Postoperative imaging of the aorta
Introduction
Imaging plays an increasingly important role in the postoperative management of aortic disease, especially as surgical techniques become increasingly complex. Surgical approach can widely differ between the thoracic and abdominal aorta, including open and endovascular techniques. To properly evaluate the postoperative aorta, adequate understanding of the anatomy, surgical technique, expected computed tomography (CT) appearance, and potential complications are all essential. In this article, thoracic and abdominal aortic repair techniques will be described, including normal postoperative appearance and complications.
Imaging protocol
CT angiography (CTA) is the gold standard for the evaluation of the postoperative aorta due to precise anatomic definition and dynamic contrast visualization. The core protocol is variable depending on institutional preferences but commonly involves three phases: precontrast, arterial phase and delayed phase enhancement. The arterial phase evaluates for anatomy of vascular structures, as well as for common complications including extravasation and pseudoaneurysm. The precontrast phase allows for problem solving by avoiding mimics of contrast opacification. Finally, delayed images are often critical for evaluation of slow leaks.
Protocols can vary widely by institution and are often tailored to answer specific questions based on the surgery performed. For example, electrocardiogram (ECG) gating can be critical for the evaluation of the aortic root and coronary artery ostia. Protocols can involve prospective or retrospective gating (1), with accompanying differences in radiation dose. Ultrafast nongated techniques can help reduce motion artifact and intravenous contrast dose. Postprocessing 3D volume rendering can often help with defining aortic anatomy and stent location.
One consequence of the rising popularity of endovascular repair of the aortic pathology is the need for routine yearly surveillance imaging, in contrast with open repair techniques where no routine imaging is required. Yearly CTA exams can impart a significant radiation burden over a lifetime, prompting exploration of radiation reduction techniques. For example, dual energy imaging can obviate the need the precontrast imaging due to generation of a virtual noncontrast image (2), while simultaneously exaggerating vessel contrast enhancement through the generation of low kVp images. Another issue with CTA exams is the need for intravenous contrast administration, which can be difficult in patients with renal impairment. Patients with low glomerular filtration rate (GFR) often require hydration or creative techniques involving ultrafast low dose contrast studies.
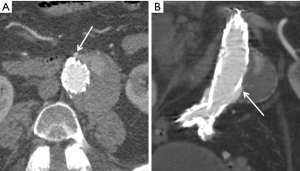
Magnetic resonance angiography (MRA) is a viable alternative for a variety of aortic imaging (Figure 1). However, in the evaluation of the postoperative patient, metallic stent grafts and other materials can obscure fine details that are often essential in postoperative assessment. Other important issues with MRA imaging include inability to assess stent patency, poor spatial resolution, nephrogenic systemic fibrosis (NSF) in renal failure patients, and inability to detect wall calcification (3). Although gadolinium based agents are the agent of choice in many protocols, there are a variety of non-gadolinium based agents which can be utilized in vascular imaging (4).
Ultrasonography (US) has been evaluated for its utility in the postoperative monitoring of abdominal aortic aneurysms (AAA). A systematic review demonstrated poor sensitivity for the detection of endoleak using color duplex ultrasound after endovascular repair of AAA, although this sensitivity did increase with use of ultrasound contrast agents (5).
Anatomy
The aorta is often characterized by section and can be anatomically divided into the thoracic and abdominal aorta at the diaphragmatic hiatus. The thoracic aorta is broadly divided into the ascending aorta, aortic arch, and descending aorta (6). The ascending aorta begins at the aortic valve to the level of the pulmonary artery bifurcation, and contains the sinuses of Valsalva where the coronary arteries originate. The aortic arch gives rise to three major vessels: the brachiocephalic trunk (which further branches into the right common carotid and subclavian arteries), left common carotid and left subclavian arteries. Variant anatomy occurs in approximately 30% of patients, with a common origin of the brachiocephalic trunk and left common carotid (sometimes referred to as “bovine arch”) being most common (7). The descending aorta gives rise to the intercostal arteries, bronchial arteries, and superior phrenic arteries among others. The anterior spinal arteries deserve special mention as branches of the intercostal arteries. They supply the anterior spinal cord and can lead to spinal cord ischemia in endovascular stent repair of the descending thoracic aorta.
The abdominal aorta branches include inferior phrenic arteries, suprarenal arteries, visceral arteries (celiac axis, superior mesenteric artery, and inferior mesenteric artery), renal arteries and lumbar arteries before bifurcating into the left and right common iliac arteries. The medial sacral artery also arises at this point.
In endovascular planning, a separate classification is used to section the aorta based on landing zones for proximal and distal attachments (Table 1) (8). Proximal attachments are classified as Zone 0 to 5, with distal attachments classified as Zone 4 to 11 (9).

Full table
Thoracic aorta
The most common indications for surgical intervention of the thoracic aorta include elective aortic aneurysm repair or urgent acute aortic syndromes including dissection, pseudoaneurysm and intramural hematoma (10). Acute aortic syndromes involving the ascending aorta and arch are repaired much more urgently due to risk of involving the pericardium, coronary arteries, and great vessels. For elective aneurysm repair, the general threshold size is typically 5.5 cm although there are indications at smaller sizes in preexisting connective tissue disease and familial syndromes (e.g., Marfan’s or bicuspid aortic valve) (11). Other indications include rapid sac growth of >0.5 cm/year or symptomatic aneurysm (e.g., aortic insufficiency, chest pain) (10) (Table 2)
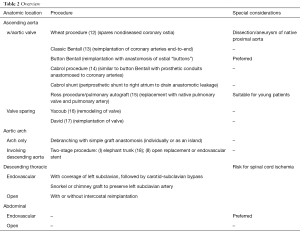
Full table
Ascending aorta
The surgical approach to aortic repair varies of a wide range of factors including anatomy, anatomic extent of disease, age, prior surgeries and need for long-term anticoagulation (10,12-14). A primary consideration in surgical planning involves assessing the extent of disease in determining whether to involve the aorta valve, coronary sinuses, coronary arteries and/or arch vessels (15).
Early aortic root replacement involved concurrent replacement of the aortic valve, either with a mechanical or tissue valve. Aortic root replacement was first described in 1964 by Wheat et al. which involved graft replacement of the aortic valve and the ascending aorta distal to the coronary ostia (16). Through this method, complications from manipulating the native coronary ostia are avoided. However, proximal native aortic dissection or pseudoaneurysms are recognized complications (17).
The Bentall procedure involves complete composite replacement of the proximal aorta with re-implantation of the coronary arteries (18). Several modifications have been made to this technique, including the “button Bentall” technique where buttons of coronary ostia are anastomosed (14), Currently, the “button Bentall” is the preferred approach for aortic root repair.
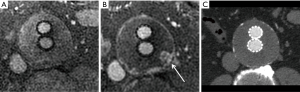
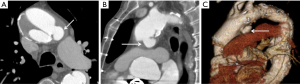
The Cabrol procedure (Figure 2) is an uncommon alternative where a side to side anastomosis to a prosthetic conduit is achieved for the left and right coronary arteries (19). Indications for the Cabrol procedure include dissection extension into the coronary ostia or inadequate/inadequate surgical mobilization of the coronary ostia for the button Bentall technique (15,20).
In patients with relatively normal aortic valves, valve-sparing root replacement is a consideration. Advantages include avoidance of lifelong anticoagulation and improved flow dynamics. The Yacoub (21,22) and David techniques (23,24) are described which can involve re-implantation of the native valve or remodeling of the sinuses of Valsalva to enable suturing of the native valve.
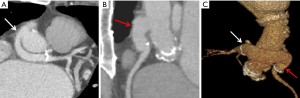
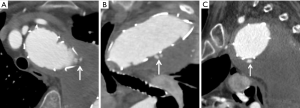
Finally, biologic autografts involving use of the native pulmonary artery and pulmonary valve to replace the aortic valve can be considered, typically in younger patients allowing for improved hemodynamics, known as the Ross procedure (Figure 3). A homograft valve replaces the native pulmonic valve. This procedure is especially favorable for younger patients to better optimize flow dynamics (25).
Broadly, grafts can be placed either through an interposition graft or much commonly an inclusion graft. The interposition graft is placed by complete resection of the diseased aorta and proximal and distal anastomosis. In contrast, the inclusion graft is placed in the retained disease aorta, leaving a peri-graft space that ideally thromboses closed. In the event of delayed thrombosis and continued hemorrhage, techniques such as the Cabrol shunt have been employed which involve creating a shunt to drain peri-graft hemorrhage into the right atrium (26,27).
Aortic arch
The degree of arch involvement dictates the surgical approach to repair. Isolated arch involvement only requires arch graft repair with re-implantation of the arch vessels either individually, as an island or a peninsula.
For more extensive aortic repairs involving the aortic arch and ascending or descending aorta, the elephant trunk procedure is performed (28-31). The two-step procedure first involves graft repair of the ascending aorta and arch. The native arch is removed with debranching of the great vessels followed by reimplantation into the graft. The distal edge of the graft is left unattached and free to dangle in the descending aorta in preparation for the second step. Subsequently, a second graft can be attached to the dangling piece either through an open or endovascular approach (32).
Endovascular repair
Thoracic endovascular aortic repair (TEVAR) is an alternative to open surgical management for the descending thoracic aorta repair, which won Federal Drug Administration (FDA) approval in 2005 (33). In contrast to open repair, TEVAR uses a retrograde approach through the femoral arteries to place a stent graft into the descending thoracic aorta. There are currently four FDA approved stent graft materials [Nitinol, stainless steel, polytetrafluoroethylene (PTFE), polyester] (34).
Rigorous preprocedural imaging is required to define anatomy and evaluate for adequate access, stent graft size, and placement, which is beyond the scope of this review. In cases where is no adequate landing zone >2 cm from the origin of the left subclavian artery, a decision can be made to intentionally cover the origin of the left subclavian (35,36). In patients with a dominant left vertebral system or incomplete Circle of Willis, revascularization of the left subclavian to carotid bypass should be performed preoperatively to minimize the risk of stroke (37). Routine revascularization of the left subclavian artery, however, has not been shown to be beneficial in a meta-analysis (30).
Alternatives to this include use of a fenestrated graft or chimney and snorkel techniques to maintain antegrade arterial flow (38).
Abdominal aorta
AAA are the most common indication for abdominal aortic surgery, first described in 1952 (39). Rupture carries a high mortality rate (over 50%) with over 13,000 deaths per year in the United States (40). The prevalence of AAA is approximately 10% in individuals over 65 and often asymptomatic. The US Preventive Services Task Force (USPSTF) has published guidelines regarding routine screening in men over age 65, which were updated in 2014 (41). A recent study demonstrated increased screening appropriateness following these updates (42). Current USPSTF guidelines are being updated for 2019, with tentative research draft plans investigating the benefit of screening asymptomatic adults aged 50 and older. Indications for surgical treatment include AAA >5.5 cm, rapid expansion >1 cm in 1 year, infection and other complications.
Endovascular repair
The discovery of endovascular aneurysm repair (EVAR) has transformed the management of AAA. From 2000 to 2010, EVAR use has rapidly grown from 5.2% to 74% of all AAA repairs (43), largely in part due to improvement in 30-day outcomes including declines in all-cause mortality, surgical site infection, pneumonia and sepsis (32). Nevertheless, endograft repair carries its own complications and thus routine surveillance is required to confirm graft stability. Current guidelines include imaging surveillance at 1 and 12 months after surgery, followed by annual routine imaging (44).
The stent graft is composed of a delivery system, main body device, and iliac extensions. Bifurcating iliac limbs are most commonly used except in cases of severe unilateral iliac stenosis or in cases of ruptured aneurysm with need for expeditious control of hemorrhage. There are eight stent grafts available in the United States, which vary in degree of structural support (Nitinol or stainless steel) throughout the graft. Grafts are usually made of polyester (e.g., Dacron) or PTFE (45).
In cases where the visceral and renal arteries are involved, aortic stent grafts with fenestrations or branches are used to preserve flow (32,46). These fenestrations or branches are then covered with a bridging stent to create a seal.
Open repair
Endovascular repair has largely replaced open due to multiple trials demonstrating perioperative and 30-day improvements in mortality and morbidity. Indications for open repair include unfavorable anatomy (suprarenal/juxtarenal AAA), excessive vessel tortuosity, small caliber vessels, horseshoe kidney, and inability to comply with post EVAR surveillance. However, given the prospective cumulative radiation dose needed for lifelong surveillance after endovascular repair, open repair can be advocated in younger patients under 65 (47). Grafts include polyester (e.g., Dacron), PTFE and autogenous vein.
Normal CT postoperative appearance following open repair
The aortic repair graft can often appear indistinguishable from the native aortic wall on postcontrast imaging. However, the precontrast phase often demonstrates a slightly hyperattenuating aortic graft. In inclusion grafts, there is often peripheral thrombosis of the excluded perigraft space. In the immediate postoperative study, homogeneous, low-density perigraft fluid is a common finding and represents postoperative seroma (48). This fluid can persist for up to a year following repair before slowly shrinking. Other causes of low attenuation material surrounding the graft include granulation tissue, omentum or bovine pericardium (49).
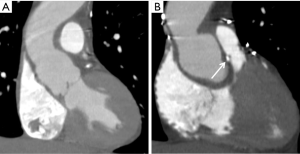
A small amount of air can be seen in the perigraft space in the immediate postoperative study. One study demonstrated resolution of periprosthetic air within 3 months (50). In the absence of fever, laboratory abnormalities, or clinical symptoms, even a large amount of air surrounding the graft can be normal (Figure 4). However, a superimposed infection can be difficult to exclude based on imaging appearance alone, and should be raised in the appropriate clinical context (50).
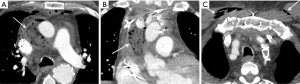
Aortic grafts can be covered by strips of felt or felt pledgets can be used to for structural support. Felt is hyperdense on CT and can mimic the appearance of contrast extravasation; noncontrast images are invaluable to exclude contrast leaks or pseudoaneurysm.
Graft-related complications of open repair
There is no formal consensus on the timing of imaging follow-up for open repair of the thoracic or abdominal aorta. Late complications are infrequently and inconsistently reported due to lack of routine follow-up.
Anastomotic pseudoaneurysm
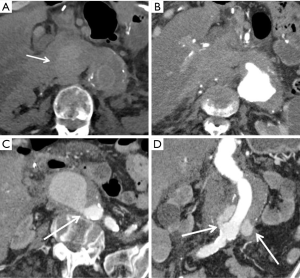
The incidence of anastomotic pseudoaneurysms (Figure 5) is not definitively known due to the absence of routine postoperative imaging. However, in AAA repair, anastomotic pseudoaneurysm represents the most commonly reported late graft complication, with reported incidence ranging from 4.6% to 9.4% of AAA repair (51,52). Infection is an important cause of pseudoaneurysm that can lead to further erosion, thrombosis or rupture. These are typically treated with re-do conventional open repair, although endovascular stenting is an option in suitable candidates (53). However, graft infection is a common complication of redo endovascular repair.
Graft occlusion
Occlusion of the aortic graft remains a rare complication. Conrad et al. demonstrated four graft occlusions out of 152 open repairs (51). The Dutch Randomized Endovascular Aneurysm Repair (DREAM) trial demonstrated only three occlusions of 178 open repairs (54). Thrombus appears as low-density material within a graft limb, with lack of opacification on delayed imaging. Once identified, graft thrombosis can be treated endovascularly with a thrombectomy catheter.
Graft dehiscence
Contrast extravasation at the anastomosis is pathognomonic of graft dehiscence and can be identified even years after the surgery (Figure 6). One study found the average time interval of dehiscence at over 5 years following surgery (55). Early detection is crucial to discontinue anticoagulation, trigger evaluation by the surgeon and subsequent graft repair. The operative mortality of re-do surgeries exceeds the primary surgery and ranges from 13% to 41% (56).
Graft infection
Infection of the graft is a significant complication that should be raised in the event fever, elevated white blood cell count and elevated inflammatory markers. On CT, graft infection will appear as new fat stranding of hypodense collections surrounding the graft. If untreated, graft infections can lead to pseudoaneurysm or rupture. The incidence of graft infection after open surgery ranges from 0.2% to 2% (53,57). The presence of air in the excluded perigraft space should raise concern gas forming organism. Treatment requires removal of the infected graft material.
Aortoenteric fistula
Upper gastroesophageal bleeding and shock should prompt workup for possible aortoenteric fistula with CTA and endoscopy. The incidence of aortoenteric fistula ranges from 0.3% to 2.5% (58,59) and can be identified by gas in the excluded aneurysm sac. In contrast to infection, tethering of adjacent bowel should raise concern for fistulation. Mortality of aortoenteric fistulas approaches 50% (60) and can be treated either by open or endovascular repair.
General complications of endovascular repair
Endoleak
The primary objective of postoperative surveillance after endovascular repair is for the detection of endoleak, which is reported to occur in approximately 20–50% of patients. Endoleak is defined as persistent blood flow within the excluded aneurysm sac, which is detected on CTA as contrast opacification, often on delayed imaging (44). Endoleak can occur at any time point, from intraoperatively to years following the procedure. As it is often asymptomatic, failure to detect an endoleak can lead to progressive aneurysm expansion and rupture. Thus, lifelong imaging surveillance is mandatory.
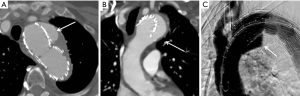
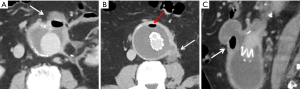
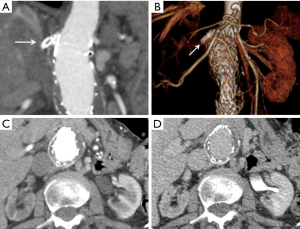
- A type I endoleak (Figure 7) refers to incompetent seal at the proximal (1a) or distal (1b) sites, and are the most concerning type. Type I endoleaks are repaired upon discovery as they do not typically resolve spontaneously. Additional grafts may need to be placed to ensure adequate seal. They are the second most common endoleak (approximately 10%);
- Type II endoleaks (Figure 8) represent the vast majority (over 50% and up to 80% of reported cases) and result from patent collateral inflow. In the chest, they can occur from intercostal arteries or backflow from the left subclavian artery, and in the abdomen, they occur from lumbar, accessory renal or inferior mesenteric artery collaterals. On CTA, delayed imaging is required to assess for the presence of type II endoleak. Once detected, typically observation and continued surveillance is recommended as these often spontaneously resolve. Intervention is indicated for sac expansion >5 mm, and includes embolization through transarterial or translumbar approaches (61);
- Type III endoleaks (Figure 9) are rare with improvements in newer stent grafts but are defined by leaks in the junction or defects of the stent fabric. Like type I endoleaks, the type III endoleaks require treatment with additional stent graft components to bridge the defect;
- Type IV endoleaks are self-limited and occur with porosity of the stent graft. They do not require treatment and typically resolve within days of graft placement. Type IV endoleaks are important to recognize as they can obscure a type I or III endoleak;
- Type V endoleaks, also known as endotension, refer to continued aneurysmal sac expansion without demonstrated leak. This phenomenon is poorly understood.
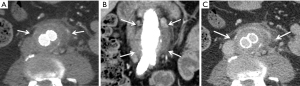
A unique mimic of endoleak following endovascular repair occurs with Endologix stent grafts due to their construction, where the metallic endoskeleton is located within the graft cover. A rim of contrast outside the endoskeleton can mimic a type III endoleak but still remained well contained in the graft, a phenomenon known as “billowing.” There is no apparent association with increased morbidity, mortality or incidence of endoleak with billowing of Endologix stent grafts (62).
Stent migration and kinking
In addition to endoleaks, CTA is important for detection of other immediate and delayed complications. Caudal migration of the stent graft by more than 10 mm can occur due to excessive over-sizing or tortuous seal zone anatomy. One study demonstrated >10 mm of stent migration in 2.8% of patients after TEVAR (63). Device collapse or kinking can occur and often present with acute aortic occlusion (60).
Aneurysm sac growth
The ultimate goal of surveillance imaging is to detect early aneurysm sac growth after endovascular repair. Makaroun et al. found that after TEVAR, there was a 19% rate of aneurysm sac growth after 5 years (64). For EVAR, Hogg et al. similarly found a 21% rate of aneurysm sac growth after 5 years (65). Aneurysm sac growth necessitates a search for occult endoleak and may lead to surgical intervention. Failure to properly follow up in these cases can lead to further sac growth and eventual rupture.
Infection
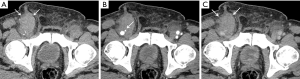
The imaging findings of infection after endovascular graft repair are similar to the previously discussed CT findings after open repair (Figure 10). The DREAM trial noted a 0.6% rate of infections after endovascular repair (54). Foci of gas bubbles in the aneurysm sac are often suggestive of gas forming organism (Figure 11).
Visceral organ ischemia
Other complications to note include end organ ischemia from the graft seal. Bowel ischemia is an important early complication from coverage of the visceral arteries and poor collateral flow (Figure 12). Bowel wall thickening with associated fat stranding in a vascular distribution in the postoperative setting is a strong indicator for bowel ischemia. Ultee et al. noted a 1.6% incidence of bowel ischemia following elective repair and 15.2% rate after ruptured repair (66). Other solid organs such as the kidneys and spleen can demonstrate infarction related to ischemia from stent coverage (Figure 13). If the stent graft extends to or above the level of the renal arteries, care must be taken to ensure adequate perfusion to the kidneys in the postoperative setting. Renal artery ischemia can occur either from stent coverage of the renal arteries or embolization of cholesterol plaques (67).
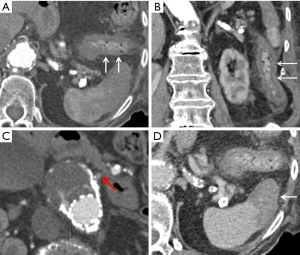
Femoral artery injury
Other important complications to note involve anatomy not directly involving the graft itself. These include evaluation of groin hematomas (68) and pseudoaneurysms at the femoral access sites for sources of bleeding (Figure 14). Unidentified hematomas are also at risk for superinfection.
Conclusions
Knowledge of increasingly complex normal appearances of the postoperative aorta is essential for detection of complications and avoidance of imaging pitfalls. As further advances are made in surgical techniques and graft prostheses, reliable imaging will become increasingly important in the future.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Roos JE, Willmann JK, Weishaupt D, et al. Thoracic aorta: motion artifact reduction with retrospective and prospective electrocardiography-assisted multi-detector row CT. Radiology 2002;222:271-7. [Crossref] [PubMed]
- McCollough CH, Leng S, Yu L, et al. Dual- and Multi-Energy CT: Principles, Technical Approaches, and Clinical Applications. Radiology 2015;276:637-53. [Crossref] [PubMed]
- Takehara Y, Yamashita S, Sakahara H, et al. Magnetic Resonance Angiography of the Aorta. Ann Vasc Dis 2011;4:271-85. [Crossref] [PubMed]
- Oliveira IS, Hedgire SS, Li W, et al. Blood pool contrast agents for venous magnetic resonance imaging. Cardiovasc Diagn Ther 2016;6:508-18. [Crossref] [PubMed]
- Sun Z. Diagnostic value of color duplex ultrasonography in the follow-up of endovascular repair of abdominal aortic aneurysm. J Vasc Interv Radiol 2006;17:759-64. [Crossref] [PubMed]
- Celikyay ZR, Koner AE, Celikyay F, et al. Frequency and imaging findings of variations in human aortic arch anatomy based on multidetector computed tomography data. Clin Imaging 2013;37:1011-9. [Crossref] [PubMed]
- Layton KF, Kallmes DF, Cloft HJ, et al. Bovine aortic arch variant in humans: clarification of a common misnomer. AJNR Am J Neuroradiol 2006;27:1541-2. [PubMed]
- Bryce Y, Rogoff P, Romanelli D, et al. Endovascular Repair of Abdominal Aortic Aneurysms: Vascular Anatomy, Device Selection, Procedure, and Procedure-specific Complications. RadioGraphics 2015;35:593-615. [Crossref] [PubMed]
- Fillinger MF, Greenberg RK, McKinsey JF, et al. Reporting standards for thoracic endovascular aortic repair (TEVAR). J Vasc Surg 2010;52:1022-33, 1033.e15.
- Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation 2010;121:e266-369. [Crossref] [PubMed]
- Hanneman K, Chan FP, Mitchell RS, et al. Pre- and Postoperative Imaging of the Aortic Root. RadioGraphics 2016;36:19-37. [Crossref] [PubMed]
- Fattori R, Cao P, De Rango P, et al. Interdisciplinary expert consensus document on management of type B aortic dissection. J Am Coll Cardiol 2013;61:1661-78. [Crossref] [PubMed]
- Hagan PG, Nienaber CA, Isselbacher EM, et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA 2000;283:897-903. [Crossref] [PubMed]
- Prescott-Focht JA, Martinez-Jimenez S, Hurwitz LM, et al. Ascending Thoracic Aorta: Postoperative Imaging Evaluation. RadioGraphics 2013;33:73-85. [Crossref] [PubMed]
- Latson LA Jr, DeAnda A Jr, Ko JP. Imaging of the Postsurgical Thoracic Aorta: A State-of-the-Art Review. J Thorac Imaging 2017;32:1-25. [Crossref] [PubMed]
- Wheat MW Jr, Wilson JR, Bartley TD. Successful Replacement of The Entire Ascending Aorta and Aortic Valve. JAMA 1964;188:717-9. [Crossref] [PubMed]
- Yoda M, Nonoyama M, Shimakura T, et al. Surgical case of aortic root and thoracic aortic aneurysm after the Wheat procedure. Ann Thorac Cardiovasc Surg 2002;8:115-8. [PubMed]
- Bentall H, De Bono A. A technique for complete replacement of the ascending aorta. Thorax 1968;23:338-9. [Crossref] [PubMed]
- Cabrol C, Pavie A, Gandjbakhch I, et al. Complete replacement of the ascending aorta with reimplantation of the coronary arteries: new surgical approach. J Thorac Cardiovasc Surg 1981;81:309-15. [PubMed]
- Gelsomino S, Frassani R, Da Col P, et al. A long-term experience with the cabrol root replacement technique for the management of ascending aortic aneurysms and dissections. Ann Thorac Surg 2003;75:126-31. [Crossref] [PubMed]
- Sarsam MA, Yacoub M. Remodeling of the aortic valve anulus. J Thorac Cardiovasc Surg 1993;105:435-8. [PubMed]
- Yacoub MH, Gehle P, Chandrasekaran V, et al. Late results of a valve-preserving operation in patients with aneurysms of the ascending aorta and root. J Thorac Cardiovasc Surg 1998;115:1080-90. [Crossref] [PubMed]
- David TE, Feindel CM. An aortic valve-sparing operation for patients with aortic incompetence and aneurysm of the ascending aorta. J Thorac Cardiovasc Surg 1992;103:617-21; discussion 622. [PubMed]
- Demers P, Miller DC. Simple modification of "T. David-V" valve-sparing aortic root replacement to create graft pseudosinuses. Ann Thorac Surg 2004;78:1479-81. [Crossref] [PubMed]
- Ross DN. Replacement of aortic and mitral valves with a pulmonary autograft. Lancet 1967;2:956-8. [Crossref] [PubMed]
- Kourliouros A, Soni M, Rasoli S, et al. Evolution and current applications of the Cabrol procedure and its modifications. Ann Thorac Surg 2011;91:1636-41. [Crossref] [PubMed]
- Blum M, Panos A, Lichtenstein SV, et al. Modified Cabrol shunt for control of hemorrhage in repair of type A dissection of the aorta. Ann Thorac Surg 1989;48:709-11. [Crossref] [PubMed]
- Hino Y, Okada K, Oka T, et al. Extended replacement of the thoracic aorta. Eur J Cardiothorac Surg 2013;43:176-81; discussion 181. [Crossref] [PubMed]
- Castrovinci S, Murana G, de Maat GE, et al. The classic elephant trunk technique for staged thoracic and thoracoabdominal aortic repair: Long-term results. J Thorac Cardiovasc Surg 2015;149:416-22. [Crossref] [PubMed]
- Leontyev S, Borger MA, Etz CD, et al. Experience with the conventional and frozen elephant trunk techniques: a single-centre study. Eur J Cardiothorac Surg 2013;44:1076-82; discussion 1083. [Crossref] [PubMed]
- Folkmann S, Weiss G, Pisarik H, et al. Thoracoabdominal aortic aneurysm repair after frozen elephant trunk procedure. Eur J Cardiothorac Surg 2015;47:115-9; discussion 119. [Crossref] [PubMed]
- Greenberg RK, Sternbergh WC 3rd, Makaroun M, et al. Intermediate results of a United States multicenter trial of fenestrated endograft repair for juxtarenal abdominal aortic aneurysms. J Vasc Surg 2009;50:730-7.e1. [Crossref] [PubMed]
- Makaroun MS, Dillavou ED, Kee ST, et al. Endovascular treatment of thoracic aortic aneurysms: results of the phase II multicenter trial of the GORE TAG thoracic endoprosthesis. J Vasc Surg 2005;41:1-9. [Crossref] [PubMed]
- Gawenda M, Brunkwall J. Comparison of CE approved TEVAR devices. J Cardiovasc Surg (Torino) 2010;51:157-68. [PubMed]
- Godoy MC, Cayne NS, Ko JP. Endovascular repair of the thoracic aorta: preoperative and postoperative evaluation with multidetector computed tomography. J Thorac Imaging 2011;26:63-73. [Crossref] [PubMed]
- Hajibandeh S, Hajibandeh S, Antoniou SA, et al. Meta-analysis of Left Subclavian Artery Coverage With and Without Revascularization in Thoracic Endovascular Aortic Repair. J Endovasc Ther 2016;23:634-41. [Crossref] [PubMed]
- Rehman SM, Vecht JA, Perera R, et al. How to manage the left subclavian artery during endovascular stenting of the thoracic aorta. Eur J Cardiothorac Surg 2011;39:507-18. [Crossref] [PubMed]
- Ricco JB, Forbes TL. Trans-atlantic debate: the role of completion imaging following carotid artery endarterectomy. Eur J Vasc Endovasc Surg 2013;45:423. [Crossref] [PubMed]
- Dubost C, Allary M, Oeconomos N. Resection of an aneurysm of the abdominal aorta: reestablishment of the continuity by a preserved human arterial graft, with result after five months. AMA Arch Surg 1952;64:405-8. [Crossref] [PubMed]
- Kent KC. Clinical practice. Abdominal aortic aneurysms. N Engl J Med 2014;371:2101-8. [Crossref] [PubMed]
- Guirguis-Blake JM, Beil TL, Senger CA, et al. Ultrasonography screening for abdominal aortic aneurysms: A systematic evidence review for the u.s. preventive services task force. Ann Intern Med 2014;160:321-9. [Crossref] [PubMed]
- Zucker EJ, Misono AS, Prabhakar AM. Abdominal Aortic Aneurysm Screening Practices: Impact of the 2014 U.S. Preventive Services Task Force Recommendations. J Am Coll Radiol 2017;14:868-74. [Crossref] [PubMed]
- Dua A, Kuy S, Lee CJ, et al. Epidemiology of aortic aneurysm repair in the United States from 2000 to 2010. J Vasc Surg 2014;59:1512-7. [Crossref] [PubMed]
- Chaikof EL, Blankensteijn JD, Harris PL, et al. Reporting standards for endovascular aortic aneurysm repair. J Vasc Surg 2002;35:1048-60. [Crossref] [PubMed]
- Brown LC, Greenhalgh RM, Kwong GP, et al. Secondary interventions and mortality following endovascular aortic aneurysm repair: device-specific results from the UK EVAR trials. Eur J Vasc Endovasc Surg 2007;34:281-90. [Crossref] [PubMed]
- Ohrlander T, Sonesson B, Ivancev K, et al. The chimney graft: a technique for preserving or rescuing aortic branch vessels in stent-graft sealing zones. J Endovasc Ther 2008;15:427-32. [Crossref] [PubMed]
- Vallabhaneni R, Farber MA, Schneider F, et al. Debate: whether young, good-risk patients should be treated with endovascular abdominal aortic aneurysm repair. J Vasc Surg 2013;58:1709-15. [Crossref] [PubMed]
- England A, Mc Williams R. Endovascular Aortic Aneurysm Repair (EVAR). Ulster Med J 2013;82:3-10. [PubMed]
- Cao P, De Rango P, Verzini F, et al. Endoleak after endovascular aortic repair: classification, diagnosis and management following endovascular thoracic and abdominal aortic repair. J Cardiovasc Surg (Torino) 2010;51:53-69. [PubMed]
- O'Hara PJ, Borkowski GP, Hertzer NR, et al. Natural history of periprosthetic air on computerized axial tomographic examination of the abdomen following abdominal aortic aneurysm repair. J Vasc Surg 1984;1:429-33. [Crossref] [PubMed]
- Conrad MF, Crawford RS, Pedraza JD, et al. Long-term durability of open abdominal aortic aneurysm repair. J Vasc Surg 2007;46:669-75. [Crossref] [PubMed]
- Hallett JW Jr, Marshall DM, Petterson TM, et al. Graft-related complications after abdominal aortic aneurysm repair: reassurance from a 36-year population-based experience. J Vasc Surg 1997;25:277-84; discussion 285-6. [Crossref] [PubMed]
- O'Connor DJ, Vouyouka A, Ellozy SH, et al. Stent Graft Repair of Para-Anastomotic Aneurysms Following Open Descending Thoracic and Thoracoabdominal Aortic Aneurysm Repair. Ann Vasc Surg 2013;27:693-8. [Crossref] [PubMed]
- De Bruin JL, Baas AF, Buth J, et al. Long-Term Outcome of Open or Endovascular Repair of Abdominal Aortic Aneurysm. N Engl J Med 2010;362:1881-9. [Crossref] [PubMed]
- Olsson C, Thelin S, Stahle E, et al. Thoracic aortic aneurysm and dissection: increasing prevalence and improved outcomes reported in a nationwide population-based study of more than 14,000 cases from 1987 to 2002. Circulation 2006;114:2611-8. [Crossref] [PubMed]
- Mohammadi S, Bonnet N, Leprince P, et al. Reoperation for false aneurysm of the ascending aorta after its prosthetic replacement: surgical strategy. Ann Thorac Surg 2005;79:147-52; discussion 152. [Crossref] [PubMed]
- Hertzer NR, Mascha EJ, Karafa MT, et al. Open infrarenal abdominal aortic aneurysm repair: the Cleveland Clinic experience from 1989 to 1998. J Vasc Surg 2002;35:1145-54. [Crossref] [PubMed]
- Plate G, Hollier LA, O'Brien P, et al. Recurrent aneurysms and late vascular complications following repair of abdominal aortic aneurysms. Arch Surg 1985;120:590-4. [Crossref] [PubMed]
- Vu QD, Menias CO, Bhalla S, et al. Aortoenteric Fistulas: CT Features and Potential Mimics. Radiographics 2009;29:197-209. [Crossref] [PubMed]
- Canaud L, Marty-Ane C, Ziza V, et al. Minimum 10-year follow-up of endovascular repair for acute traumatic transection of the thoracic aorta. J Thorac Cardiovasc Surg 2015;149:825-9. [Crossref] [PubMed]
- Brown A, Saggu GK, Bown MJ, et al. Type II endoleaks: challenges and solutions. Vasc Health Risk Manag 2016;12:53-63. [PubMed]
- Helo N, Chang AC, Hyun C, et al. Retrospective Review of Billowing Phenomenon-A Mimic of Endoleak Following Placement of Endologix Covered Stent for the Treatment of Abdominal Aortic Aneurysm. Ann Vasc Surg 2017;45:239-46. [Crossref] [PubMed]
- Matsumura JS, Cambria RP, Dake MD, et al. International controlled clinical trial of thoracic endovascular aneurysm repair with the Zenith TX2 endovascular graft: 1-year results. J Vasc Surg 2008;47:247-57; discussion 257. [Crossref] [PubMed]
- Makaroun MS, Dillavou ED, Wheatley GH, et al. Five-year results of endovascular treatment with the Gore TAG device compared with open repair of thoracic aortic aneurysms. J Vasc Surg 2008;47:912-8. [Crossref] [PubMed]
- Hogg ME, Morasch MD, Park T, et al. Long-term sac behavior after endovascular abdominal aortic aneurysm repair with the Excluder low-permeability endoprosthesis. J Vasc Surg 2011;53:1178-83. [Crossref] [PubMed]
- Ultee KH, Zettervall SL, Soden PA, et al. Incidence of and risk factors for bowel ischemia after abdominal aortic aneurysm repair. J Vasc Surg 2016;64:1384-91. [Crossref] [PubMed]
- Liewald F, Scharrer-Pamler R, Gorich J, et al. Intraoperative, perioperative and late complications with endovascular therapy of aortic aneurysm. Eur J Vasc Endovasc Surg 2001;22:251-6. [Crossref] [PubMed]
- Liaw JV, Clark M, Gibbs R, et al. Update: Complications and management of infrarenal EVAR. Eur J Radiol 2009;71:541-51. [Crossref] [PubMed]


