Left ventricular energy model predicts adverse events in women with suspected myocardial ischemia: results from the NHLBI-sponsored women’s ischemia syndrome evaluation (WISE) study
Introduction
In the United States ischemic heart disease (IHD) related mortality rates for women are higher than rates for men despite efforts to ensure gender-appropriate application of diagnostic and therapeutic cardiovascular disease strategies (1,2). To address this gap, gender-specific optimization of risk stratification strategies for IHD in women may be required. The Women’s Ischemia Syndrome Evaluation (WISE) study was initiated to examine IHD in women (3). Myocardial perfusion imaging (MPI) status using Single Photon Emission Computer Tomography (SPECT) has been extensively studied. More recently magnetic resonance imaging (MRI) has increased our understanding of the influence of global myocardial perfusion on survival (4). The additional capabilities of MRI and gated-SPECT to evaluate left ventricular (LV) function also provides additional prognostic value compared to knowledge of myocardial perfusion status alone (5). However, the measurement of LV function is confounded by the influence of vascular loading conditions, which cannot be assessed using non-invasive imaging approaches.
Methodologies to determine the load-independent contractile status of the LV were pioneered by Frank and, independently, Starling in the 1890’s (6). Over half a century later, Suga and Sagawa developed the end-systolic pressure-volume relationship (ESPVR), which was initially proposed as a preload independent measurement of the maximal myocardial elastance, Emax (7). However, routine use of the ESPVR is impractical since it requires subjecting the cardiovascular system to a series of stresses with simultaneous measurement of intraventricular blood pressures and volumes. Recently, Shoucri extended the ESPVR framework and introduced a model of the LV describing its energy utilization and efficiency (8). The relationships between key energy model variables were validated in vivo by Devereux et al. using echocardiographic data (9,10). The model incorporates vascular loading conditions and can be used to evaluate LV energy expenditure, distinguishing between internal and external work performed. While the concept of external work performed by the heart is well developed, and is incorporated in variables such as the cardiac work index and stroke work, the concept of internal energy utilization is less appreciated and not widely used when assessing cardiovascular health.
We hypothesize that the recently developed LV energy model provides variables describing LV function and internal energy utilization that have higher prognostic value than conventional measures of LV function and myocardial perfusion status. Myocardial perfusion status, energy model variables and conventional cardiovascular variables (including LV volumes and blood pressure) were independently measured using the modalities of MRI and gated-SPECT in a cohort of women with suspected IHD. The prognostic value of myocardial perfusion status, energy model variables and conventional LV variables are evaluated and assessed separately for each MRI and SPECT modality.
Methods
Study population
Among the 936 WISE study participants a sub-population consisting of 227 women with suspected myocardial ischemia were recruited underwent a clinically indicated gated-SPECT evaluation and a study-directed MRI. This prospective sub-study was performed at a single WISE site, the University of Alabama at Birmingham (UAB) between the dates of November 1993 and October 1998, and included WISE participants with no contraindications for MRI examination. All subjects provided written informed consent using forms and procedures approved by the Institutional Review Board at UAB. The MRI and gated-SPECT studies were performed on the same day and readers were blinded to all women’s data.
Baseline MPI and LV function evaluation
The WISE study design and methodology has been previously described (3). In brief, on enrolment into WISE, demographic data, risk factors for CAD, medical and reproductive history, and functional capacity were collected as well as blood sampling for Lipid Core Laboratory evaluation. The Framingham risk score (FRS) was calculated using guidelines current at the time of data acquisition (11). The current study was structured with a pilot (n=64) and implementation (n=165) phase. During the pilot phase, the imaging protocol for the non-invasive approaches of MRI and gated-SPECT underwent optimization, and each protocol was fixed for the implementation phase.
Gated-SPECT
The gated-SPECT examination was performed in parallel with the MRI examination. A baseline gated-SPECT examination was obtained (ADAC, Milpitas, CA). Following this, women were sent to the MRI suite where at three minutes following infusion of dipyridamole (0.56 mg/kg over four minutes) technetium-99m sestamibi (MIBI) was administered. Following the MRI study, women returned to the nuclear cardiology laboratory for hyperemic gated-SPECT imaging. During the pilot phase, gated-SPECT studies were performed with either thallium-201 and methoxyisobutylisonitrile (MIBI) used for post dipyridamole or with MIBI (low dose/high dose) used for both baseline and hyperemic gated-SPECT MPI (12). During the implementation phase, the MIBI (low dose/high dose) protocol was used exclusively for gated-SPECT MPI. The gated-SPECT MPI data were evaluated by a consensus of two or more readers experienced in the interpretation of gated-SPECT and without knowledge of prior clinical history or angiographic results (13). Gated-SPECT data were entered in to an analysis program (ADAC, Milpitas, CA) that fitted the data to a 3D model of the LV, and end-systolic and end-diastolic volumes were extracted with minimal user interaction. Data were of sufficient quality for the automatic volumetric extraction in 149 (66%) cases (predominantly from the implementation phase).
Magnetic resonance
Magnetic resonance cine images were acquired using a Philips ACS 1.5T scanner (Philips Medical System, Best, The Netherlands). Non-invasive MPI was performed in the short axis orientation using a bolus injection of gadolinium (0.1 mmol/kg at a rate of 4-6 mL/sec) followed by a 10 mL saline flush.
LV function was evaluated from serial cine slices acquired in the short axis orientation. End-diastolic and end-systolic volumes were extracted from endocardial contours semi-automatically drawn using the MASS program (Medis, Leiden, The Netherlands). The myocardial wall thickness was measured at end-diastole in the horizontal long axis view at the mid-ventricular level in the septal wall and the opposite free wall. The two measurements were averaged to form the mean myocardial wall thickness.
Left ventricular structure and energy model
In the Shoucri energy model, the LV is represented as a thick-walled cylinder and the work performed during contraction and the generation of pressure is balanced against the product of myocardial elastance and the change in LV volume, Equation [1].
Fr × Wth – P = E × (VED–V) [1]
Where Fr is the radial component of force during LV contraction, Wth is myocardial thickness, P is LV pressure which physiologists often term isovolumic pressure, Piso, E is myocardial elastance, VED is end-diastolic volume and V is the LV volume. Consideration of this force-pressure equilibrium equation can be used to derive the energy equation of the left ventricle, see Appendix material. The energy model describes the energy utilization and elastance of the LV; see Figure 1 which illustrates three components of total energy (14). The area encompassed by an individual pressure-volume loop corresponds to the stroke work (SW), which represents the external work performed. The area of triangle CW, represents the energy passively absorbed by the myocardium (15). Of greatest interest here, the area of triangle IEU represents the Internal Energy Utilization representing the energy related to the internal metabolism of the left ventricle. The slope of the end systolic pressure volume relationship represents maximal ventricular elastance at end-systole, Emax, and the indicated diagonal slope of the pressure-volume loop represents the maximal aortic elastance at end-systole, Eam. This model predicts maximal ejection efficiency when the ratio Emax:Eam is 2:1 which was confirmed experimentally by Burkhoff, Sagawa and others (16,17). Since the majority of women lacked measurement of ventricular pressure by catheter-based methods, the end-systolic pressure was taken as the systolic pressure measured by sphygmomanometry. Carabello has argued that end-diastolic volume is closely related to end-diastolic pressure (18). This is supported by our data in 102 WISE (45%) women which shows that catheter derived LV end-diastolic pressure was linearly related to the measured end-diastolic volume when fitting a regression equation relating PED to MRI and SPECT measures of end diastolic volume, (MRI, P<0.05; gated-SPECT P<0.001), Equation [2] and [3].
PED = 9+0.05× VED [2] (MRI)
PED = 7+0.1× VED [3] (gated-SPECT)
Other model parameters were fitted using simplified assumptions with reference to Figure 1:
Eam = (PES–PED)/(VED–VES) [4]
Emax = (PES)/(VES–V0) [5]
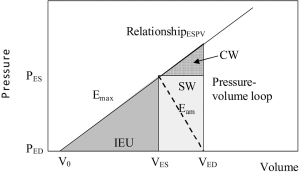
Where V0 is the ventricular volume-axis intercept (Figure 1)
IEU = [(VES–V0) × PES]/2 [6]
Measurement of V0 requires a series of invasive measurements. Shoucri has proposed a method to estimate V0, but here we chose to universally set V0 to zero using the common assumption that the pressure-axis intercept of the ESPR relationship is small (19). The IEU variable was indexed to body surface area to form IEUi, to normalize for the body habitus, but it is also possible to normalize to total work.
Follow-up procedures
Follow-up consisted of a scripted telephone interview performed by an experienced research coordinator at 6-weeks after enrolment and annually thereafter. The major adverse cardiovascular events (MACE) followed were cardiovascular mortality (assessed by a panel of experts), first incidence of nonfatal myocardial infarction (MI) or hospitalization for congestive heart failure. Follow-up was 3.3±1.4 years. In the event of death, a death certificate and/or hospital record was obtained.
Statistical analysis
Continuous values were presented as mean ± S.D. and categorical variables as percent frequency. The relationship between variables was assessed using correlation analysis. Continuous clinical and demographic characteristics were compared between groups using the independent samples t-test; the chi-square test was used for categorical comparisons. The predictors of MACE were identified by univariable Cox regression analyses. For each modality, multivariable models Cox models were constructed using the predictors established using univariable modelling. The hazard ratio was used to categorize women into high-risk and not high-risk for MACE. Women with a positive hazard value were designated high-risk, with not high-risk status assigned to all others and the association of risk stratification on MACE was determined. The Cox proportional hazards assumption was met for each model constructed. Kaplan-Meier curves were used to compare the risk-stratified groups on time to MACE. The log-rank test was used to compare survival curves. All statistical tests were two-tailed and a P value
Results
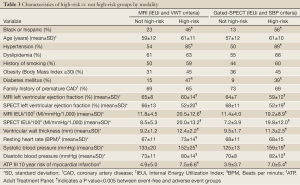
Population characteristics and data present
The mean age of women was 59±12 years (range, 31-86 years); 33% were ethnic minorities, primarily African-Americans. Demographic data for all women and women categorized by MACE are summarized in Table 1. At the end of the 5-year follow-up period, MACE occurred in 33 women (15%) consisting of 20 deaths, seven hospitalizations for congestive heart failure, and 6 MIs. Of the 227 women, complete MRI and gated-SPECT perfusion data were available for approximately 90% of women (MRI, 89%, SPECT, 91%). Similarly, complete MRI, EF and IEUi data were available for approximately 90% of women (89% and 86%, respectively). However, for gated-SPECT, LV function variables were available only for the implementation phase, reducing the number of women with functional SPECT data to approximately 60% (EF, 66%, IEU, 59%). Correlations between key MRI and SPECT variables are shown in Table 2.
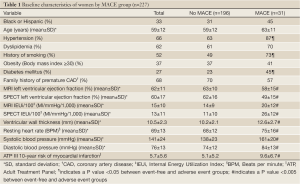
Full Table
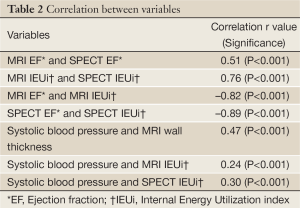
Full Table
Univariable predictors of outcome
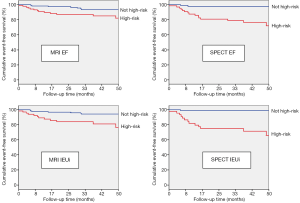
For MRI, multivariable Cox regression analysis showed that average myocardial wall thickness (HR 1.37, 95% CI, 1.16-1.50), EF (HR 0.97, 0.94-0.99), and IEUi (HR 1.05, 1.03-1.07) were significant predictors of MACE. However, the presence of at least one myocardial region with a perfusion abnormality was not statistically significant (HR 1.73, 95% CI, 0.76-3.93). For SPECT, IEUi (HR 1.06, 95% CI, 1.03-1.09), EF (HR 0.97, 0.95-0.99), presence of at least one myocardial region with a perfusion abnormality (HR 2.46, 95% CI, 1.13-5.35), end-systolic volume index (HR 1.03, 95% CI, 1.01-1.05) and end-diastolic volume index (HR 1.03, 95% CI, 1.01-1.05); and physiologic variables: systolic blood pressure (HR 1.03, 95% CI, 1.01-1.04) and diastolic blood pressure (HR 1.05, 95% CI, 1.02-1.08) were significant predictors of MACE. Figure 2 shows Kaplan-Meier event-free survival curves based on the univariable MRI and SPECT Cox independent predictor variables relating to cardiac function.
Multivariable predictors of outcome
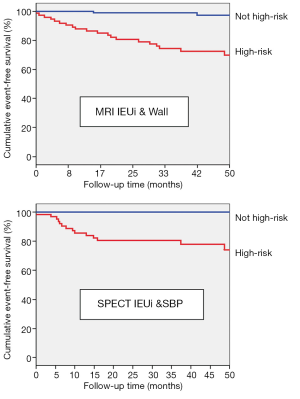
The final multivariable Cox model using MRI data showed that IEUi and wall thickness were significant predictors of MACE while the final model for SPECT showed that IEUi and systolic blood pressure were significant predictors of MACE. These models remained significant after adjusting for the Framingham risk score, age and presence of diabetes. Results are summarized in Table 3, and Figure 3 shows Kaplan-Meier survival curves for the MRI (log rank 25, P
Full Table
Discussion
In women with suspected IHD enrolled in the NHLBI-sponsored WISE study, our results demonstrate for the first time the prognostic importance of LV energy model variables. In both MRI and SPECT Cox models, the LV energy model variables provided greater prognostic value than either conventional metrics of LV function or the presence of any MPI abnormality. These findings may be particularly relevant to IHD risk assessment since the energy model is routinely available from image data and better identifies risk than either volumetric or pressure conditions considered alone.
Energy model vs. conventional LV function
By two separate modalities, the Energy Model variable of IEUi was demonstrated to better predict prognosis than conventional EF. Both EF and IEUi are expressions of LV function influenced by multiple physical conditions including LV chamber volume, afterload, ischemia (severity, extent, location), as well as myocardial elastance. The IEUi incorporates systolic blood pressure, which may be a factor dominating the prognostic value of IEUi. However, IEUi was only moderately correlated with systolic blood pressure (Table 2), and IEUi remained predictive when entering blood pressure variables in multivariable Cox regression models. Between modalities, measures of IEUi were strongly correlated (r=0.76) while measures of EF were moderately correlated (r=0.51). Within each modality, measures of IEUi were strongly but inversely correlated with EF (MRI, r=–0.82, SPECT, r=–0.89). Thus, IEUi has higher prognostic value than LV EF, and measures of IEUi demonstrate higher correlation between modalities, indicating that it may provide a better modality-independent assessment of risk.
Structural LV variables
The LV myocardial wall is known to thicken in response to long-term elevation in arterial pressure. However, the moderate correlation of wall thickness and systolic blood pressure (Table 2) likely reflects the influence of other cardiac components that also influence wall thickness, including geometric changes in shape and curvature, each serving to reduce wall stress. Currently, wall thickness is not routinely available using gated-SPECT, but when available by MRI can be combined with IEUi to better predict prognosis. When wall thickness is not available, IEUi can be combined with systolic blood pressure to improve prognosis, as was show to be the case for gated-SPECT. Incorporating direct cardiac measures may better assess cardiac status compared to indirect measures such as blood pressure.
SPECT vs. MRI
Measures of LV volume are expected to be more accurate by MRI than SPECT due to better in-plane resolution (1-2 mm2 compared to 10-15 mm2, respectively) and better temporal resolution (16-20 frames per cycle, compared to 8, respectively). Further, while visualization of the myocardium and LV cavity by MRI is thought to be independent of LV perfusion status, the same may not be true for SPECT. This may explain the observed low correlation between MRI and SPECT measures of LV EF. Despite these systematic differences between modalities, measures of IEUi showed strong correlations, indicating that use of IEUi may provide a modality-independent means of assessing women.
Perfusion variables
The presence of at least one perfusion abnormality by either modality was associated with a comparably increased event rate for MRI and SPECT. Despite this, IEUi was a better predictor of MACE, indicating a hierarchy in interpretation: MPI assessment should primarily guide detection of high-grade coronary artery stenosis (diagnosis) (13) while the energy model data better predicts risk (prognosis). IEUi ultimately reflects myocardial performance due to perfusion status in addition to other hemodynamic and structural changes that have occurred in the cardiovascular system. Thus, combined use of energy model and perfusion assessment may lead to better patient management, whereby therapy might be better directed toward improving both energy model and perfusion conditions.
Limitations
Accuracy of the LV energy model variables is limited in our study due to the absence of invasively obtained LV pressure measurements. We used sphygmomanometry data for systolic pressure, and we used a fitted value for end-diastolic pressure based on end-diastolic volume. Accurate determination of the model parameter Emax would require exposing subjects to increasing stress levels and obtaining (possibly invasively) LV pressure measurements, which was not included in the protocol for this study. Data were only obtained from one site and the population was too small to allow extensive model development beyond combing two variables. Data from the pilot and implementation phase were combined, resulting in fewer LV volumetric data available for gated-SPECT compared to MRI, preventing a direct comparison between MRI and SPECT in predicting prognosis. Apart from this limitation, combining pilot and implementation phase data is not expected to otherwise affect results, since the primary difference between phases affected the perfusion data, which was not used in this analysis. Kaplan-Meier analysis was conducted using Cox models generated in the same population. This type of analysis is common for such observational studies. Future research needs to focus on developing a prospective study to validate these findings in women and determine how they differ from men.
Conclusions
In women with suspected IHD, we demonstrate for the first time the prognostic value of LV energy model variables using MRI and gated-SPECT. Our results demonstrate that the energy model provides improved prognostic value to conventional measures of MPI and LV EF. The LV IEUi was strongly correlated to EF, and each variable can be obtained clinically with equal ease. More importantly, these variables provide a more sophisticated measure of LV function than blood volumes displacement, with EF assessing ejection efficiency and IEUi assessing a component of the LV’s energy expenditure distinctly separate from any measure of external work. Additional work in larger datasets, as well as translation to clinical care practice is indicated.
Acknowledgements
We are grateful to Bracco, Princeton, NJ, for providing the ProHance contrast agent. This work was supported by contracts from the National Heart, Lung and Blood Institutes, nos. N01-HV-68161, N01-HV-68162, N01-HV-68163, N01-HV-68164, RO1-HL-073412-01, grants U0164829,U01 HL649141, U01 HL649241, and grants from the Gustavus and Louis Pfeiffer Research Foundation, Danville, New Jersey, The Women’s Guild of Cedars-Sinai Medical Center, Los Angeles, California, The Ladies Hospital Aid Society of Western Pennsylvania, Pittsburgh, Pennsylvania, and QMED, Inc., Laurence Harbor, New Jersey, and the Edythe L. Broad Endowment, Cedars-Sinai Medical Center, Los Angeles, California, and the Barbra Streisand Women’s Cardiovascular Research and Education Program, Cedars-Sinai Medical Center, Los Angeles.
Disclosure: Gerald M. Pohost receives research support from GE Healthcare. C Noel Bairey Merz receives support or has interests in Bristol-Myers Squibb, Curtis Green LLP, Cook Inc., Giead Sciences, Narvis Healthcare, NHLBI, Pollock Communications, Practice Point Communications, Society for Women’s Health Research, Itamar Medical Inc, Virginia Commonwealth University, Medtronic, Johnson and Johnson.
Appendix
Considering the left ventricle as a thick-walled cylinder, with the myocardium generating a radially directed force, Fr, that is in equilibrium with the pressure (force/unit area) on the inner surface of the myocardium (endocardium), P, often termed isovolumic pressure, Piso:
Fr–P = E(VED–V) [A1]
where E(VED–V) = pressure resulting from the elastic deformation of the passive medium of the myocardium. The energy equation is represented by the area below the ESPVR line, TW, comprising the individual regions IEU, SW, and CW in Figure 1. IEU represents the energy related to the internal metabolism of the heart, SW, stroke work, represents the energy delivered to the systemic circulation, and CW, represents the energy passively absorbed by the myocardium. Multiply both sides of Equation [A1] by (VED–V0):
Fr (VED–V0) –PES (VED–V0) = Emax (VED–VES) (VED–V0) [A2]
Fr (VED–V0) –PES (VED–V0) =Emax (VED–VES) (VED–VES + VES– V0) [A3]
Noting that Emax (VES–V0) = PES, the equation can be written in the form:
Fr (VED–V0) = Emax (VED–VES)2 + Emax (VED–VES)(VED–V0) + PES (VED–V0) [A4]
2 area TW = 2 area CW + PES (VED–VES +VED–V0) [A5]
2 area TW = 2 area CW + 2 PES(VED–VES) + PES(VES–V0) [A6]
2 area TW = 2 area CW + 2 area SW + 2 area IEU [A7]
TW = CW + SW + IEU [A8]
This represents the energy equation:
1 = CW/TW + SW/TW + IEU/TW [A9]
Represents the normalized form of the energy equation.
References
- Mieres JH, Shaw LJ, Arai A, et al. Role of noninvasive testing in the clinical evaluation of women with suspected coronary artery disease: Consensus statement from the Cardiac Imaging Committee, Council on Clinical Cardiology, and the Cardiovascular Imaging and Intervention Committee, Council on Cardiovascular Radiology and Intervention, American Heart Association. Circulation 2005;111:682-96. [PubMed]
- Gordon EE. Coronary artery disease in women: the role of diagnostic imaging. Echocardiography 1993;10:321-30. [PubMed]
- Merz CN, Kelsey SF, Pepine CJ, et al. The Women’s Ischemia Syndrome Evaluation (WISE) study: protocol design, methodology and feasibility report. J Am Coll Cardiol 1999;33:1453-61. [PubMed]
- Doyle M, Weinberg N, Pohost GM, et al. Prognostic value of global MR myocardial perfusion imaging in women with suspected myocardial ischemia and no obstructive coronary disease: results from the NHLBI-sponsored WISE (Women’s Ischemia Syndrome Evaluation) study. JACC Cardiovasc Imaging 2010;3:1030-6. [PubMed]
- Thomas GS, Miyamoto MI, Morello AP 3rd, et al. Technetium 99m sestamibi myocardial perfusion imaging predicts clinical outcome in the community outpatient setting. The Nuclear Utility in the Community (NUC) Study. J Am Coll Cardiol 2004;43:213-23. [PubMed]
- Steele L, Webster NR. Altered cardiac function. J R Coll Surg Edinb 2001;46:29-34. [PubMed]
- Suga H, Sagawa K, Shoukas AA. Load independence of the instantaneous pressure-volume ratio of the canine left ventricle and effects of epinephrine and heart rate on the ratio. Circ Res 1973;32:314-22. [PubMed]
- Shoucri RM. Active and passive stresses in the myocardium. Am J Physiol Heart Circ Physiol 2000;279:H2519-28. [PubMed]
- Shoucri RM. Performance of left ventricle based on pressure-volume relation. J Biomed Eng 1990;12:482-8. [PubMed]
- Saba PS, Ganau A, Devereux RB, et al. Impact of arterial elastance as a measure of vascular load on left ventricular geometry in hypertension. J Hypertens 1999;17:1007-15. [PubMed]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001;285:2486-97. [PubMed]
- DePuey EG, Parmett S, Ghesani M, et al. Comparison of Tc-99m sestamibi and Tl-201 gated perfusion SPECT. J Nucl Cardiol 1999;6:278-85. [PubMed]
- Doyle M, Fuisz A, Kortright E, et al. The impact of myocardial flow reserve on the detection of coronary artery disease by perfusion imaging methods: an NHLBI WISE study. J Cardiovasc Magn Reson 2003;5:475-85. [PubMed]
- Shoucri RM. ESPVR and the Mechanics of Ventricular Contraction. WSEAS Transactions on Biology and Biomedicine 2010;7:158-67.
- Shoucri RM, Kohar R. Criteria for study of heart failure derived from ESPVR. Conf Proc IEEE Eng Med Biol Soc 2012;2012:5586-9.
- Burkhoff D, Sagawa K. Ventricular efficiency predicted by an analytical model. Am J Physiol 1986;250:R1021-7. [PubMed]
- Sunagawa K, Maughan WL, Sagawa K. Optimal arterial resistance for the maximal stroke work studied in isolated canine left ventricle. Circ Res 1985;56:586-95. [PubMed]
- Carabello BA. Abnormalities in cardiac contraction: Systolic dysfunction. In: Hosenpud JD, Greenberg BH. eds. Congestive Heart Failure: Pathophysiology, Diagnosis and Comprehensive Approach to Management. New York: Springer-Verlag, 1994:54-67.
- Shoucri RM. A method to calculate the parameters of isovolumic curves in the ventricles II. Proceedings of the World Medical Conference: Proceedings of the 2nd International Conference on Pathology (Pathology ’11) 2011:150-5.


