Below-knee deep vein thrombosis (DVT): diagnostic and treatment patterns
Introduction
Deep vein thrombosis (DVT) is a common diagnosis, contributing substantial morbidity and mortality to the population both in and outside the hospital. DVT occurs at an annual rate of approximately 1 in 1,000 adults, and death occurs within one month for 5.5% of patients (1). The most devastating complication of DVT is pulmonary embolism (PE), which is estimated to have a mortality rate as high as 30% within one month (1). Chronic venous insufficiency and the post-thrombotic syndrome are common sequelae of DVT that have a dramatic effect on quality of life (2-4). Treatment with anticoagulation is the accepted standard of care for DVT involving the proximal leg veins, specifically, the popliteal, femoral, and iliac veins. However, management of below-knee DVT (BKDVT) is less clearly understood and lacks the same evidence-based consensus (5-14).
BKDVT is defined as thrombosis of the deep venous system of the leg distal to the popliteal vein, including the tibial, peroneal, soleus, and gastrocnemius veins. BKDVT should be not be confused with superficial thrombophlebitis (thrombosis of the superficial venous system), which does not confer the same mortality risk and should be treated symptomatically. The American College of Chest Physicians (ACCP) offers the only consensus guidelines for BKDVT management, and these are considered “grade 2C”; weak recommendations based on low-quality evidence (14). As a result, physicians who care for patients with BKDVT face a considerable dilemma because the risks of anticoagulation versus PE are unclear. The ACCP cautiously recommends anticoagulation only for severely symptomatic patients with BKDVT. For other patients, it is recommended to perform surveillance ultrasound in two weeks to monitor for clot propagation, at which point anticoagulation should be initiated if there is any evidence of proximal extension (see Figure 1).
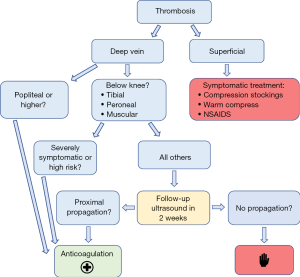
Most of what is known about the natural history of BKDVT is based on observational studies, with a wide range of estimates on its prevalence and the risk of clot propagation and embolism. It is felt that most DVT’s begin in the calf veins, but that these do not usually become symptomatic until they have propagated to a proximal vein (5,13). As a result, observational studies on symptomatic patients may underestimate the prevalence of isolated BKDVT. To support this, studies on inpatients have found that BKDVT constitutes only 20% of all DVT diagnoses, whereas studies on outpatients have reported a percentage as high as 70% (1). Overall, there are an estimated 300,000 cases of BKDVT diagnosed each year in the United States (5).
Most cases of BKDVT resolve spontaneously without anticoagulation. BKDVT is unlikely to embolize until after extension to the proximal deep veins (15-19), and propagation usually occurs within 2 weeks of the initial diagnosis (5,8,20,21). These observations form the basis for recommending surveillance ultrasound on select patients with BKDVT rather than treating all patients with anticoagulation. The decision not to treat BKDVT, therefore, depends critically on the likelihood of proximal propagation and the presumed low rate of embolization without propagation. However, the absolute risk of clot propagation and embolization remains unclear, and vary greatly in the literature. One systematic review found reported rates of propagation ranging from 3% to 32% (5). A recent case-control study found a rate of propagation to proximal veins in just 5% of cases after 180 days (vs. 1.4% in patients on anticoagulation), and PE in 4.3% of cases (vs. 1.6%). The risk reduction from anticoagulation was still significant but was offset by an increased risk of bleeding (22).
Two recent systematic reviews found the methodologic quality of the available literature to be too low to make a conclusive recommendation on whether to anticoagulate patients with BKDVT (11,13). Amid the continuing controversy, it is unclear how today’s practitioners are managing these patients. It is also unclear how radiologists can report a finding of BKDVT in a way that promotes appropriate clinical practice. It is likely that making a diagnosis of “DVT”, which is the technically correct terminology, may ironically encourage or even force the hand of practitioners to treat some patients inappropriately. This study seeks to identify diagnostic and treatment patterns amongst radiologists and clinicians who encounter patients with BKDVT within a large multi-hospital healthcare system.
Methods
A retrospective analysis was conducted of radiology reports and medical records from a large multi-hospital healthcare system between 2014 to 2016. Lower extremity ultrasounds were assessed based on the location of identified thrombosis as described in the body of the radiology report. BKDVT was defined as thrombosis involving the paired tibial, peroneal, or deep muscular veins of the calf (gastrocnemius and soleal veins). Only cases of isolated BKDVT were included for analysis; cases with additional thrombosis involving or extending into the femoropopliteal system were excluded. Only new diagnoses of BKDVT were included for analysis; follow-up studies were excluded, such as patients with previously-diagnosed BKDVT or resolving femoropopliteal DVT.
The corresponding medical record was evaluated in each case. Clinical risk factors (1) were assessed such as age, gender, malignancy, recent surgery (within 3 months), and history of DVT. Treatment decisions and clinical outcomes (such as concurrent or subsequent PE) were identified. Treatment was defined as any initiation or escalation of anticoagulation therapy, or placement of an IVC filter.
Radiology reporting was categorized based on the language used in the final report “conclusion” or “impression”. Reports were categorized as positive, negative, or descriptive. For example, any report using the terminology “DVT” fell into the category of “positive for DVT” or “negative for DVT”. Other terminology such as “thrombosis of the anterior tibial vein” without using the word “DVT” were categorized as descriptive. Chi-square and logistic regression were used to correlate risk factors with outcomes and to determine the odds of treatment.
Results
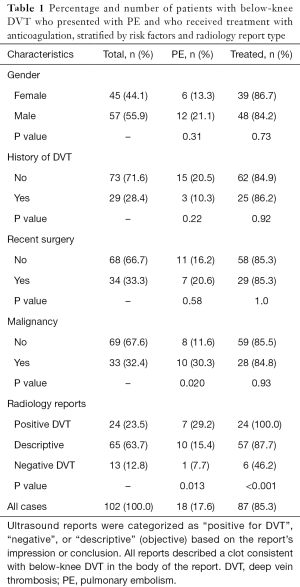
One hundred and two cases of new, isolated, below-knee DVT were identified. Average patient age was 62 years (SD =14 years), and 55.9% of patients were male (see Table 1). A total of 28.4% of patients had previous history of DVT (n=29), 33.3% had recent surgery within the past 30 days (n=34), and 32.4% had active malignancy (n=33). Eighteen patients were diagnosed with PE during the same clinical encounter (17.6% of all patients; 62.1% with chest CT). PE was not associated with age, gender, recent surgery, or history of DVT. PE was associated with malignancy, with much higher odds of PE in the malignancy group (OR =3.3, 95% CI: 1.2–9.4; P=0.015). PE was diagnosed in 30.3% of the patients with a known malignancy (n=10/33) compared to 11.6% of patients without malignancy (n=8/69).
Full table
Treatment was not independently associated with any of the clinical risk factors (age, gender, recent surgery, malignancy, or history of DVT). Treatment was associated with the type of radiology report impression/conclusion, which were highly variable. Treatment was much less likely if the report described a below-knee thrombosis but was said to be otherwise “negative for DVT” (P<0.001). A total of 86.3% (n=88) of all patients were treated, compared to 46.2% (n=6) of patients in this group (n=13). One hundred percent of patients were treated when the report conclusion was “positive DVT” (n=24; 23.5% of the sample) and 89.2% (n=58) were treated after an objective description of the clot location without using the word “DVT” (63.7% of the sample, n=65). IVC filters were placed in 3 patients. Of the 14 untreated patients, 5 received active surveillance with follow-up ultrasound (4.9% of the sample). Of these, 3 subsequently developed proximal (femoropopliteal) DVT requiring treatment and none developed PE.
Discussion
Management of below-knee DVT remains controversial because current guidelines are based on weak evidence. The ACCP recommends surveillance with follow-up ultrasound in 2 weeks unless the patient has severe symptoms. Our observations suggest that in clinical practice, almost all patients (~90%) receive treatment with anticoagulation. One explanation is that many patients do present with severe symptoms, however, our study did not grade clinical symptomatology.
Many patients also present with acute PE and require anticoagulation for this reason. Nevertheless, surveillance ultrasound is not commonly recommended and likely underutilized. In our sample, for example, only 5 of the 14 untreated patients received surveillance with follow-up ultrasound. Failure to follow up and treat is in part due to inadequate radiologic diagnoses rather than clinical decision making. Some BKDVT are simply said to be “positive” for DVT, and in other cases the final diagnosis was said to be “negative” or even described as “superficial thrombophlebitis”.
While prospective trials are lacking, the available literature suggests that the majority of BKDVT cases resolve spontaneously, and proximal propagation of BKDVT is required before clinically significant PE can occur. On the other hand, BKDVT does occasionally lead to PE, and anticoagulation reduces this risk. We are unable to further assess the natural history of BKDVT because such a limited number of patients in our sample received follow-up without anticoagulation. Larger randomized-controlled trials are needed.
Our study did observe a significant proportion of patients who presented with concurrent PE (17.6% of the sample), although this likely overestimates the overall risk of embolization (5). For example, many cases in our sample were diagnosed only after the patient developed clinically significant PE, creating a selection bias. The majority of BKDVT cases are undiagnosed and resolve spontaneously. In addition, it is possible for femoropopliteal DVT to manifest as BKDVT after embolization of the proximal clot component (23,24). This calls into question the causality between BKDVT and concurrent PE.
The elevated risk of thromboembolic disease in patients with malignancy is well documented, but it is interesting to note that even among patients with BKDVT, patients with malignancy were much more likely to present with concurrent PE. One potential explanation is that there is a lower threshold for chest CT in patients with malignancy, which could add selection bias to the sample. Another explanation is that malignancy increases the risk of embolism independently from the already-elevated risk of thrombosis. It may be reasonable to use a lower threshold for treatment of BKDVT in patients with malignancy.
In conclusion, there remains confusion among physicians in how to best manage BKDVT because of limited data and practice guidelines. Randomized prospective trials are still needed to determine the absolute risk of clot propagation and embolism. Radiologists should be aware of the clinical distinction between proximal DVT and BKDVT, and should clearly report findings in a way that most appropriately influences patient management. Considering the current controversy, describing BKDVT findings only in terms of being “positive” or “negative” for DVT may be inadequate. Based on current guidelines, it is reasonable to suggest follow-up ultrasound for cases of incidental or asymptomatic BKDVT as an alternative to anticoagulation.
Acknowledgements
Funding: R Oklu gratefully acknowledges funding from the National Institutes of Health (EB021148, CA172738, EB024403, HL137193) and the Mayo Clinic.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical statement: Institutional IRB approval was granted for conducting a retrospective analysis of patient medical records (IRB #002338). All data was collected and stored in an anonymous and secure fashion using only the medical record numbers for reference.
References
- Heit JA, Spencer FA, White RH. The epidemiology of venous thromboembolism. J Thromb Thrombolysis 2016;41:3-14. [Crossref] [PubMed]
- Kahn SR. Frequency and determinants of the postthrombotic syndrome after venous thromboembolism. Curr Opin Pulm Med 2006;12:299-303. [Crossref] [PubMed]
- Kahn SR, Hirsch A, Shrier I. Effect of postthrombotic syndrome on health-related quality of life after deep venous thrombosis. Arch Intern Med 2002;162:1144-8. [Crossref] [PubMed]
- Shbaklo H, Kahn SR. Long-term prognosis after deep venous thrombosis. Curr Opin Hematol 2008;15:494-8. [Crossref] [PubMed]
- Masuda EM, Kistner RL. The case for managing calf vein thrombi with duplex surveillance and selective anticoagulation. Dis Mon 2010;56:601-13. [Crossref] [PubMed]
- Galanaud JP, Sevestre-Pietri MA, Bosson JL, et al. Comparative study on risk factors and early outcome of symptomatic distal versus proximal deep vein thrombosis: results from the OPTIMEV study. Thromb Haemost 2009;102:493-500. [PubMed]
- Franco L, Giustozzi M, Agnelli G, et al. Anticoagulation in patients with isolated distal deep vein thrombosis: a meta-analysis. J Thromb Haemost 2017;15:1142-54. [Crossref] [PubMed]
- Macdonald PS, Kahn SR, Miller N, et al. Short-term natural history of isolated gastrocnemius and soleal vein thrombosis. J Vasc Surg 2003;37:523-7. [Crossref] [PubMed]
- Righini M, Paris S, Le Gal G, et al. Clinical relevance of distal deep vein thrombosis. Review of literature data. Thromb Haemost 2006;95:56-64. [PubMed]
- Schwarz T, Schmidt B, Beyer J, et al. Therapy of isolated calf muscle vein thrombosis with low-molecular-weight heparin. Blood Coagul Fibrinolysis 2001;12:597-9. [Crossref] [PubMed]
- Masuda EM, Kistner RL, Musikasinthorn C, et al. The controversy of managing calf vein thrombosis. J Vasc Surg 2012;55:550-61. [Crossref] [PubMed]
- Palareti G, Cosmi B, Lessiani G, et al. Evolution of untreated calf deep-vein thrombosis in high risk symptomatic outpatients: the blind, prospective CALTHRO study. Thromb Haemost 2010;104:1063-70. [Crossref] [PubMed]
- Kearon C. Natural history of venous thromboembolism. Circulation 2003;107:I22-30. [Crossref] [PubMed]
- Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141:e419S-96S.
- Brandjes DP, Heijboer H, Buller HR, et al. Acenocoumarol and heparin compared with acenocoumarol alone in the initial treatment of proximal-vein thrombosis. N Engl J Med 1992;327:1485-9. [Crossref] [PubMed]
- Hull R, Delmore T, Genton E, et al. Warfarin sodium versus low-dose heparin in the long-term treatment of venous thrombosis. N Engl J Med 1979;301:855-8. [Crossref] [PubMed]
- Hull RD, Raskob GE, Hirsh J, et al. Continuous intravenous heparin compared with intermittent subcutaneous heparin in the initial treatment of proximal-vein thrombosis. N Engl J Med 1986;315:1109-14. [Crossref] [PubMed]
- Monreal M, Ruiz J, Olazabal A, et al. Deep venous thrombosis and the risk of pulmonary embolism. A systematic study. Chest 1992;102:677-81. [PubMed]
- Raschke RA, Reilly BM, Guidry JR, et al. The weight-based heparin dosing nomogram compared with a "standard care" nomogram. A randomized controlled trial. Ann Intern Med 1993;119:874-81. [Crossref] [PubMed]
- Masuda EM, Kessler DM, Kistner RL, et al. The natural history of calf vein thrombosis: lysis of thrombi and development of reflux. J Vasc Surg 1998;28:67-73; discussion 73-4. [Crossref] [PubMed]
- Solis MM, Ranval TJ, Nix ML, et al. Is anticoagulation indicated for asymptomatic postoperative calf vein thrombosis? J Vasc Surg 1992;16:414-8; discussion 418-9. [Crossref] [PubMed]
- Utter GH, Dhillon TS, Salcedo ES, et al. Therapeutic Anticoagulation for Isolated Calf Deep Vein Thrombosis. JAMA Surg 2016;151:e161770. [Crossref] [PubMed]
- Bartter T, Hollingsworth HM, Irwin RS, et al. Pulmonary embolism from a venous thrombus located below the knee. Arch Intern Med 1987;147:373-5. [Crossref] [PubMed]
- Browse NL, Thomas ML. Source of non-lethal pulmonary emboli. Lancet 1974;1:258-9. [Crossref] [PubMed]


