Drug-eluting balloons with provisional bail-out or adjunctive stenting in de novo coronary artery lesions—a systematic review and meta-analysis
Introduction
Percutaneous coronary intervention and stenting is the most commonly used treatment for coronary artery disease. Plain-old balloon angioplasty (POBA) was the first technique (1) which facilitated a minimally-invasive expansion of a stenosed coronary artery (2). The technique was limited by acute complications including coronary dissection, which in some patients progressed to abrupt vessel closure requiring emergency bypass surgery; and acute elastic recoil, reducing the luminal gain following balloon dilatation. Post-procedure, restenosis due to neointimal proliferation of vascular smooth muscle cells represented another limiting factor most common in patients with diabetes and complex coronary artery disease (3-5).
Bare-metal stents (BMS) virtually solved the problem of dissection; acute vessel closure and the need for emergency bypass surgery (2), but did not reduce the risk of restenosis. The implantation of stents also led to a new problem of acute, late and very late stent thrombosis (6). However, treatment with dual antiplatelet therapy (DAPT) reduced this risk (7). The introduction of drug-eluting stents (DES) and its generational enhancements (i.e., thinner struts) partially resolved the problem of restenosis through release of anti-mitotic agents but concerns surrounding prolonged DAPT still remained (8,9).
To overcome the limitations of POBA, BMS and DES, the drug-eluting balloons (DEB) was developed. Initially introduced as a treatment for in-stent restenosis (ISR) in BMS and DES-lesions (10) they have emerged as a potential treatment for de novo coronary lesions. This is because the DEB intervention has several advantages over DES such as homogenous transfer of drug across vessel wall, lack of foreign body implantation, reduced bleeding risk (1 month DAPT versus 6–12 months of therapy for DES) and access to complex lesions (4,11). However, one disadvantage is that in a small proportion of DEB-treated patients, bail-out stenting is required, following recoil or dissections (12). Despite the promising characteristics of this intervention type, the indication for DEB use in de novo coronary lesions is still unclear.
Previous meta-analyses have failed to address the DEB-only strategy for de novo coronary artery disease (13,14). Moreover, analyses have not yet explored a DEB-only approach in bifurcation lesions. As initial studies suggest that DEB-alone show success for treatment of high-risk restenotic areas (15,16), we hypothesised that DEB will also provide superior results compared with other interventional treatments, for the treatment of small vessel disease and bifurcation lesions in de novo coronary disease. This study, which includes data from DEB and adjunctive BMS as well as DEB-only studies, therefore aims to provide the most up-to-date and comprehensive systematic review and meta-analysis for the treatment of de novo coronary artery disease. The safety and efficacy of DEB (with or without BMS) will be compared to other conventional management options (POBA, BMS and DES) in treating de novo lesions.
Methods
This study was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Literature search
MEDLINE, Embase, PubMed and ClinicalTrials.gov were searched for RCTs published from 1st January 2000 through to 1st May 2017 using the key terms: ‘drug eluting balloon’, ‘drug coated balloon’, ‘paclitaxel eluting balloon’, ‘de novo coronary lesion’, ‘coronary stenosis’, ‘coronary disease’, ‘small vessel disease’, ‘bifurcation’, ‘complex long lesion’, ‘acute myocardial infarction’, ‘acute MI’ and ‘MI’. No filters were applied. Abstracts and conference proceedings of the American College of Cardiology and Transcatheter Cardiovascular Therapeutics Symposia were also screened. References included in literature reviews were searched manually to ensure all studies that complied with the inclusion criteria were identified. Two investigators (S Patel and T Svermova) independently reviewed the resulting articles. A qualitative risk of bias in seven domains (random sequence generation; allocation concealment; blinding of participants/personnel; outcome; incomplete outcome; selective reporting and other risk of bias) was conducted.
Study selection
We included all studies that compared angioplasty using the DEB-only strategy as well as DEB with adjunctive BMS to: POBA, BMS and DES. For analysis, data pertaining to the longest available follow up periods were used. Exclusion criteria were patients treated for ISR and use of interventions other than DEB, POBA, BMS or DES (e.g., endothelial progenitor capturing stents). Non-randomised controlled trials and non-observational studies were also excluded. The primary endpoint collected was in-segment or in-stent late lumen loss (LLL). Secondary angiographic and clinical endpoints were: binary restenosis (in-stent or in-segment), target lesion revascularization (TLR), major adverse cardiac events (MACE), myocardial infarction (MI) and cardiac death.
Statistical analysis
Statistical analysis was performed using standard software packages (Review Manager version 5.3, The Nordic Cochrane Centre, The Cochrane Collaboration, 2014, Copenhagen, Norway; Comprehensive Meta-analysis version 3 software, Biostat Inc., Englewood, NJ, USA) with two-tailed P values <0.05 considered significant. Analyses are presented as forest plots—the conventional method for showing results from individual studies and meta-analysis. Risk ratios (RRs) with 95% confidence intervals (CIs) or mean differences (MDs) with standard deviations are presented as summary statistics. Subgroup analyses comparing DEB-only studies with DES were also performed. Heterogeneity between studies was compared by Q and I2 statistics (respectively, P<0.1 indicates heterogeneity; <25%, 25–50% and >50% indicate low, medium and high levels). Studies were combined using the random effect model. Publication bias was assessed using funnel plots and Egger’s test (P<0.05 indicates significant bias).
Results
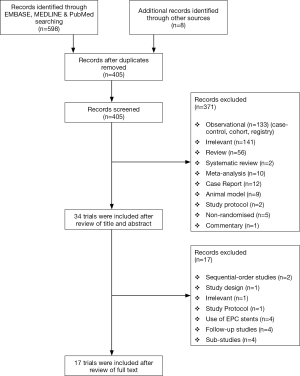
A PRISMA diagram of the literature search and the subsequent selection is presented in Figure 1. In total, 606 articles were retrieved. Following screening for replicates, 405 remained; and after screening abstracts for relevance, a further 371 studies were removed. Finally, full text of the remaining 34 studies were screened, of which 17 randomised controlled trials (17-34) fulfilled the inclusion criteria and were qualitatively and quantitatively analysed.
Characteristics of the patients and study designs
A summary of the included studies is provided in Table 1 and this table also documents: (I) interventions compared; (II) DEB, DES and BMS type; (III) indication; (IV) primary endpoint; (V) percent bare metal stenting; and (VI) angiographic follow-up time.

Full table
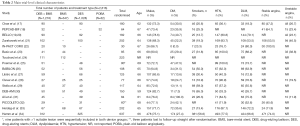
A total of 2,616 patients, ranging from 30 to 637 per study, were enrolled in the meta-analysis. There were 1,218 patients treated with DEB + BMS; whereas, 347 were treated with BMS; 1,028 with DES; and 32 with POBA. The mean age of patients recruited was 65 years; and all of the studies had a majority of male patients. Studies recruited from different patient populations. In the trial by Ali and colleagues (31), all patients were diabetic; whereas, in trials conducted by Besic et al. (23) and Poerner et al. (25) all patients were hypertensive. Data for each trial are detailed in Table 2.
Full table
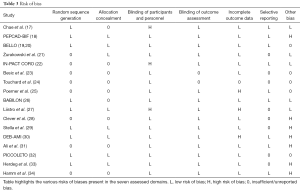
Risk of bias analysis revealed high-risk bias in open-label studies (17,22,27) and incomplete outcome data (25,27,30) where greater than 15% of patients were lost to follow-up (Table 3).
Full table
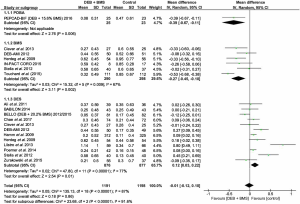
LLL
In subset analyses, DEB + BMS compared more favourably to POBA with a statistically significant difference observed for LLL [MD =−0.39; (−0.67 to −0.11); P=0.006]. DEB + BMS also had a significantly lower rate of LLL compared to the BMS group [MD =−0.27; (−0.45 to −0.10); P=0.002]. When compared to the DES group, the DEB + BMS group had an increased LLL and therefore was significantly less effective than DES [MD =0.12; (0.03 to 0.22); P=0.01] (Figure 2).
Binary restenosis
In subset analyses, risk of binary restenosis in the DEB + BMS group was significantly lower compared to the POBA group [RR =0.20; (0.05 to 0.85); P=0.03]. This was also seen in the DEB + BMS and DES groups, where binary restenosis was significantly lower in the former mode of intervention [RR =1.89; (1.13 to 3.18); P=0.02]. There was no statistically significant difference in binary restenosis, however, between the DEB + BMS and BMS groups [RR =0.44; (0.18 to 1.06); P=0.07] (Figure 3).
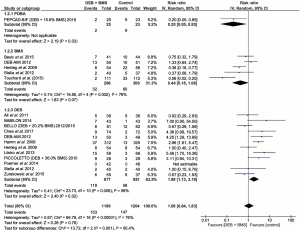
TLR
Compared to the BMS group, the DEB + BMS group had a significantly lower need for TLR [RR =0.65; (0.44 to 0.97); P=0.04]. The need for TLR, on the other hand, was higher in the DEB + BMS group compared to the DES group although this did not reach statistical significance [RR =1.57; (0.94 to 2.62); P=0.08] (Figure 4).
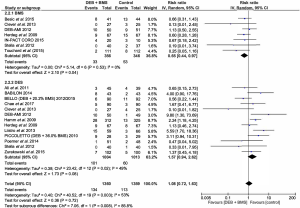
MACE
Definitions of MACE had slight variations across the 17 studies within this review. However, they were still interpreted under one global outcome. When compared to the BMS alone group, DEB + BMS had a significantly more favourable outcome (lower rate of MACE) [RR =0.64; (0.46 to 0.90); P=0.010]. However, even though the rate of MACE was higher in the DEB + BMS group compared to the DES group, there was no statistically significant difference [RR =1.39; (0.96 to 2.01); P=0.08] (Figure 5).
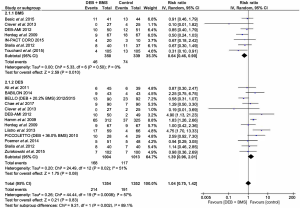
MI
There was no significant differences reported between the DEB + BMS groups and BMS or DES groups for MI [RR =0.66; (0.19 to 2.29); P=0.51]; [RR =1.30; (0.60 to 2.84, 95% CI); P=0.51] (Figure 6).
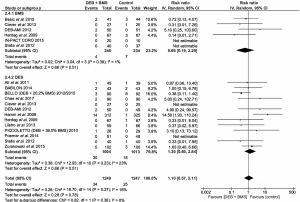
Death
There was also no significant differences reported between the DEB + BMS groups and BMS or DES groups for death [RR =0.20; (0.02 to 1.70); P=0.14]; [RR =1.43; (0.45 to 4.52); P=0.54] (Figure 7).
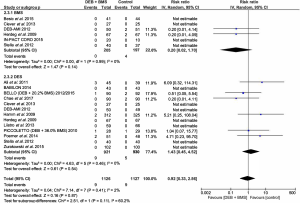
Stent thrombosis
Stent thrombosis out of all the outcomes had the lowest incident rate. No statistically significant difference was reported in the DEB + BMS group versus control [RR =1.85; (0.84 to 4.08); P=0.13]. This was also the case for DEB + BMS compared with BMS alone and DES alone [RR =4.10; (0.46 to 36.40); P=0.21]; [RR =1.64; (0.70 to 3.83); P=0.26] (Figure 8).
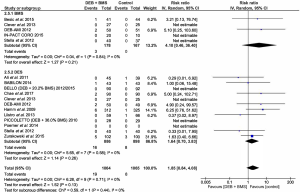
Subgroup analysis
As part of the subgroup analysis, ‘DEB-only’ interventions were compared with DES. In terms of LLL, DEB-only had no statistically significant difference to DES [MD =−0.12; (−0.25 to 0.01); P=0.06]. This was also seen in binary restenosis, MACE, TLR, MI and death; binary restenosis—[RR =1.36; (0.31 to 6.04); P=0.69], MACE—[RR =1.15; (0.27 to 4.98); P=0.85], TLR—[RR =1.26; (0.24 to 6.79); P=0.78], MI—[RR =0.65; (0.11 to 3.86); P=0.64] and death—[RR =0.69; (0.12 to 4.17); P=0.69].
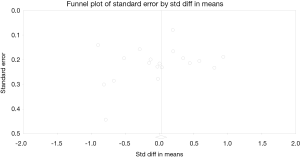
Publication bias
Egger’s test revealed no evidence of significant publication bias within this meta-analysis (P>0.05). This lack of bias was also substantiated by the symmetrical funnel plot for our primary angiographic endpoint of LLL (Figure 9).
Discussion
This is the first meta-analysis to compare DEB with and without BMS to all three management options: POBA, BMS alone and DES for treatment of de novo coronary lesions. Of the 17 RCTs included in the study, 14 studies compared DEB + BMS with DES and/or BMS, two studies compared DEB-only to DES and one, DEB alone to POBA.
Findings
The significant findings of this study can be summarised as follows: (I) DES was superior to DEB + BMS in angiographic (LLL) and clinical (binary restenosis) outcomes; (II) DEB + BMS was superior to BMS in LLL and clinical outcomes (MACE, TLR); and (III) DEB-only approach was superior to POBA for LLL and binary restenosis in bifurcation lesions.
Data interpretation
The current standard of care for PCI treatment of de novo coronary lesions is implantation of a second generation DES (35). However, there is increasing concern about the longer term risk of DES, such as increased bleeding risk with prolonged antiplatelet therapy (10,36). Additional risks include development of ISR and late ST which remain a factor of concern for DES users despite a lower incidence of disease compared to BMS (37,38). DEB, advocated for their ability to reduce DAPT and promote homogeneous drug transfer have been proposed as an alternative to DES (39). However, drawbacks such as dissection and acute elastic recoil means that DEB treatment is often seen accompanied with BMS (39).
In this analysis, it was shown that DEB + BMS were significantly inferior to DES in clinical and angiographic outcomes. Apparent lack of efficacy of DEB + BMS compared to DES could be explained in part by the high use of first-generation paclitaxel based DEB which are likely to be inferior to DES in terms of drug delivery. Also, the relatively short contact time between DEB and vessel wall could result in a greater ‘wash-off’ effect than with DES. A study investigating DEB techniques found optimal concentrations of paclitaxel in the vessel wall following short inflation times of 30 to 45 seconds (40). The study which compared 1st and 2nd generation DIOR balloons in a porcine model also found higher tissue concentrations of drug and reduced arterial wall injury with 2nd generation DEBs (40). Coupling of 2nd generation DEBs with a shorter inflation time could therefore improve release kinetics and safety in future studies.
Different generation DES were also used. Eight trials used 1st generation DES, whilst five used 2nd generation devices. The TAXUS Liberté paclitaxel-eluting stent was the most common followed by the Xience V everolimus-eluting stent. A meta-analysis which compared 1st and 2nd generation DES found that 1st generation sirolimus-eluting stents compared well in efficacy to 2nd generation everolimus/zotarolimus eluting stents in TLR, MACE and restenosis (41,42); whereas, 2nd generation everolimus-based stents had better clinical outcomes (MI and ST rates) (41,42). Thus, it cannot be ruled out that some heterogeneity within results arose from a lack of uniformity in the DEB and DES employed.
Implications of DEB-only approach
DEB-only intervention is currently accepted in routine practice for treatment of ISR, re-restenosis or for side branch POBA. There may also be other niche indications including high-risk restenotic lesions such as bifurcations, long lesions, diffuse disease in diabetic patients and small vessel disease (43). The PEPCAD-BIF trial was the first randomised controlled trial to explore DEB-only use in side branch and/or distal main branch lesions (18). The trial showed that for bifurcations, DEB alone had a statistically significant reduction in LLL and binary restenosis (18). Whilst not having been directly compared to DES, the DEB-only approach is attractive for bifurcations owing to preservation of vessel patency and avoidance of carina shift—a phenomenon largely responsible for side branch occlusion following DES treatment (16,18). In addition, low rates of restenosis and TLR seen in the PEPCAD-BIF trial also suggested some promise for the DEB-only treatment of bifurcation lesions (18). However, more randomised controlled trials comparing DES and DEB alone are needed to determine whether these theoretical benefits translate to better angiographic and clinical outcomes.
Small vessel disease is another area where the DEB-only strategy has shown potential because short drug transfer time and lack of foreign body implantation reduces higher rates of neointimal proliferation and inflammatory response found in smaller-calibre vessels treated with BMS treatment (44). This was substantiated by our findings which suggested that DEB alone performed comparably to the current mainstay of treatment, DES, for both clinical and angiographic outcomes. It should be noted, however, that our meta-analysis only presented data from two studies comparing DES and DEB-alone (BELLO and PICCOLETO) since randomised controlled trials concerning this topic were scarce.
Despite these two trials portraying DEB-alone in a favourable light in small vessel disease, data from the study endpoints were rather heterogeneous. This could be explained by the use of IN.PACT Falcon, a 2nd generation DEB in the BELLO trial and DIOR I, a 1st generation DEB in the PICCOLETO trial. Furthermore, pre-dilatation rates were very low in the PICCOLETO trial compared with the BELLO trial (25% versus 96.8% respectively) (19,32). This is an important difference as pre-dilatation is a necessary prerequisite for optimal drug absorption through creation of micro-channels in the plaque and vessel wall (45). Importantly in this regard, studies did not also report the use of cutting or scoring balloon which may be important in improving drug access to the vessel wall. Experience has indicated that adequate and appropriate lesion preparation is essential when using DEB. It is important therefore that in future studies there is a standardised approach to lesion preparation, use of adjunctive technology such as cutting or scoring balloon and an adequate description of the methodology since it is possible that this will have an important effect on the efficacy of the DEB technique. Hence, the DEB-only approach with the right variant and preparation technique could show even more promise for small vessel lesions.
Limitations
This meta-analysis had several limitations. For example, a large majority of studies had a small sample size and results were moderately heterogeneous. However, this was likely the result of different generations of DEB and DES used as well as different study design comparators such as DES, POBA and BMS. For this reason, stratified and subgroup analyses were taken into account to further isolate sources of variability. Secondly, 14 trials reported clinical outcomes from relatively short follow-up periods (≤1 year) which may have failed to capture episodes of late stent thrombosis and thus influenced results. As already mentioned, the latest generations of DES and DEB were not universally studied and therefore results should be interpreted with caution in the context of latest devices in current clinical use. Additionally, any future studies of DEB must be against the latest generation of DES so that a valid comparison can be made against the technology in current clinical use. Finally, some studies were susceptible to industry bias owing to author involvement in relevant device companies such as B. Braun, Boston Scientific Medtronic and Eurocor GmbH (18,21,22,28,29,31,33). Similarly, conflicts of interest may have arisen due to these companies funding studies which investigated their own products (18,21,26,28,30,31,33,34).
Conclusions
Overall, the results of this meta-analysis found that DEB in combination with BMS was not superior to DES in both clinical and angiographic outcomes for de novo coronary lesions. Compared to BMS alone, however, the combination of DEB and BMS was superior in LLL and MACE. The strategy of DEB alone has shown some promise. For example, DEB-only studies performed comparably to DES within the setting of small coronary vessels. In addition, the approach has shown early success in bifurcation lesions with DEB alone. These findings suggest that for major de novo coronary lesions, DEB + BMS should not be considered for treatment unless in patients who have significant contraindications to DES. Whilst the result from DEB-alone may be advantageous in cases of high-risk restenotic lesions such as small vessel disease following successful pre-dilatation and bifurcation lesions of certain classifications (Medina type 0, X, X) we conclude that DEB are still not a widely accepted modality of treatment.
Acknowledgements
Funding: This work was supported by the British Heart Foundation (grant number FS/14/6/30573) to T Svermova.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
References
- Belenkov IuN, Samko AN, Batyraliev TA, et al. Coronary angioplasty: view through 30 years. Kardiologiia 2007;47:4-14. [PubMed]
- Sigwart U. Coronary angioplasty: some historical remarks. EuroIntervention 2011;7:K8-10. [Crossref] [PubMed]
- Dangas G. Restenosis: Repeat Narrowing of a Coronary Artery: Prevention and Treatment. Circulation 2002;105:2586-7. [Crossref] [PubMed]
- Waksman R, Pakala R. Drug-Eluting Balloon: The Comeback Kid? Circ Cardiovasc Interv 2009;2:352-8. [Crossref] [PubMed]
- Gardiner GA, Bonn J, Sullivan KL. Quantification of elastic recoil after balloon angioplasty in the iliac arteries. J Vasc Interv Radiol 2001;12:1389-93. [Crossref] [PubMed]
- Byrne RA, Joner M, Kastrati A. Stent thrombosis and restenosis: what have we learned and where are we going? The Andreas Grüntzig Lecture ESC 2014. Eur Heart J 2015;36:3320-31. [Crossref] [PubMed]
- Kereiakes DJ, Yeh RW, Massaro JM, et al. Antiplatelet therapy duration following bare metal or drug-eluting coronary stents: the dual antiplatelet therapy randomized clinical trial. JAMA 2015;313:1113-21. [Crossref] [PubMed]
- Lüscher TF, Steffel J, Eberli FR, et al. Drug-eluting stent and coronary thrombosis: biological mechanisms and clinical implications. Circulation 2007;115:1051-8. [Crossref] [PubMed]
- Kang SH, Park KW, Kang DY, et al. Biodegradable-polymer drug-eluting stents vs. bare metal stents vs. durable-polymer drug-eluting stents: a systematic review and Bayesian approach network meta-analysis. Eur Heart J 2014;35:1147-58. [Crossref] [PubMed]
- Windecker S, Kolh P, Alfonso F, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 2014;35:2541-619. [Crossref] [PubMed]
- Richelsen RK, Overvad TF, Jensen SE. Drug-Eluting Balloons in the Treatment of Coronary De Novo Lesions: A Comprehensive Review. Cardiol Ther 2016;5:133-60. [Crossref] [PubMed]
- Xiu PY, Meier P. What are the barriers to the use of drug-eluting balloons? Interv Cardiol 2014;6:9-11. [Crossref]
- Li Y, Wang C, Zuo G, et al. Drug-eluting balloons in the treatment of de-novo coronary lesions: A meta-analysis of randomized-controlled trials. Coron Artery Dis 2016;27:467-77. [Crossref] [PubMed]
- Lu W, Zhu Y, Han Z, et al. Drug-coated balloon in combination with bare metal stent strategy for de novo coronary artery disease. Medicine 2017;96:e6397. [Crossref] [PubMed]
- Alfonso F, Cardenas A, Cuevas C. Paclitaxel-eluting balloons for small-vessel disease. J Am Coll Cardiol 2013;61:1831-2. [Crossref] [PubMed]
- Schulz A, Hauschild T, Kleber FX. Treatment of coronary de novo bifurcation lesions with DCB only strategy. Clin Res Cardiol 2014;103:451-6. [Crossref] [PubMed]
- Chae IH, Yoon CH, Park JJ, et al. Comparison of Drug-Eluting Balloon Followed by Bare Metal Stent with Drug-Eluting Stent for Treatment of de Novo Lesions: Randomized, Controlled, Single-Center Clinical Trial. J Korean Med Sci 2017;32:933-41. [Crossref] [PubMed]
- Kleber FX, Rittger H, Ludwig J, et al. Drug eluting balloons as stand alone procedure for coronary bifurcational lesions: results of the randomized multicenter PEPCAD-BIF trial. Clin Res Cardiol 2016;105:613-21. [Crossref] [PubMed]
- Latib A, Colombo A, Castriota F, et al. A randomized multicenter study comparing a paclitaxel drug-eluting balloon with a paclitaxel-eluting stent in small coronary vessels: The bello (balloon elution and late loss optimization) study. J Am Coll Cardiol 2012;60:2473-80. [Crossref] [PubMed]
- Naganuma T, Latib A, Sgueglia GA, et al. A 2-year follow-up of a randomized multicenter study comparing a paclitaxel drug-eluting balloon with a paclitaxel-eluting stent in small coronary vessels the BELLO study. Int J Cardiol 2015;184:17-21. [Crossref] [PubMed]
- Żurakowski A, Buszman PP, Milewski KP, et al. Stenting and Adjunctive Delivery of Paclitaxel Via Balloon Coating Versus Durable Polymeric Matrix for de Novo Coronary Lesions: Clinical and Angiographic Results from the Prospective Randomized Trial. J Interv Cardiol 2015;28:348-57. [Crossref] [PubMed]
- Burzotta F, Brancati MF, Trani C, et al. Impact of drug-eluting balloon (pre- or post-) dilation on neointima formation in de novo lesions treated by bare-metal stent: the IN-PACT CORO trial. Heart Vessels 2016;31:677-86. [Crossref] [PubMed]
- Besic KM, Strozzi M, Margetic E, et al. Drug-eluting balloons in patients with non-ST elevation acute coronary syndrome. J Cardiol 2015;65:203-7. [Crossref] [PubMed]
- Touchard AG, Goicolea J, Sabate M, et al. Paclitaxel eluting balloon after bare metal stent in STEMI (THE PEBSI STUDY). J Am Coll Cardiol 2015;65 Suppl:A1708. [Crossref]
- Poerner TC, Otto S, Gassdorf J, et al. Stent coverage and neointimal proliferation in bare metal stents postdilated with a paclitaxel-eluting balloon versus everolimus-eluting stents: Prospective randomized study using optical coherence tomography at 6-month follow-up. Circ Cardiovasc Interv 2014;7:760-7. [Crossref] [PubMed]
- López Mínguez JR, Nogales Asensio JM, Doncel Vecino LJ, et al. A prospective randomised study of the paclitaxel-coated balloon catheter in bifurcated coronary lesions (babilon trial): 24-month clinical and angiographic results. EuroIntervention 2014;10:50-7. [Crossref] [PubMed]
- Liistro F, Porto I, Angioli P, et al. Elutax paclitaxel-eluting balloon followed by bare-metal stent compared with Xience V drug-eluting stent in the treatment of de novo coronary stenosis: A randomized trial. Am Heart J 2013;166:920-6. [Crossref] [PubMed]
- Clever YP, Cremers B, Speck U, et al. Influence of a paclitaxel coated balloon in combination with a bare metal stent on restenosis and endothelial function: Comparison with a drug eluting stent and a bare metal stent. Catheter Cardiovasc Interv 2014;84:323-31. [Crossref] [PubMed]
- Stella PR, Belkacemi A, Dubois C, et al. A multicenter randomized comparison of drug-eluting balloon plus bare-metal stent versus bare-metal stent versus drug-eluting stent in bifurcation lesions treated with a single-stenting technique: Six-month angiographic and 12-month clinical results of th. Catheter Cardiovasc Interv 2012;80:1138-46. [Crossref] [PubMed]
- Belkacemi A, Agostoni P, Nathoe HM, et al. First results of the DEB-AMI (Drug Eluting Balloon in Acute ST-segment elevation myocardial infarction) Trial: A multicenter randomized comparison of drug-eluting balloon plus bare-metal stent versus bare-metal stent versus drug-eluting stent in primary percutaneous coronary intervention with 6-month angiographic, intravascular, functional, and clinical outcomes. J Am Coll Cardiol 2012;59:2327-37. [Crossref] [PubMed]
- Ali RM, Degenhardt R, Zambahari R, et al. Paclitaxel-eluting balloon angioplasty and cobalt-chromium stents versus conventional angioplasty and paclitaxel-eluting stents in the treatment of native coronary artery stenoses in patients with diabetes mellitus. EuroIntervention 2011;7 Suppl K:K83-92.
- Cortese B, Micheli A, Picchi A, et al. Paclitaxel-coated balloon versus drug-eluting stent during PCI of small coronary vessels, a prospective randomised clinical trial. The PICCOLETO study. Heart 2010;96:1291-6. [Crossref] [PubMed]
- Herdeg C, Göhring-Frischholz K, Haase KK, et al. Catheter-Based Delivery of Fluid Paclitaxel for Prevention of Restenosis in Native Coronary Artery Lesions After Stent Implantation. Circ Cardiovasc Interv 2009;2:294-301. [Crossref] [PubMed]
- Hamm CW, Cremers B, Moellmann H, et al. PEPCAD III: a randomised trial comparing a paclitaxel-coated balloon/stent system with a sirolimus eluting stent. Circulation 2009;120:2157.
- Wijns W, Kolh P, Danchin N, et al. Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2010;31:2501-55. [Crossref] [PubMed]
- Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation 2011;124:e574-651. [Crossref] [PubMed]
- D’Ascenzo F, Bollati M, Clementi F, et al. Incidence and predictors of coronary stent thrombosis: Evidence from an international collaborative meta-analysis including 30 studies, 221,066 patients, and 4276 thromboses. Int J Cardiol 2013;167:575-84. [Crossref] [PubMed]
- López Mínguez JR, Nogales Asensio JM, Doncel Vecino LJ, et al. A prospective randomised study of the paclitaxel-coated balloon catheter in bifurcated coronary lesions (BABILON trial): 24-month clinical and angiographic results. EuroIntervention 2014;10:50-7. [Crossref] [PubMed]
- Waksman R, Pakala R. Drug-eluting balloon: the comeback kid?. Circ Cardiovasc Interv 2009;2:352-8. [Crossref] [PubMed]
- Pósa A, Nyolczas N, Hemetsberger R, et al. Optimization of drug-eluting balloon use for safety and efficacy: Evaluation of the 2nd generation paclitaxel-eluting DIOR-balloon in porcine coronary arteries. Catheter Cardiovasc Interv 2010;76:395-403. [Crossref] [PubMed]
- Zhang X, Xie J, Li G, et al. Head-to-Head Comparison of Sirolimus-Eluting Stents versus Paclitaxel-Eluting Stents in Patients Undergoing Percutaneous Coronary Intervention: A Meta-Analysis of 76 Studies. PLoS One 2014;9:e97934. [Crossref] [PubMed]
- Navarese EP, Kowalewski M, Kandzari D, et al. First-generation versus second-generation drug-eluting stents in current clinical practice: updated evidence from a comprehensive meta-analysis of randomised clinical trials comprising 31 379 patients. Open Heart 2014;1:e000064. [Crossref] [PubMed]
- Li B, Ding Y, Tian F, et al. Assessment of a Drug-Eluting Balloon for the Treatment of de novo Coronary Lesions Guided by Optical Coherence Tomography: Study Protocol for a Randomized Controlled Trial. Cardiology 2017;136:252-7. [Crossref] [PubMed]
- Giannini F, Latib A, Colombo A. Paclitaxel-eluting balloons or paclitaxel-eluting stents for the treatment of small-vessel coronary artery disease. Interv Cardiol 2013;5:137-40. [Crossref]
- Marzullo R, Aprile A, Biondi-Zoccai G, et al. Drug-eluting balloon technology. Cardiac Interventions Today 2011;40-9.