Percutaneous access planning, techniques and considerations for endovascular aortic repair (EVAR)
Introduction
Historically, repair of the aorta required open surgery accompanied by extended intensive care unit (ICU) admissions followed by multiple months before patients recovered to their initial baseline status. A large number of patients were not suitable candidates for open surgical repair of the aorta due to medical comorbidities and/or underlying frailty. With the introduction of the Seldinger technique, new frontiers of percutaneous endovascular repairs were unlocked. Initially, endovascular access for aortic repair was obtained with a surgical femoral cut-down with associated risks of femoral neuralgia, lymphoceles, hematoma and infection. With the advent of innovative percutaneous suture mediated vascular closure devices and lower profile endograft devices, entirely percutaneous endovascular access developed into a viable and arguably a preferable option for patients undergoing aortic repair (1).
When planning an endovascular aortic repair (EVAR) and thoracic aortic endovascular repair (TEVAR), appropriate access site selection is critical. Percutaneous EVAR often requires multiple access sites to coordinate positioning of multiple components including the main body graft as well as contralateral limb device which often require large endovascular access which can accommodate up to 24 French sheath size. As interventionalists perform increasingly more complex aortic repairs which include the implementation of snorkels and fenestrated aortic endografts, secondary vascular access from radial, brachial or axillary arteries may also be required. However, prior to further consideration of access site selection, a brief discussion of access techniques and bailout options for potential access related complications is necessary.
Access techniques and bailout techniques
Ultrasound guided access is recommended to optimize arterial access when performing percutaneous EVAR (2,3). Next, considering the large arteriotomies which are required to deliver the large profile graft components, preclosure of the arteriotomy sites using two Perclose Proglide (Abbott Laboratories, Abbott Park, Illinois, USA) suture mediated closure devices utilizing the “preclose technique” has been correlated with shorter procedure times and decreased access site complications when compared with femoral cutdown (4-6). Additional studies demonstrate high technical success rates with “preclose technique” with very low early and late complication rates in the setting of large sheath access (7). Some operators utilize protamine for heparin reversal at the end of the procedure although this technique is not well studied in the setting of EVAR. At the end of the procedure if the arteriotomy site continues to ooze or bleed, there are several bailout techniques that may be considered to achieve postprocedural hemostasis although these are not well evaluated in the literature. First, if there is continued oozing or bleeding after the initial two perclose have been deployed, a third perclose device may be deployed. If hemostasis is still not achieved after a third perclose, the operator may choose to place an appropriately sized sheath, deploy an 8 French Angioseal (Terumo, Somerset, NJ, USA) at the arteriotomy site, utilize a FemStop (St.Jude Medical, St. Paul, MN, USA) device or if the contralateral access is still available, consider going up and over and placing a Viabahn (Gore, Flagstaff, AZ, USA) covered stent being careful to avoid covering the profunda femoral artery. Post-procedurally if femoral access was performed, the ipsilateral leg should remain straight for 6 hours after completion of the procedure to maintain hemostasis. These are some of the basic access techniques and bail-out techniques that may prevent complications and/or quickly resolve access related challenges.
Access site considerations
Now that we have discussed access techniques, we will return to potential access site options. Evaluation of the entire vasculature path is crucial, from the targeted arteriotomy to the target aortic repair location. Contrast CT (and/or CT angiography) has a greater predictive value for vascular complications compared to invasive angiography to plan vascular access for transcatheter aortic valve replacement (TAVR) which also require large arteriotomies and large endovascular devices (8). However, axial imaging may overestimate vessel diameters because the axial plane may bisect the vessel at an oblique angle. Post-processing using 2D multiplanar reformats or 3D reformatting allows the interventionalists to better assess the true diameter of access vessels and evaluate the vasculature along the planned path for the procedure (9) (the authors institution uses a GE AW Volushare 2 workstation). Several vessel characteristics should be assessed including; anterior calcification at the planned access site, small vessel size, marked vessel tortuosity and calcifications as this may increase the degree of procedural difficulty or the risk of peri-procedural complications. Access vessel diameters <5 mm have been associated with a higher risk of percutaneous aortic repair failure (10). Retrospective analysis comparing vascular complications in subgroups defined by the ratio of external sheath diameter to the minimal common femoral artery (CFA) luminal diameter and subgroups defined by the ratio of sheath area to the minimal luminal area and found that sheath to vessel diameter ratios >1.45 and sheath to vessel area ratios >1.35 may also be used as predictors of vascular access complications (11).
Since EVARs typically require at least one large arteriotomy site, typically up to a 24 French sheath for the primary graft component, arterial vessels that can accommodate this size need to first be identified. The most frequently utilized primary large access site is the CFA. The contralateral CFA has traditionally been used as the secondary access site; this allows the operator to place a contralateral iliac limb graft for an abdominal EVAR. In thoracic EVARs, the secondary access site may be more variable. Advantages of utilizing the CFA include relative ease and size of access (as the average CFA is 6.4 mm in diameter) with the additional benefit of compression support against the femoral head (12). Unfortunately, CFA access may be challenging due to obesity, hostile or scarred groin, occlusive aorto-iliac segments, or having a high-riding femoral bifurcation (13).
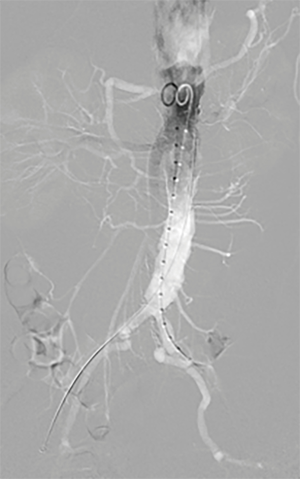
Various technical methods exist to identify and access the CFA including physical landmarks, fluoroscopy and ultrasound. The inguinal crease may be used as a landmark for the inguinal ligament; however, this anatomical relationship can be variable, averaging 6.5 cm away in patients with larger body habitus. In a single study, 76% of patients demonstrated a CFA bifurcation superior to the inguinal crease (14). Another method of determining access location is utilizing the midpoint between the anterior superior iliac spine and pubic symphysis, which can be accomplished by palpation (3). Meanwhile, using fluoroscopy alone to guide femoral access is predicated on the CFA bifurcation to be located at or below the center of the femoral head (which occurs in 98.5% of cases) as the access site is generally targeted at (or below) the center of the femoral head. Ultrasound-guided access allows direct visualization of the CFA and the CFA bifurcation and also allows for identification of calcified plaque of the arterial wall, which should be avoided. Reported complication rates with CFA access have ranged from 1.5% to 17%, especially with the use of larger sheaths. Access site complications of the CFA include hematoma, pseudoaneurysm, bleeding, access artery occlusion, distal embolization, infections, and venous thrombosis.9 Anterior femoral artery calcification correlates with a higher likelihood (83%) of failure due to inadequate suture mediated closure (15). Other complications include retroperitoneal bleeding, which is more commonly seen with a high arteriotomy, and post-procedure neuralgic pain. Anecdotally, even when patients demonstrate anatomic variations such as a very high bifurcation which may require puncture of the superficial femoral artery or even profunda femoral artery, the Preclose technique has continued to be successful in these situations in selected patients for EVAR (Figure 1).
Brachial access
Generally percutaneous brachial artery access is performed with caution due to the risk of post-procedural complications. In a single center study, 6.5% of patients with brachial artery access for any procedure experienced complications, of which 62% require additional operative correction for definitive treatment (16). Unfortunately, hematomas can easily spread within the medial brachial fascia leading to median nerve compression, which can occur without diminished distal peripheral pulses (17). To avoid inadvertently puncturing the radial or ulnar artery, performing brachial access via ultrasound guidance can detect potential anatomical variations, such as a high bifurcation of the brachial artery. Despite potential drawbacks, ultrasound-guided brachial artery access with direct needle puncture may be used as a smaller secondary access site. The brachial artery allows for a superior approach into the aorta, which can then be used for more optimal catheterization angles when cannulating inferiorly directed vessels, such as the superior mesenteric artery or renal arteries or when using physician modified aortic endografts with branch or fenestrations (12).
For brachial artery access, the left-side is preferred to avoid catheter manipulation through aortic arch and to provide a more direct path to the descending thoracic aorta. When accessing at the antecubital fossa where the artery is fairly fixed and superficial, a 21 gauge micropuncture needle is often used for single wall vessel puncture and catheterization, followed by insertion of a 0.018-in wire and 4F arterial sheath, which can then be exchanged for a 4F-9F sheath for more stability. The size of the sheath will depend on the quality and size of the brachial artery as well as what size endoprosthesis needs to traverse the access (18). Hemostasis can be attained with manual compression. At the authors' institution, heparin is reversed with protamine after the activated clotting time (ACT) reaches 150 seconds manual compression is held for 30 minutes. Potential brachial access site complications include hematoma, pseudoaneurysm, bleeding, distal embolization. In cases where excessive ilio-femoral or aortic tortuosity make it difficult to advance the endograft device, the brachial access may permit through-and-through access with an exchange length wire to facilitate improved leverage and advancement of the endograft in these challenging situations. If faced with this situation, utilization of a protective catheter may reduce the possible tearing of vessels especially (i.e., the subclavian artery) as you are putting tension on the vasculature. While embolic stroke events are a major theoretical concern for radial and brachial access, no significant differences in stroke rates were seen between radial and femoral access for coronary intervention (14).
Transradial access
Transradial approach is common for percutaneous coronary interventions and has relatively low access site bleeding complications. With the advancements in the development of even slimmer profile devices, the use of radial access has become a favorable alternative access site for adjunctive aortic procedures. The radial artery provides a superiorly oriented access to the aorta and optimization of catheterization angles for certain vessels. Benefits include: shorter post-procedural monitoring, immediate mobilization, avoidance of accessing diseased lower extremity vessels, simplified closing procedures and improved patient comfort. Drawbacks include: prohibitive lesion complexity or distance from access site, concerns of stroke, smaller vessel diameter incompatible for larger sheaths, and longer vascular route unsuitable for devices with shorter shafts (19,20).
Assessment of the upper extremity arterial patency is done with palpation and/or ultrasound, prior to performing radial access. The Barbeau test, which is a modified Allen’s test, evaluates the radial artery collateral vascularization with the ipsilateral ulnar artery through the palmar arch. With a pulse oximetry on the patient’s ipsilateral thumb, the interventionalist evaluates the pulse oximetry waveform before and during radial artery compression. If interrupted arterial filling with waveform type D is detected, radial access should not be performed. Radial artery size should be further assessed via ultrasonography to confirm that the intended device can fit within the vessel lumen; sheath sizes greater than 6 French are typically avoided. Additionally, the radial artery should be evaluated with ultrasound for any evidence of extreme tortuosity or loops that may limit the ability to advance the catheter to the desired target (21). Exclusion criteria of performing transradial access includes: Raynaud’s disease, upper limb claudication, Barbeau D waveforms, radial artery loop (which is a relative contraindication due to potential technical challenges) (22).
For radial approach, the patient is placed supine on the angiography table, with left arm supported by an armboard. Left-sided artery is preferred to avoid catheter manipulation through aortic arch and the potential theoretical risk of strokes. A micropuncture needle is used for single wall vessel puncture and catheterization followed by insertion of a 4 F arterial sheath. This sheath can be exchanged for longer sheath, with sizes from 4 to 7 F for more stability. Typically hydrophilic low-profile sheaths such as the Terumo Slender sheaths (Terumo, Irvine, CA, USA) which have smaller outer diameters but maintain a large inner diameter by decreasing the sheath thickness. To minimize arterial spasm, a cocktail injection of 3,000 to 5,000 units heparin, 2.5 mg Verapamil, and 200 mcg Nitroglycerin mixed with the patient’s blood (to reduce pain from injecting the cocktail) can be given (23). Diagnostic catheters with a length of 100–150 cm can be used to support wire passage into the descending aorta, and the catheter and sheath may be exchanged over a wire for a longer sheath or guiding catheter. In radial access, hemostasis is often attained with TR band (Terumo, Irvine, CA, USA). Radial access site complications include hematoma, pseudoaneurysm, bleeding, access artery occlusion (4%), distal embolization. While stroke is a major theoretical concern for radial access, no significant differences in stroke rates were seen between radial and femoral access for coronary intervention (15,16).
Surgical conduits
Surgical conduits may also be used for endovascular access for aortic procedures in patients with unfavorable vasculature. When traditional percutaneous CFA access would be contraindicated due to small, calcified tortuous iliac arteries surgical conduits may be considered as alternative access. The axillary conduit is a valuable approach where you can place multiple sheaths for complex fenestrated aortic endovascular repair, branched endografts or for multiple chimney placements. EVAR has successfully been performed in patients with challenging vascular anatomy through retroperitoneal iliac and aortic conduits as well as axillary conduits in patients who would have otherwise been poor endovascular candidates (24,25).
Left ventricular/transapical access
A left ventricular/transapical approach is utilized in some rare situations, providing a shorter and more direct access point to the true lumen of the thoracic aorta (26). Patients receiving transapical access are ineligible for a transfemoral approach often due to lack of access vessels, absence of femoral pulse, a severely atherosclerotic or kinked thoracic descending aorta, inherently narrow arterial diameter, type A aortic dissection, or ascending aortic aneurysm (22,27-29). Compared to transfemoral access, the access via the left ventricular apex increases control for the proceduralist due to a straighter and shorter approach (23). Larger endovascular delivery systems and more precise placement of stents can be achieved compared to other endovascular access methods (22). Criteria for severe atherosclerosis in one study that prompted the diversion from the transfemoral approach included: “atheromatous disease of the aorta with protruding atheroma >5 mm thickness, existing >50% of circumference, and a diffuse involvement of most of the length of the descending thoracic aorta” (23). In the same study, of the 6 patients receiving a transapical deployment of the stent graft, a moderate to high grade aortic valve regurgitation was noted in half, all of whom recovered to preoperative levels. Aortic valve regurgitation was noted to deteriorate when careful traction was applied to the guidewire as the device was advanced through the aortic arch. In another small study, two patients out of seven who had intra-operative ventricular fibrillation exhibited significant aortic regurgitation immediately prior to the episodes of ventricular fibrillation (25). A number of other major complications exist via the transapical approach, such as stroke, failure to exclude the aneurysm, retrograde aortic dissection, and aortoesophageal/bronchial fistula (22-25). Ventricular pseudoaneurysm and injury to adjacent cardiac components are additional risks encountered with transapical left ventricular access (30).
Transcaval access
In patients where conventional transfemoral access methods are not feasible, a transcaval technique may be used to deliver large profile devices into the abdominal aorta (31). With potentially fewer access related complications and a shorter procedure length, the transcaval approach combines elements of arterial and venous access strategies without related femoral or iliac artery complications—higher vessel compliance in the venous system yields lower resistance in accommodating larger catheter sheaths (32). Procedurally, an 0.014” guidewire directed from the femoral vein through the inferior vena cava results in transcatheter puncture of the abdominal aorta. The cavo-aortic site is determined based on CT demonstrating close proximity of the aorta and IVC. A nitinol cardiac occluder device is then utilized to achieve tract closure (26). This percutaneous approach can be used for thoracic aortic aneurysm repair in patients with severe peripheral arterial disease, highly unusual anatomy, or those with contraindications to arterial access due to calcification or narrowed effective lumen diameter of the iliac or femoral arteries. Because of the novelty of this technique, further investigation regarding the larger-scale cost-effectiveness and safety is warranted; however, an early study has indicated a successful transcaval access and closure in 99 out of 100 attempts during TAVR procedures (28,33). The one complication in this study occurred when the guidewire failed to cross and the operator had to perform transfemoral artery TAVR, which was further complicated by iliac artery rupture. Device success occurred in 98 out of 100 patients—the outliers included a failure for the guidewire to cross and another due to the operator not repositioning a fully withdrawn nitinol occluder (29).
Iliofemoral “Pave and Crack” technique
Access-related complications through stenotic or diseased iliac arteries are more common via transfemoral approaches to EVAR (34). Iliofemoral access in some patients may be difficult either due to iliofemoral occlusive disease or excessive tortuosity and calcification (35). Complications associated with unfavorable iliac anatomy include rupture, dissection, thrombosis, or distal ischemia (31). Generally, solutions to poor iliac access involve angioplasty and stent placement or retroperitoneal iliac conduit. The “pave and crack” technique accomplishes dilation and relining of stenosed iliac arteries through the use of polytetrafluoroethylene (PTFE) covered stents such as the Viabahn (GORE, Flagstaff, Arizona, USA) and ICAST (Atrium, Hudson, NH, USA) stents to minimize risk of pseudoaneurysm or hemorrhage (30). The distal limbs of bifurcated stent grafts can then be extended into the covered stents in the iliac arteries to establish an optimal distal seal. It is important to occlude the ipsilateral internal iliac artery to prevent type II endoleak propagation.
Solopath
Another endovascular option for narrow access vessels is the Solopath (Terumo, Irvine, CA, USA) balloon expandable trans-femoral system, which is an expandable (up to 21 French) femoral access infrastructure built for large-bore vessel access (36). The versatility of an expandable system and the reinforced polymer sheath allows access to tortuous and calcified vessels by creating a linear path that otherwise would be unfavorable for femoral access. An “external collapsible outer jacket and folded distal end (sheath) pre-mounted over a central balloon dilatation catheter (expander)” is also available. The sheath is available in both 25 and 35 cm lengths, which allows extension from the external iliac artery through the abdominal aorta. The outer diameter range is 17–24 F and inner diameter range is 14–21 F. The functional use of the Solopath system is to force calcifications against the vessel wall, similar to what a stent or balloon angioplasty would do. The protection offered by the Solopath sheath is what enables the functional use and versatility. An attractive advantage of this access strategy opposed to alternative access strategies is that the Solopath system can physically straighten the artery and expand and collapse to minimize bleeding, rupture, or dissection while inserting and withdrawing devices. The superior maneuvering capability takes advantage of the compliance and elasticity of vessels to provide an easier operator experience as opposed to attempting to force an 18 F sheath through a smaller tortuous heavily calcified vessel.
Conclusions
In summary, endovascular access for aortic repair may be highly variable but generally requires at least one if not multiple large bore sheaths which often requires multiple carefully selected access sites. Preprocedural planning may be performed with contrast CT or CT angiography. Whichever access sites are chosen for EVAR, ultrasound guidance should ideally be used during percutaneous access. Preclose technique is also recommended to minimize access site complications. Given the large endoluminal requirements for the primary graft component, the common femoral artery is the most commonly used access site. However, in patients who have unfavorable anatomy multiple alternative access sites and techniques are available to proceed with successful percutaneous EVAR including: brachial access, radial access, surgical conduits, transapical, transcaval, “Pave and Crack” techniques and Solopath device. Vascular interventionalists who perform EVAR should be aware of these access techniques to expand their ability to treat a wider spectrum of patients even in the presence of unfavorable vascular anatomy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Walker TG, Kalva SP, Yeddula K, et al. Clinical Practice Guidelines for Endovascular Abdominal Aortic Aneurysm Repair: Written by the Standards of Practice Committee for the Society of Interventional Radiology and Endorsed by the Cardiovascular and Interventional Radiological Society of Europe and the Canadian Interventional Radiology Association. J Vasc Interv Radiol 2010;21:1632-55. [Crossref] [PubMed]
- Bensley RP, Hurks R, Huang Z, et al. Ultrasound-guided percutaneous endovascular aneurysm repair success is predicted by access vessel diameter. J Vasc Surg 2012;55:1554-61. [Crossref] [PubMed]
- Sarmiento JM, Wisniewski PJ, Do NT, et al. The Kaiser Permanente experience with ultrasound-guided percutaneous endovascular abdominal aortic aneurysm repair. Ann Vasc Surg 2012;26:906-12. [Crossref] [PubMed]
- Nelson PR, Kracjer Z, Kansal N, et al. A multicenter, randomized, controlled trial of totally percutaneous access versus open femoral exposure for endovascular aortic aneurysm repair (the PEVAR trial). J Vasc Surg 2014;59:1181-93. [Crossref] [PubMed]
- Lee WA, Brown MP, Nelson PR, et al. Total percutaneous access for endovascular aortic aneurysm repair ("Preclose" technique). J Vasc Surg 2007;45:1095-101. [Crossref] [PubMed]
- Lee WA, Brown MP, Nelson PR, et al. Midterm outcomes of femoral arteries after percutaneous endovascular aortic repair using the Preclose technique. J Vasc Surg 2008;47:919-23. [Crossref] [PubMed]
- Lee W, Brown M, Nelson P, et al. Midterm outcomes of femoral arteries after percutaneous endovascular aortic repair using the Preclose technique. J Vasc Surg 2008;47:919-23. [Crossref] [PubMed]
- Okuyama K, Jilaihawi H, Kashif M, et al. Transfemoral access assessment for transcatheter aortic valve replacement: evidence-based application of computed tomography over invasive angiography. Circ Cardiovascular Imaging 2014;8. [Crossref] [PubMed]
- Picel A, Kansal N. Essentials of endovascular abdominal aortic aneurysm repair imaging: preprocedural assessment. AJR Am J Roentgenol 2014;203:W347-57. [PubMed]
- Bensley R, Hurks R, Huang Z, et al. Ultrasound-guided percutaneous endovascular aneurysm repair success is predicted by access vessel diameter. J Vasc Surg 2012;55:1554-61. [Crossref] [PubMed]
- Krishnaswamy A, Parashar A, Agarwal S, et al. Predicting vascular complications during transfemoral transcatheter aortic valve replacement using computed tomography: a novel area-based index. Catheter Cardiovasc Interv 2014;84:844-51. [Crossref] [PubMed]
- Lombardo A, Van Den Berg J. Preventing vascular access site complications during interventional procedures. Interv Cardiol 2010;2:829-40. [Crossref]
- Vatakencherry G, Ghandi R, Molloy C. Endovascular access for challenging anatomies in peripheral vascular interventions. Tech Vasc Interv Radiol 2016;19:113-22. [Crossref] [PubMed]
- Van Den Berg J. Optimal technique for common femoral artery access. Endovascular Today 2013:58-61
- Manunga J, Gloviczki P, Oderich G, et al. Femoral artery calcification as a determinant of success for percuatneous access for endovascular abdominal aortic aneurysm repair. J Vasc Surg 2013;58:1208-12. [Crossref] [PubMed]
- Alvarez-Tostado J, Moise M, Bena J, et al. The brachial artery: a critical access for endovascular procedures. J Vasc Surg 2009;49:378-85; discussion 385. [Crossref] [PubMed]
- Sos T. Brachial and axillary arterial access. Endovascular Today. 2010: 55-57. Available online: https://evtoday.com/pdfs/et0510_feature_sos.pdf
- Criado FJ, Wilson E, Abul-Khoudoud O, et al. Brachial artery catheterization to facilitate endovascular grafting of abdominal aortic aneurysm: safety and rationale. J Vasc Surg 2000;32:1137-41. [Crossref] [PubMed]
- Roy AK, Garot P, Louvard Y, et al. Comparison of transradial vs transfemoral access for aortoiliac and femoropopliteal interventions. J Endovasc Ther 2016;23:880-8. [Crossref] [PubMed]
- Stavroulakis K, Usai M, Torsello G, et al. Efficacy and safety of transbrachial access for iliac endovascular interventions. J Endovasc Ther 2016;23:454-60. [Crossref] [PubMed]
- Fischman A, Patel R. The Time Is Now for Transradial Intervention. Endovascular Today. 2013: 50-58. Available online: https://evtoday.com/pdfs/et0413_F1_Fischman.pdf
- Kotowycz MA, Dzavik V. Radial Artery Patency After Transradial Catheterization. Circ Cardiovasc Interv 2012;5:127-33. [Crossref] [PubMed]
- Boyer N, Beyer A, Gupta V, et al. The effects of intra-arterial vasodilators on radial artery size and spasm: implications for contemporary use of trans-radial access for coronary angiography and percutaneous coronary intervention. Cardiovasc Revasc Med 2013;14:321-4. [Crossref] [PubMed]
- Yano OJ, Faries PL, Morrissey N, et al. Ancillary techniques to facilitate endovascular repair of aortic aneurysms. J Vasc Surg 2001;34:69-75. [Crossref] [PubMed]
- Diethrich EB. Technical tips for thoracic aortic endografting. Semin Vasc Surg 2008;21:8-12. [Crossref] [PubMed]
- Kappert U, Ghazy T, Ouda A, et al. Transapical endovascular stenting of penetrating atherosclerotic ulcer of ascending aorta. Ann Thorac Surg 2013;96:e101-3. [Crossref] [PubMed]
- Murakami T, Nishimura S, Hosono M, et al. Transapical endovascular repair of thoracic aortic pathology. Ann Vasc Surg 2017;43:56-64. [Crossref] [PubMed]
- Weigang E, Weiler H, Frieß T, et al. The heart as access to the aorta. Eur J Cardiothorac Surg 2013;44:559-61; discussion 561-2. [Crossref]
- Flege JB Jr, Aberg T. Transventricular aortic cannulation for repair of aortic dissection. Ann Thorac Surg 2001;72:955-6. [Crossref] [PubMed]
- Uflacker A, Lim S, Ragosta M, et al. Transcaval aortic access for percutaneous thoracic aortic aneurysm repair: initial human experience. J Vasc Interv Radiol 2015;26:1437-41. [Crossref] [PubMed]
- Greenbaum AB, O’Neill WW, Paone G, et al. Caval-aortic access to allow transcatheter aortic valve replacement in otherwise ineligible patients: initial human experience. J Am Coll Cardiol 2014;63:2795-804. [Crossref] [PubMed]
- Martinez-Clark PO, Singh V, Cadena J, et al. Transcaval retrograde transcatheter aortic valve replacement for patients with no other access. JACC Cardiovasc Interv 2014;7:1075-7. [Crossref] [PubMed]
- Greenbaum AB, Babaliaros VC, Chen MY, et al. Transcaval access and closure for transcatheter aortic valve replacement. J Am Coll Cardiol 2017;69:511-21. [Crossref] [PubMed]
- Hinchliffe RJ, Ivancev K, Sonesson B, et al. “Paving and Cracking”: An Endovascular Technique to Facilitate the Introduction of Aortic Stent-Grafts through Stenosed Iliac Arteries. J Endovasc Ther 2007;14:630-3. [Crossref] [PubMed]
- Chan Y, Cheng S. Does difficult iliac access still exist? Endovascular Today, Mar 2012, 83-86. Available online: https://evtoday.com/pdfs/et0312_F5_Cheng.pdf
- Terumo and Company. Terumois.com, Solopath Balloon Expandable Transfemoral System, Product Overview. Available online: http://www.terumois.com/


