Pulmonary vascular pathophysiology
Introduction
Pulmonary physiology is a complex process where in multiple dynamic processes are at interplay to achieve the function of an adaptable right heart circulation and provide an efficient gaseous exchange. In this section, we will review some of the basic concepts of pulmonary pathophysiology with emphasis on common diseases.
Pulmonary vascular anatomy
The pulmonary trunk divides into right and left main pulmonary arteries which subsequently branch into large elastic arteries (diameter >1,000 µm), muscular medium arteries, small arterioles (diameter <100 µm) and capillaries. A large number of functional pulmonary capillaries provide an extensive network of channels communicating with the alveoli for an efficient gaseous exchange. The pulmonary venules join to form the superior and inferior pulmonary veins (typically four) which carry the oxygenated blood to the left atrium. Bronchial arteries [2% of cardiac output (CO)] provide collateral oxygenated blood supply to the tracheobronchial tree, and bronchial veins drain into azygous and pulmonary venous system. Some of the pulmonary vascular facts are presented in Table 1 (1).
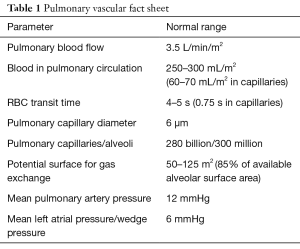
Full table
Pulmonary circulation
Right heart output is similar to left heart output (in series) and pulmonary blood flow (PBF) is approximately 3.5 L/min/m2. However, there are significant differences between the right and left sided circulations (Table 2) (1). Systemic and pulmonary pressures are illustrated in Figure 1. Pulmonary circulation is characterized by thin walled, distensible, large diameter vessels without predominant muscular layer resulting in low resistance and low pressures. Resistance is defined as the pressure difference divided by blood flow (Poiseuille’s law), and is the key to understand the pathophysiology. Therefore, pulmonary vascular resistance (PVR) is the difference between the mean pulmonary arterial pressure (mPAP/P1) and mean left atrial pressure (mLAP/P2) divided by the PBF (PBF/Q), i.e., PVR = P1−P2/Q. PVR is directly proportional to mPAP and inversely proportional to mLAP and blood flow. This is an important concept since increase in PBF results in decreased PVR to maintain the mPAP, and similarly decreased PBF results in increased PVR. Parameters that determine PVR include passive factors such as gravity, position of the body, transmural pressure difference, lung volume, and CO and active factors such as hormonal-catecholamines, histamine, prostaglandins, nitric oxide, isoproterenol, hypoxia and hypercapnia, and autonomic nervous system for vessels >30 microns (1). Lung volume changes during inspiration and expiration result in opposite changes in alveolar and extra-alveolar vessels. High lung volumes result in a stretching/elongation of the alveolar vessels resulting in an increased PVR whereas the extra-alveolar vessels are subject to radial traction and negative pleural pressure resulting in a decreased PVR. The converse is true at low lung volumes. Alveolar and extra-alveolar PVRs are added together to result in a cumulative pulmonary vascular PVR at a specific lung volume, which is low at the functional residual capacity (FRC) and high at both residual volume (RV) and total lung capacity (TLC) thereby resulting in a U shaped cumulative PVR curve (2). Mechanical ventilation with positive end expiratory pressure (PEEP) results in increased alveolar pressure as well as intrapleural pressure, and therefore increases PVR at both alveolar and extra-alveolar levels throughout the respiratory cycle (Zone 1). Additionally, an increased intrapleural pressure throughout the respiratory cycle can compress the intrathoracic vessels and inferior vena cava (IVC) resulting in decreased preload and CO. When CO increases secondary to exercise, the mPAP is only minimally increased because of decreased PVR from additional recruitment (with small increase in CO) and distension (with large increase in CO) of pulmonary vessels. PBF is more to the gravity dependent portions of the lungs with a high intravascular pressure and low resistance secondary to the additional recruitment/distension process (Zone 3, Pa > Pv > PA; a, arterial; v, venous; and A, alveolar pressures, respectively). This PBF gradient from upper to lower lung lobes is reversed in cardiogenic edema, especially secondary to mitral stenosis.

Full table

Pathophysiology and clinical correlation
Exercise
Pulmonary arterial pressure demonstrates only slight increase with exercise in normal individuals secondary to decrease in PVR from additional arterial recruitment and arterial distension (3). Pulmonary rehabilitation programs steadily improve the exercise capacity in patients with pulmonary arterial hypertension (PAH) limiting sudden increase in pulmonary arterial pressure.
High altitude/hypoventilation
Generalized hypoxic vasoconstriction secondary to decreased PO2 results in increased arterial pressure and recruitment/distension of alveolar capillaries for better gaseous exchange. However, increased pulmonary arterial pressure also increases capillary hydrostatic pressure and thereby resulting in pulmonary edema. Increased PVR and right heart work load can present as right heart failure (4,5).
This phenomenon of generalized hypoxic vasoconstriction is critical for in-utero and new born pulmonary vascular hemodynamics and ventilation (2).
Airway obstruction or atelectasis
Decreased PO2 (possible contributions from low pH and increased PCO2) results in a not so robust hypoxic precapillary vasoconstriction and locally increased PVR (at the level of small arteries and arterioles) with shunting of blood to better ventilated areas. This phenomenon results in the low and very low probability ventilation-perfusion (VQ) scans (matched VQ defects and triple-match VQ scan with corresponding radiographic abnormality).
Pulmonary arterial hypertension (PAH)
A mPAP >25 mmHg is defined as PAH, and can be primary from pulmonary vasculature involvement or secondary to lung disease. Increased pulmonary arterial pressure could be due to increased PVR, left atrial pressure (pulmonary venous hypertension) or PBF (6). Pulmonary arterial pressure measurement is performed with a catheter positioned within the pulmonary artery via internal jugular or right femoral vein approach. Vasoconstriction, vascular remodeling, thrombosis and wall stiffness contribute to the multifactorial pathophysiology of PAH. Hypoxia should be avoided (for example, air travel) due to concern for hypoxemic pulmonary vasoconstriction and sudden increase in PVR resulting in acute right heart failure. Calcium channel blockers, endothelin receptor antagonists, phosphodiesterase inhibitors, and prostanoid treatments decrease the PVR and PAH, forming the main stay of medical treatment (7-9). The number of Tc-99m macro-aggregated albumin (MAA) particles in a perfusion study for patients with PAH should be reduced to 100–200 k. Contrast injection rate should also be decreased in patients with elevated pulmonary arterial pressures to decrease the risk of acute right heart failure, and a low pressure hand injection of contrast material is an alternative option.
Pulmonary edema
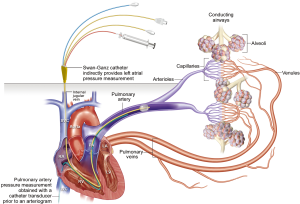
Fluid dynamics and movement can be explained based on Starling’s equation with capillary hydrostatic (10 mmHg) and interstitial osmotic pressure (20 mmHg) working in extravascular direction whereas the interstitial hydrostatic (−5 to −7 mmHg) and plasma osmotic pressure (25–28 mmHg) facilitate intravascular movement (1,6). Increased capillary hydrostatic pressure is seen with causes of left heart failure or increased preload/hypervolemia. mLAP readings are obtained by wedging a Swan-Ganz balloon catheter within the segmental pulmonary artery. Balloon inflation eliminates the contribution of pulmonary artery pressure (forward flow), and reflects the left atrial pressure (backward pressure). The technique for measurement of left atrial pressure is illustrated in Figure 2. mLAP measurements are helpful to estimate the severity of left heart failure and can guide to maintain optimal pulmonary capillary pressures.
Re-expansion pulmonary edema after rapid evacuation of pneumothorax or pleural fluid is secondary to decreased interstitial hydrostatic pressure and is usually self-limiting. Colloid pressure is decreased in patients with malnutrition or renal disease resulting in inability to maintain intravascular volume. Additional etiologies include capillary permeability [acute respiratory distress syndrome (ARDS), toxins] and inadequate lymphatic drainage (malignancy and interstitial disease) (10). Edema initially occurs within the interstitium and later involves the alveoli, impeding gaseous exchange.
Pulmonary embolism
Pulmonary embolism is secondary to venous thromboembolic disease from venous stasis, endothelial injury, and hypercoagulable state (Virchow’s triad). Lower lobes are frequently involved with pulmonary thromboembolic disease given the preferential zonal PBF. The underlying pathophysiology depends on the size and location of the clot and preexisting cardiopulmonary status (6,11,12). A massive central thrombus can result in significantly increased PAP and right ventricular workload with acute right heart failure. Right ventricular dilatation and flattening/bowing of the interventricular septum indicate right heart strain, and can result in decreased CO and left ventricular failure. Medium sized emboli result in loss of perfusion to the affected segment with preservation of ventilation resulting in a mismatched defect on VQ scan and symptoms of hypoxemia. Small and chronic emboli result in gradual increase of PVR and PAH. Small thromboembolic burden can also result in hemodynamic decompensation in patients with compromised pulmonary reserve from underlying cardiopulmonary disease. Recanalization and pathophysiologic changes in both untreated and treated pulmonary embolism (PE) are complex processes and beyond the scope of current discussion (11,13).
Fat embolism is usually secondary to a long bone fracture with presence of fat globules within the pulmonary circulation (mechanical and biochemical mechanisms) resulting in respiratory failure, neurological symptoms (micro-emboli or paradoxical emboli), and petechial rash. Venous air embolism is usually secondary to a known risk factor and results in increased pulmonary and right ventricular pressures or an air lock with circulatory collapse. Left lateral decubitus and Trendelenburg positioning is important to prevent further air embolization and related complications.
Pulmonary arteriovenous malformation (PAVM)
PAVMs (aka pulmonary arteriovenous fistulae) are frequently seen in patients with hereditary hemorrhagic telangiectasia, and include an arterial feeder, aneurysmal sac and a draining vein without the normal intervening capillary network. Patients can be asymptomatic or present with symptoms of right to left shunting such as hypoxemia, fatigue, systemic embolism and brain abscess/stroke. PAH can be seen concomitantly or after embolization of low resistance PAVMs.
Intrapulmonary vascular dilatations in hepatopulmonary syndrome result in hypoxemia secondary to ventilation perfusion mismatch, alveolar-capillary oxygen disequilibrium, and rarely anatomic shunts (14).
Others
Pulmonary vasculitis is characterized by inflammation, destruction and necrosis. The differential is broad and multiple organs are involved. Recognition of post-surgical vascular complications including pseudo aneurysm, thrombosis, fistula, and anastomotic stenosis requires close clinical monitoring and a high degree of suspicion. These topics would be discussed in detail within the appropriate sections of this focused issue on pulmonary vascular disease.
Conclusions
Changes in PVR, pressure and blood flow predominantly determine the physiologic response to various respiratory and metabolic demands as well as contribute to pathological disease states. A thorough understanding of the pathophysiology is essential for all those involved in the multidisciplinary management of pulmonary diseases.
Acknowledgements
The authors would like to thank Erin Moore, M.A., Medical Illustrator, Sr. Graphic Designer, University of Texas Southwestern Medical Center, Dallas, Texas.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Levitzky MG. Pulmonary physiology. 8th edition. New York: McGraw Hill Education, 2013.
- Weinberger SE, Cockrill BA, Mandel J. 6th edition. Principles of pulmonary medicine. Saunders, 2013.
- Waxman AB. Exercise physiology and pulmonary arterial hypertension. Prog Cardiovasc Dis 2012;55:172-9. [Crossref] [PubMed]
- Watanabe S. Pathophysiology of hypoxaemic pulmonary vascular diseases. Bull Eur Physiopathol Respir 1987;23 Suppl 11:207s-9s. [PubMed]
- Wilkins MR, Ghofrani HA, Weissmann N, et al. Pathophysiology and treatment of high-altitude pulmonary vascular disease. Circulation 2015;131:582-90. [Crossref] [PubMed]
- West JB, Luks AM. West's Pulmonary Pathophysiology. 9th edition. LWW, 2017.
- Patel D, Lakhkar A, Wolin MS. Redox Mechanisms Influencing cGMP Signaling in Pulmonary Vascular Physiology and Pathophysiology. Adv Exp Med Biol 2017;967:227-40. [Crossref] [PubMed]
- Taguchi K, Hattori Y. Unlooked-for significance of cardiac versus vascular effects of endothelin-1 in the pathophysiology of pulmonary arterial hypertension. Circ Res 2013;112:227-9. [Crossref] [PubMed]
- Wolin MS, Gupte SA, Mingone CJ, et al. Redox regulation of responses to hypoxia and NO-cGMP signaling in pulmonary vascular pathophysiology. Ann N Y Acad Sci 2010;1203:126-32. [Crossref] [PubMed]
- Kobayashi K. Pathophysiology of adult respiratory distress syndrome-- with special reference to pulmonary vascular structure and hemodynamics. Kokyu To Junkan 1981;29:495-501. [PubMed]
- Elliott CG. Pulmonary physiology during pulmonary embolism. Chest 1992;101:163S-71S. [Crossref] [PubMed]
- Nelson JR, Smith JR. The pathologic physiology of pulmonary embolism. A physiologic discussion of the vascular reactions following pulmonary arterial obstruction by emboli of varying size. Am Heart J 1959;58:916-32. [Crossref] [PubMed]
- Melot C, Naeije R. Pulmonary vascular diseases. Compr Physiol 2011;1:593-619. [PubMed]
- Krowka MJ, Fallon MB, Kawut SM, et al. International Liver Transplant Society Practice Guidelines: Diagnosis and Management of Hepatopulmonary Syndrome and Portopulmonary Hypertension. Transplantation 2016;100:1440-52. [Crossref] [PubMed]


