Hemost 2013;39:446-51. [Crossref] [PubMed]
- Sista AK, Vedantham S, Kaufman JA, et al. Endovascular Interventions for acute and chronic lower extremity deep venous disease: state of the art. Radiology 2015;276:31-53. [Crossref] [PubMed]
- Vedantham S, Sista AK, Klein SJ, et al. Quality improvement guidelines for the treatment of lower extremity deep vein thrombosis with use of endovascular thrombus removal. J Vasc Interv Radiol 2014;25:1317-25. [Crossref] [PubMed]
- Herrera S, Comerota AJ, Thakur S, et al. Managing iliofemoral deep venous thrombosis of pregnancy with a strategy of thrombus removal is safe and avoids post-thrombotic morbidity. J Vasc Surg 2014;59:456-64. [Crossref] [PubMed]


