Very late bare metal stent thrombosis in the setting of discontinuation of optimal medical therapy for 2 years
Introduction
Previous studies have reported that very late stent thrombosis is associated with the uncovered stent strut and discontinuation of antiplatelet agents in drug eluting stent (DES) (1,2). On the other hand, bare metal stent (BMS) were considered relatively safe in terms of stent thrombosis because the amount of neointimal coverage in BMS was relatively higher than those in DES. Therefore, the shorter duration of dual antiplatelet agents has been recommended in BMS compared with DES (3). Recently, pathological studies suggested that neoatherosclerosis might involve the inability to maintain a fully functional endothelialized luminal surface within the stented segment because of underlying unstable plaque, and contribute to another factor of very late stent thrombosis in both DES and BMS (4). Under this condition, optimal medical therapy may be important factor of prevention to neoatherosclerosis. However, few reports exist regarding the association between neoatherosclerosis and the discontinuation of optimal medical therapy. Here, we report a case with very late BMS thrombosis associated with neoatherosclerosis in the setting of discontinuation of optimal medical therapy for 2 years.
Case presentation
A 60-year-old man was admitted to our hospital because of chest pain which was not relieved by rest. He was well until 8 years before, when he was suffered from acute myocardial infarction. At that time, after BMS with the diameter of 3.5 mm and length of 28 mm was implanted in the left anterior descending artery (LAD), aspirin, clopidogrel, statin, angiotensin II receptor blocker, and β blocker were prescribed. He was consulted by the local doctor, however, he stopped to see the doctor and all medications were discontinued 2 years before the present event.
At admission electrocardiogram exhibited prominent ST elevation in precordial leads (Figure 1), suggesting the recurrence of acute coronary syndrome along the LAD. Blood tests showed elevation of cardiac enzyme, and his low density lipoprotein cholesterol level increased to 140 mg/dL, which was much higher than target value for secondary prevention of coronary heart disease.
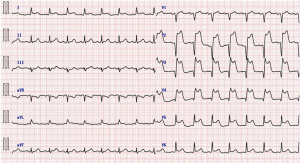
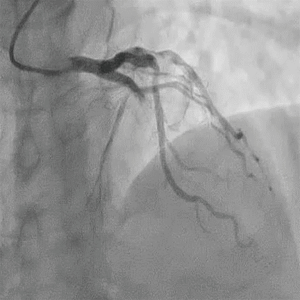
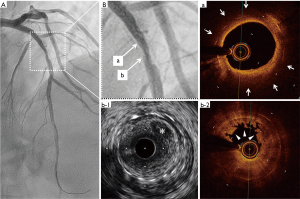
Emergent coronary angiography showed the total occlusion of the LAD at the site where the previous stent was deployed (Figure 2). After accessing the LAD by guidewire which partially gave reflow, intravascular ultrasound (IVUS) and optical coherent tomography (OCT) were performed in LAD (Figure 3). OCT showed lipid rich neointima with thin cap, suggesting neoatherosclerosis at the proximal to the occlusive site. OCT also showed white thrombus formation around at the occlusive site. IVUS showed the ruptured cavity within the stent at the occlusive site. Drug coated balloon was dilated at the site of the previous stent which completely recovered the coronary flow in the LAD.
Discussion
It is well known that discontinuation of antiplatelet agents in early phase after coronary stent implantation can result in stent thrombosis particularly in DESs with persistent uncovered struts (1). Moreover, stent thrombosis after DES implantation occurs soon after discontinuation of antiplatelet agents (2). As for BMS, the surface of the stent has been already covered by neointima even in early phase (5), preventing the stent thrombosis in early as well as late phase after implantation. The present case, however, demonstrates that stent thrombosis can occur even in BMS with covered struts, and 2 years after discontinuation of antiplatelet agents. It is notable that the patient stopped taking not only antiplatelet agents but also statin, angiotensin II receptor blocker, and β blocker.
We previously reported that the first generation DES is often associated with neoatherosclerosis formation probably due to the chronic inflammation raised by the polymer and drug (6). However, one might suggest that stent thrombosis can occur even in BMS with covered struts which were considered relatively safe compared with DES in terms of neoatherosclerosis associated with stent thrombosis. In this case, it is possible that “de novo” atherosclerosis within BMS may progress resulting to formation and disruption of vulnerable plaque for 2 years because of insufficient secondary prevention of coronary heart disease. Thus, we suggest continuation of optimal medical therapy to prevent the substantial neoatherosclerotic burden and occurrence of late phase stent thrombosis even in the BMS. Especially in selected cases, with high clinical risk profile it is safer to prolong DAPT for long term.
Acknowledgements
We thank Professor Masakazu Yamagishi for his cordial comments on this manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Finn AV, Joner M, Nakazawa G, et al. Pathological correlates of late drug-eluting stent thrombosis: strut coverage as a marker of endothelialization. Circulation 2007;115:2435-41. [Crossref] [PubMed]
- McFadden EP, Stabile E, Regar E, et al. Late thrombosis in drug-eluting coronary stents after discontinuation of antiplatelet therapy. Lancet 2004;364:1519-21. [Crossref] [PubMed]
- Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2016;68:1082-115. [Crossref] [PubMed]
- Nakazawa G, Otsuka F, Nakano M, et al. The pathology of neoatherosclerosis in human coronary implants bare-metal and drug-eluting stents. J Am Coll Cardiol 2011;57:1314-22. [Crossref] [PubMed]
- Mori M, Sakata K, Nakanishi C, et al. Early endothelialization associated with a biolimus A9 bioresorbable polymer stent in a porcine coronary model. Heart Vessels 2017;32:1244-52. [Crossref] [PubMed]
- Sakata K, Namura M, Takagi T, et al. Repeated occurrence of slow flow phenomenon during and late after sirolimus-eluting stent implantation. Heart Vessels 2015;30:406-9. [Crossref] [PubMed]


