Interaction between platelets and endothelium: from pathophysiology to new therapeutic options
Introduction
Platelets are the “rulers” of haemostasis and thrombosis, but recently their role in vasomotor function, chemotaxis, inflammation and atherosclerosis has been very well-recognized and extensively studied. The reciprocal and often complex interactions with the endothelium and leucocytes are object of continuous studies and future targeted drug therapies. Low-grade inflammation, endothelial dysfunction, and platelet hyper-reactivity are all independently associated with an increased risk of cardiovascular events. In this context, antiplatelet treatment for patients with coronary artery disease (CAD), beyond its major treatment impact on the reduction of thrombotic events through platelet inhibition, seems to have an important role on platelet and endothelium interplay, by decreasing inflammation, improving endothelial function and decelerating atherosclerosis progression.
This review article describes the cross talk between platelets and endothelial cells (ECs), in particular those who promote atherosclerosis. Moreover, we summarize the current knowledge about the influence of contemporary antiplatelet regimens with their individual characteristics on those complex processes.
Platelets
Platelets are anucleated cells, as they derive from megakaryocytes’ cytoplasmic fragmentation in the bone marrow. Morphologically they have a discoid form and a diameter of 2–3 µm. Their normal count in humans is 150,000–400,000/µL, with a lifespan of 9–10 days (1).
Platelet’s membrane includes a large number of invaginations that communicate to the outer part through numerous pores resulting in a particularly large surface area called open canalicular system (OCS) (2). Three types of granules can be found in platelet’s cytoplasm. Largest ones are alpha granules whom number is 50–60 per platelet (3). They contain coagulation factors like von Willebrand factor (vWF), fibrinogen, V, XI, XIII factors and growth factors like vascular endothelial growth factor (VEGF), tissue growth factor β (TGF-β), platelet factor 4 (PF-4), P-selectin, thrombospondin, as well as a variety of chemokines. Platelet lysosomes, contain mainly acid hydrolases, cathepsin D and E which are able to degrade glycoproteins, glycolipids and glycosaminoglycans (4). They are implicated in the remodeling of the extracellular matrix and to thrombus regulation.
Dense granules count 4–8 per platelet, containing high concentrations of calcium and phosphates, serotonin and adenine nucleotides, substances that promote vasoconstriction and platelet aggregation (5). They contain also adhesion proteins such as P-selectin, glycoprotein IIb/IIIa and Ib (GPIIb/IIIa and GPIb). P-selectin, a large adhesion molecule of the selectin family, is expressed in elevated concentrations on platelets surface during platelets activation mediating interactions between ECs, leucocytes and other platelets (6,7). Platelets actions are mediated through a variety of receptors and mediator molecules. GPVI and GPa2b1 integrin represent the main collagen receptors that mediate platelet interactions with subendothelial collagen during primary haemostasis (8). The GPIb/IX/V receptor complex is composed from four parts, GPIba, GPIbβ, GPΙΧ and GPV. GPIba receptor is the principal platelet receptor for vWF (9). The GPIIB/IIIa receptor is composed from two subunits, alpha and beta. It is found on platelets surface in an inactive form and changes structure during platelets activation, having a central role in platelets aggregation (10,11). Fibrinogen, fibronectin, vitronectin and vWF constitute the main ligands of this important receptor.
Protease activated receptors (PAR) which are members of G protein receptors, mediate the action of thrombin, one of the most important activators inducing platelet activation and granule release (12).
Thromboxane A2 (TXA2) is an arachidonic acid product that induces potent platelet activation, aggregation and degranulation, in cooperation with other platelet agonists. These actions are mediated via two isoforms of receptors, TPa which represents the prevalent form on human platelets and TPb, members of the G protein-coupled receptor family (13,14).
Adenosine nucleotides ADP and ATP are involved in platelet adhesion and aggregation. Their actions, mainly driven by ADP, derive from the stimulation of two G protein-coupled receptors, the P2Y1 and P2Y12. The stimulation of P2Y1 receptor results in increased intracellular calcium levels and initiation of platelet shape change and aggregation (15). At the same time, the stimulation of the P2Y12 receptor inhibits adenylyl cyclase activity, resulting in reduction of the intracellular levels of CAMP and subsequent amplification and stabilization of platelets aggregation process, enhancing platelet response to other agonists (15,16).
Endothelium
The entire vascular system is covered by a single strut of ECs. Endothelium which was originally considered as a simple passive barrier, is now viewed as an organ whose normal functioning is crucial for maintaining vascular health and whose dysfunction is crucial in the initiation, progression and clinical complications of vascular disease (17). ECs play an important role in vascular tone, regulating thrombosis and thrombolysis, platelets adherence and activation in order to maintain an undisturbed blood flow under physiologic conditions. At the same time endothelium is of paramount importance in body homeostasis as it regulates the transfer of different molecules through blood vessels (17). The role of the endothelium as a semipermeable barrier is one of its most basic functions. It regulates transport of macromolecules between the vascular lumen and vascular smooth muscle.
Under physiologic conditions platelets circulate without adhering to intact and inactive endothelium. A layer of proteoglycans and glycoproteins is present between ECs and blood, known as glycocalyx (18). This structure regulates endothelium permeability and endothelium interactions with other cells such as platelets and leucocytes, mainly repelling them through its negative charge and limiting the endothelial exposure to adhesion molecules. In addition, prostacyclin, a product of arachidonic acid metabolism in endothelial cells with vasodilating properties, inhibits platelets aggregation by elevating intracellular levels of CAMP (19,20). This substance has a synergistic action with nitric oxide (NO). NO is the most important endothelial derived relaxing factor and inhibits platelets activation by enhancing the production of guanosine monophosphate (GMP). As a result, intracellular Ca2+ decreases and transformation of GPIIb/IIIa platelet receptor together with the binding of the integrin to fibrinogen is suppressed (19,20) The ecto-ADPase (CD-39), placed on the surface of endothelial cells hydrolyzes both ATP and ADP in order to generate AMP, attenuating platelets reactivity (Figure 1) (19,21). The endothelium is also producing substances with vasoconstrictive and pro-thrombotic behavior, like TXA2 (22) which promotes platelet aggregation, expresses adhesive co-factors for platelets such as vWF, fibronectin and thrombospondin, and procoagulant factors such as factor V. Endothelial-derived vasoconstrictors are opposing the action of the endothelial-derived vasodilators (23). Most important among them are endothelin-1 (ET-1), angiotensin-II (ANG-II) and vasoconstrictor prostaglandins (24-26). Endothelial dysfunction is a disturbance in normal endothelial function as a consequence of different stimuli or clinical conditions. This disturbed balance may lead to platelets aggregation and adhesion to the endothelium thereby activating it and encouraging leukocyte adhesion, as well as releasing platelet-derived growth factors (PDGFs) that stimulate intimal hyperplasia.
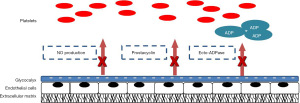
Platelets and endothelium interaction
Atherosclerosis is characterized by two parallel operations: infiltration of inflammatory cells and lipid accumulation in the intima of the arterial wall (27). Chronic inflammation defines the evolution of the atherosclerotic plaque, from the earliest stages till its rupture and atherothrombosis. There is growing evidence that platelets, through complex interactions with endothelial and inflammatory cells, play a major role in the initiation and preservation of this process (28-30).
The initiation of atherosclerosis requires the presence of either activated platelets or activated endothelial cells or both (31). A series of pathologic stimuli like hypertension, diabetes mellitus, smoking and dyslipidemia can lead to endothelial dysfunction and triggering of the atherosclerotic process. Oxidant stress and in particular low-density lipoprotein (LDL) accumulation, results in reduced NO levels and triggering of inflammatory pathways (32,33). At the same time oxidized LDL enhances ET-1 expression in ECs, inducing vasoconstriction and proliferation of fibroblasts and vascular smooth muscle cells (VSMCs). In addition, oxidized LDL increases the production and secretion, of fibronectin, thrombospondin and a variety of other glycoproteins, reinforcing leukocytes’ and platelets’ adhesion (Figure 2) (34).
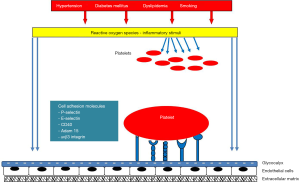
Alternatively circulating activated platelets can secrete inflammatory and mitogenic factors in the local microenvironment, leading to ECs activation and monocytes’ recruitment (35). The initial contact between platelets and endothelial cells is mediated by P-selectin (CD62P), E-selectin and PSGL-1, which are expressed on the surface of EC during inflammatory processes (36-38). The presence of integrins which are transmembrane receptors mediating cells adhesion on platelets surface, further enhances this binding, leading to a more stable adhesion between platelets and ECs (39,40). Furthermore, ECs express ADAM-15, member of the ADAM (a disintegrin and metalloproteinase) family, a transmembrane cell-surface protein that binds to platelets through the GIIb/IIIa receptor, promoting their activation (41).
Platelets enter in an activation state, releasing a plethora of inflammatory mediators and growth factors such as chemokines, TNF superfamily factors, adhesion proteins and coagulation factors and other mediators further activating the ECs and recruiting monocytes/macrophages (42-45). Chemokines family includes platelet factor 4/CXCL4 that promotes monocyte attraction and differentiation into macrophages (46) as well as inhibition of LDL degradation (47,48), RANTES/CCL5 a major protagonist in leucocyte/monocyte recruitment (49-51), CXCL12/SDF-1, monocyte chemoattractant protein-1 (MCP-1) (52), interleukins (53) and TGF-β1. TNF superfamily factors refers mainly to CD40L/TNFSF5, a monocyte chemoattractant that also enhances the action of metalloproteins and downregulates NO production (54,55) and LIGHT/TNFSF14 (45,56) which is a potent ECs and monocyte/macrophage activator. Adhesion proteins, platelet endothelial cell adhesion molecule (PECAM), P-selectin, fibronectin, vWF, vitronectin and fibrinogen, coagulation factors including plasminogen, protein S, factor V and matrix metalloproteinase are also released. Other mediators participating in this activation state are serotonin, histamine and PDGF. Platelets express also chemokine receptors such as CXCR4, CCR-1, -3, -4 (43) in response to inflammatory signals from ECs and leucocytes that further stimulate them.
The cumulative result of all those substances’ action is the modulation of biological properties of ECs, leucocytes/monocytes and platelets themselves, in terms of chemotaxis, differentiation, cell adhesion, proliferation and aggregation. In this way platelets promote inflammation in a self-sustaining way preparing the next step which is the formation of atherosclerotic plaque (Figure 3).
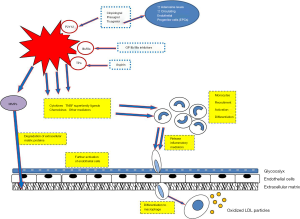
Platelet-leucocyte aggregates (PLAs)
Activated platelets form aggregates with circulating leucocytes (PLAs) (57-59). The initial binding of platelets to leukocytes is mediated by platelet P-selectin binding to leukocyte PSGL-1. This is followed by further platelets activation by leucocytes, while platelets promote leucocytes transformation in more adhesive and migratory forms, in a continuous interaction. PLAs are circulating in a “sticky” form, roll on endothelium, a process largely mediated by endothelial selectins, activating ECs, promoting monocytes transmigration and formation of atherosclerotic lesions in the vessel wall.
Platelets and endothelial progenitor cells (EPCs)
EPCs can been found in peripheral blood as mononuclear cells with a myeloid-endothelial intermediate phenotype, as they express VEGF receptor-2 (VEGFR-2) but also CD34 and CD133. These cells are involved in vascular repair processes after vascular injury or ischemia. They sustain reendothelialization and neovascularization, via their potential to proliferate and differentiate into endothelial-phenotype cells. The number of those cells in peripheral blood of patients with cardiovascular disease has been shown to predict cardiovascular outcomes. In patients with CAD, EPCs have attenuated migration, proliferation and differentiation. Platelets not only mediate EPCs recruitment at the site of vascular injury, but they also strongly support their proliferation, maturation, differentiation to endothelial-phenotype cells and the acquisition of functional properties such as NO production in vitro (60). Those platelet actions are independent from direct contact between the two cell populations and seem to be mediated through PDGF and basic fibroblast growth factor (BFGF).
Antiplatelet agents and endothelium
The last two decades became more evident that complex interactions between platelets, ECs and monocytes/macrophages, determine the evolution of the atherosclerotic lesions, from the early stages to the plaque rupture and atherothrombosis. Antiplatelet therapy is a cornerstone therapy for CAD patients as it prevents thrombotic events. Older antiplatelet agents like aspirin and clopidogrel and newer more potent agents like prasugrel and ticagrelor have been proven effective in all the clinical spectrum of CAD patients, especially those undergoing percutaneous coronary intervention (PCI) or suffering from acute coronary syndromes (ACS). Current antiplatelet medications offer clinical benefits not only due to their well-recognized antithrombotic effect, but also via the attenuation of platelet inflammatory action, impediment of P2Y12 activation effects in other cells and through other pathways.
Aspirin remains the basis of antiplatelet therapy for thrombotic events prevention. Aspirin is considered to prevent platelet aggregation by inhibition of platelet TXA2 synthesis due to irreversible inactivation of platelet cyclooxygenase-1 (COX-1). Since platelet-derived TXA2 increases vessel wall constriction and enhances proliferation of vascular cells (61), inhibition of its synthesis by aspirin may have indirect effects on the interaction between platelets and vascular wall. Hypercholesterolemic mice lacking the TXA2 receptor (TP) develop less atherosclerosis (62), suggesting an anti-atherosclerotic activity of aspirin. Lots of studies have also shown inhibition of experimental vascular inflammation by aspirin, some even at low antiplatelet doses, including the reduction of inflammatory markers (CRP, M-CSF, MCP-1) and pro-inflammatory factors (TXA2, S1P, sICAM-1, IL-6) (63). Aspirin inhibits NO consumption by platelets from healthy subjects, but its beneficial effects on NO bioactivity may be compromised in some hypercholesterolemic patients (64). In the clinical level, platelet inhibition with aspirin has been shown to modulate acetylcholine-induced peripheral vasodilation in patients with atherosclerosis, via inhibition of COX-dependent vasoconstrictors (65). In addition, administration of low dose aspirin seems to improve the endothelium dependent vasodilation in patients with arterial hypertension (66).
Platelet inhibition by specific GPIIb/IIIa receptor blockade has provided clear evidence of an unequivocal benefit in reducing ischemic events, especially in the setting of ACS patients undergoing PCI. Tirofiban, a selective IIb/IIIa receptor inhibitor, relaxes the coronary artery via an endothelium-dependent NO-cGMP signaling as confirmed by the activation of PI3K/Akt/eNOS. Heitzer et al. demonstrated that platelet GPIIb/IIIa receptor blockade with tirofiban and eptifibatide improved endothelium-dependent vasodilation in patients with symptomatic CAD (67). The inhibitory effect of the NO synthase inhibitor L-NMMA was significantly increased during platelet GPIIb/IIIa blockade, indicating that the beneficial effect of GPIIb/IIIa blockade is mainly owing to enhanced NO bioactivity. Finally, Warnholtz et al. showed that tirofiban can reverse PCI induced endothelial dysfunction in forearm vessels of patients with stable CAD (68).
Platelet P2Y12 inhibitors form a major part of the treatment strategy for patients with CAD due to the importance of the platelet P2Y12 receptor in mediating arterial thrombosis. The most commonly used agents are clopidogrel and the newer and more potent prasugrel and ticagrelor. These agents are selective P2Y12 inhibitors, with different pharmacodynamic and pharmacokinetic properties. Prasugrel and ticagrelor with in vitro and in vivo stronger antiplatelet action than clopidogrel, have proven better clinical efficacy than clopidogrel, showing less thrombotic events in large ACS populations, with the cost of increased bleeding events. Besides platelet’s activation and thrombus formation, P2Y12 receptor activation leads also to reduce platelets NO responsiveness and reinforces the production of reactive oxygen species (ROS). ROS can further activate platelets, enhance platelets-leukocytes interactions and accelerate lipids oxidation and inflammation processes. P2Y12 receptors expression can be also found in other cells, such as leukocytes, dendritic cells, VSMC, endothelial cells and neurons (69). In ECs the activation of P2Y12 receptor decreases the intracellular cAMP concentration, with negative effects on endothelial barrier functions promoting VSMC contraction and vasoconstriction.
In patients with stable CAD, clopidogrel treatment is associated with improvement of forearm blood flow and reduction in values of inflammation and oxidative stress biomarkers (70). Warnholtz et al. showed that clopidogrel administration in patients with CAD, improved endothelial function assessed by measurement of flow mediated dilation of the brachial artery, in a dose dependent relationship (71). Consistently, Patti et al. in ARMYDA-150mg study revealed that in patients undergoing PCI, administration of 150 mg clopidogrel instead of 75 mg as a maintenance dose in addition to aspirin, resulted in higher platelet inhibition, amelioration of endothelial function and reduction of inflammatory indices (72). Bonello et al. [2010] showed that adequate platelet inhibition in response to clopidogrel treatment attenuates endothelial injury estimated with the number of circulating endothelial cells (73). Muller et al. demonstrated that endothelial dysfunction, (evaluated with endothelial peripheral arterial tonometry, vWF antigen level and ristocetin co-factor activity) is associated with a greater residual platelet reactivity after 600 mg clopidogrel administration before scheduled PCI (74). In another study from the same group, a significant proportionate improvement of endothelial function was achieved after 600 mg clopidogrel loading dose in patients with low residual platelet reactivity measured with VerifyNow assay [P2Y12 reaction unit (PRU) <240]. A similar effect was not observed in patients with high platelet reactivity (PRU ≥240) (75). Nevertheless, this clopidogrel’s positive effect on endothelial function might not be preserved after long term treatment (76). Fujisue et al. showed that endothelial function evaluated with peripheral arterial tonometry was impaired in patients with residual platelet reactivity ≥230 PRU after administration of aspirin and clopidogrel, in a group that lacked CYP2C19*2 or *3 loss of function allele (77). However, in another study Haynes et al. did not find any significant relationship between endothelial function evaluated with flow mediated dilation and platelets activation evaluated with flow cytometric determination of GPIIb/IIIa activation, surface P-selectin expression and presence of monocyte-platelet aggregates (78). Those controversies might be explained due to the different methods used for endothelial function and platelet activation evaluation and the different study populations.
Prasugrel, similar to clopidogrel, is a prodrug requiring hepatic metabolism to generate an active metabolite that acts as an irreversibly binding P2Y12 antagonist. Prasugrel improves mid-term vascular dysfunction and inflammation in patients with unstable angina. It is also associated with a reduction of platelet-derived inflammatory markers and platelet-leukocyte interaction (79).
Ticagrelor seems to offer additional benefits than clopidogrel and prasugrel on endothelial function (80). Ticagrelor beyond its profound platelet inhibition seems to enhance adenosine levels by blocking adenosine reuptake in blood cells (81), resulting in vessels dilatation. In addition, it causes ATP release from red blood cells (82), enhancing the endothelium release of vasodilatory mediators such as prostacyclin, NO and endothelial hyperpolarizing factor. In CLOTILDIA STUDY 42 patients with diabetes mellitus and stable coronary disease were randomly assigned to receive either ticagrelor or clopidogrel (83). Brachial artery reactivity, as well as post treatment platelet inhibition with VerifyNow assay was evaluated in all patients. The results of this study suggested that ticagrelor, besides a more potent platelet inhibition, caused a significant improvement of brachial artery vascular tone, both by endothelial function amelioration and with enhancement of nitroglycerin-mediated dilation, an endothelium independent mechanism. Furthermore, Torngren et al. found that in ACS patients, ticagrelor administration was associated with improvement of endothelial function, a benefit that was not observed in patients receiving clopidogrel or prasugrel (84). Similar encouraging results were observed in animal models, concerning ticagrelor’s impact on atherosclerosis initiation and progression. Rusnak et al. showed stabilization of fibrous cap size and necrotic core extension of an already established atherosclerotic plaque in mice after 25 weeks of ticagrelor treatment (85). In other animal models ticagrelor seems to attenuate neointimal formation or hyperplasia (86,87). Interestingly ticagrelor exerts beneficial effects on endothelium also through other pathways. Bonello et al. found that patients treated with ticagrelor had an important increase in numbers of circulating EPCs of CD34+133PC and CD34+KDR+EPC types, compared to clopidogrel treated patients (88). This effect of ticagrelor was independent of platelet activity and may contribute to endothelial repair-regeneration processes. Ticagrelor beyond P2Y12 reversible inhibition appears to have additional antithrombotic properties. Tissue factor (TF) is the molecule that promotes the extrinsic coagulation cascade and it is expressed in different cells, including endothelial cells. Patients with cardiovascular risk factors or ACS seem to have elevated levels of TF. Reiner et al. studied TF expression and activity in human aortic endothelial cells, treated with ticagrelor or clopidogrel active metabolite (CAM), after stimulation with TNF-α, a molecule that promotes TF expression and activity. They found that ticagrelor unlike CAM, attenuated both TNF-α induced endothelial expression through proteasomal degradation, as well as TF activity itself (89). These results showed that ticagrelor exhibits endothelial-specific antithrombotic properties independent from P2Y12 receptor inhibition. Those findings were confirmed in ticagrelor-treated mice where attenuated endothelial TF expression levels were observed. A summary of all the clinical studies showing the impact of antiplatelet treatment on endothelial function is shown in the Table 1.
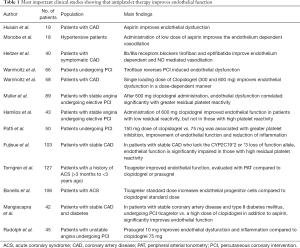
Full table
Future perspectives
Platelet interactions with endothelium have a well-recognized, important contribution to atherosclerosis progression and to atherothrombosis related clinical events. Antiplatelet agents seem in general to interfere to this process beyond their pure antiplatelet action by improving endothelial function. This might be a direct effect but there are indications that the more potent the antiplatelet action is the more improvement in endothelial function is observed. Moreover, there are pleiotropic actions of some newer antiplatelet agents like ticagrelor, that may further help to reduce vascular inflammation, endothelial dysfunction and atherogenesis beyond its class effect. Whether those observations can be translated in clinically significant reduction of atherosclerotic plaque progression is a first important question. A second question is whether new agents can target platelet endothelium interactions in a more specific way, “blocking” some part of atherosclerosis progression. There are some preliminary data in a porcine model, showing that ticagrelor improves endothelial function and reduces neointimal formation and peri-strut inflammation after drug-eluting stent (DES) implantation while potent platelet inhibition by prasugrel did not result in any such effects (90). Those findings are promising in the clinical level as ticagrelor therapy might reduce in stent restenosis after PCI due to reduced inflammation and improved macro and micro vascular endothelial function. In addition, the armamentarium against atherosclerosis has been significantly enriched recently. Beyond the newer potent antiplatelet agents and their potential to act against plaque progression, newer agents targeting to cholesterol lowering have been added to everyday clinical practice. Monoclonal antibodies acting as PCSK-9 inhibitors reduce impressively LDL-C when added to maximally tolerated statin therapy, improving patient’s clinical outcome (91,92). Moreover, they have shown significant coronary plaque regression and could provide additional gain in ACS patients through plaque stabilization, anti-inflammatory or antiplatelet effects (93). Possible synergistic effects of those agents with intensive platelet inhibition could provide further clinical benefit in patients with CAD. Answers to those and other similar questions are expected from future bench and clinical studies.
Conclusions
Platelets together with endothelium have a major role in inflammation and atherosclerosis progression. Today it is a common knowledge that platelet’s inhibition can reduce endothelial dysfunction, vessel wall inflammation and attenuate atherosclerosis progression and atherothrombotic events. Concerning that antiplatelet agents beyond platelet inhibition have differential interactions with endothelium and vessel wall, tailored antiplatelet regimens, depending on atherosclerosis extent, type of interventions and possible modification of vessel wall inflammation might help improving outcomes in the clinical level. Most of the present data although experimental or coming from small clinical series, hold promise for further development of the antiplatelet armamentarium and give motivation for larger clinical studies.
Acknowledgements
None.
Footnote
Conflicts of Interest: M Hamilos has received speaker fees and participated in advisory boards for Astra-Zeneca. The other authors have no conflicts of interest to declare.
References
- Hanson SR, Slichter SJ. Platelet kinetics in patients with bone marrow hypoplasia: evidence for a fixed platelet requirement. Blood 1985;66:1105-9. [PubMed]
- White JG, Clawson CC. The surface-connected canalicular system of blood platelets–a fenestrated membrane system. Am J Pathol 1980;101:353-64. [PubMed]
- Blair P, Flaumenhaft R. Platelet alpha-granules: basic biology and clinical correlates. Blood Rev 2009;23:177-89. [Crossref] [PubMed]
- Ciferri S, Emiliani C, Guglielmini G, et al. Platelets release their lysosomal content in vivo in humans upon activation. Thromb Haemost 2000;83:157-64. [Crossref] [PubMed]
- Rendu F, Brohard-Bohn B. The platelet release reaction: granules constituents, secretion and functions. Platelets 2001;12:261-73. [Crossref] [PubMed]
- Merten M, Thiagarajan P. P-selectin in arterial thrombosis. Z Kardiol 2004;93:855-63. [Crossref] [PubMed]
- Tailor A, Cooper D, Granger DN. Platelet-vessel wall interactions in the microcirculation. Microcirculation 2005;12:275-85. [Crossref] [PubMed]
- Farndale RW, Lisman T, Bihan D, et al. Cell-collagen interactions: the use of peptide Toolkits to investigate collagen-receptor interactions. Biochem Soc Trans 2008;36:241-50. [Crossref] [PubMed]
- Andrews RK, Gardiner EE, Shen Y, et al. Glycoprotein Ib-IX-V. Int J Biochem Cell Biol 2003;35:1170-4. [Crossref] [PubMed]
- Niiya K, Hodson E, Bader R, et al. Increased surface expression of the membrane glycoprotein IIb/IIIa complex induced by platelet activation. Relationship to the binding of fibrinogen and platelet aggregation. Blood 1987;70:475-83. [PubMed]
- Ma YQ, Qin J, Plow EF. Platelet integrin alpha(IIb)beta(3): activation mechanisms. J Thromb Haemost 2007;5:1345-52. [Crossref] [PubMed]
- Kahn ML, Nakanishi-Matsui M, Shapiro MJ, et al. Protease-activated receptors 1 and 4 mediate activation of human platelets by thrombin. J Clin Invest 1999;103:879-87. [Crossref] [PubMed]
- Murugappan S, Shankar H, Kunapuli SP. Platelet receptors for adenine nucleotides and thromboxane A2. Semin Thromb Hemost 2004;30:411-8. [Crossref] [PubMed]
- Habib A, FitzGerald GA, Maclouf J. Phosphorylation of the thromboxane receptor alpha, the predominant isoform expressed in human platelets. J Biol Chem 1999;274:2645-51. [Crossref] [PubMed]
- Murugappan S, Kunapuli SP. The role of ADP receptors in platelet function. Front Biosci 2006;11:1977-86. [Crossref] [PubMed]
- Dorsam RT, Kunapuli SP. Central role of the P2Y12 receptor in platelet activation. J Clin Invest 2004;113:340-5. [Crossref] [PubMed]
- Galley HF, Webster NR. Physiology of the endothelium. Br J Anaesth 2004;93:105-13. [Crossref] [PubMed]
- Alphonsus CS, Rodseth RN. The endothelial glycocalyx: a review of the vascular barrier. Anaesthesia 2014;69:777-84. [Crossref] [PubMed]
- Jin RC, Voetsch B, Loscalzo J. Endogenous mechanisms of inhibition of platelet function. Microcirculation 2005;12:247-58. [Crossref] [PubMed]
- Moncada S. Adventures in vascular biology: a tale of two mediators. Philos Trans R Soc Lond B Biol Sci 2006;361:735-59. [Crossref] [PubMed]
- Marcus AJ, Broekman MJ, Drosopoulos JH, et al. Role of CD39 (NTPDase-1) in thromboregulation, cerebroprotection, and cardioprotection. Semin Thromb Hemost 2005;31:234-46. [Crossref] [PubMed]
- FitzGerald GA. Mechanisms of platelet activation: thromboxane A2 as an amplifying signal for other agonists. Am J Cardiol 1991;68:11B-15B. [Crossref] [PubMed]
- Alexander RW, Griendling KK. Signal transduction in vascular smooth muscle. J Hypertens Suppl 1996;14:S51-4. [PubMed]
- Rosen B, Barg J, Zimlichman R. The effects of angiotensin II, endothelin-1 and protein kinase C inhibitor on DNA synthesis and intracellular calcium mobilization in vascular smooth muscle cells from young normotensive and spontaneously hypertensive rats. Am J Hypertens 1999;12:1243-51. [Crossref] [PubMed]
- Hahn AW, Resink TJ, Kern F, et al. Peptide vasoconstrictors, vessel structure, and vascular smooth-muscle proliferation. J Cardiovasc Pharmacol 1993;22:S37-43. [Crossref] [PubMed]
- Inoue A, Yanagisawa M, Kimura S, et al. The human endothelin family: three structurally and pharmacologically distinct isopeptides predicted by three separate genes. Proc Natl Acad Sci U S A 1989;86:2863-7. [Crossref] [PubMed]
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005;352:1685-95. [Crossref] [PubMed]
- von Hundelshausen P, Weber C. Platelets as immune cells: bridging inflammation and cardiovascular disease. Circ Res 2007;100:27-40. [Crossref] [PubMed]
- May AE, Seizer P, Gawaz M. Platelets: inflammatory firebugs of vascular walls. Arterioscler Thromb Vasc Biol 2008;28:S5-10. [Crossref] [PubMed]
- Smyth SS, McEver RP, Weyrich AS, et al. Platelet functions beyond hemostasis. J Thromb Haemost 2009;7:1759-66. [Crossref] [PubMed]
- Palomo I, Toro C, Alarcón M. The role of platelets in the pathophysiology of atherosclerosis Mol Med Rep 2008;1:179-84. (Review). [PubMed]
- Lusis AJ. Atherosclerosis. Nature 2000;407:233-41. [Crossref] [PubMed]
- Willerson JT, Kereiakes DJ. Endothelial dysfunction. Circulation 2003;108:2060-1. [Crossref] [PubMed]
- Badrnya S, Butler LM, Söderberg-Naucler C, et al. Platelets directly enhance neutrophil transmigration in response to oxidised low-density lipoprotein. Thromb Haemost 2012;108:719-29. [Crossref] [PubMed]
- Gawaz M, Langer H, May AE. Platelets in inflammation and atherogenesis. J Clin Invest 2005;115:3378-84. [Crossref] [PubMed]
- Frenette PS, Johnson RC, Hynes RO. Platelets roll on stimulated endothelium in vivo: an interaction mediated by endothelial P-selectin. Proc Natl Acad Sci U S A 1995;92:7450-4. [Crossref] [PubMed]
- Frenette PS, Moyna C, Hartwell DW, et al. Platelet-endothelial interactions in inflamed mesenteric venules. Blood 1998;91:1318-24. [PubMed]
- Subramaniam M, Frenette PS, Saffaripour S, et al. Defects in hemostasis in P-selectin-deficient mice. Blood 1996;87:1238-42. [PubMed]
- Gawaz M, Neumann FJ, Dickfeld T, et al. Vitronectin receptor (alpha(v)beta3) mediates platelet adhesion to the luminal aspect of endothelial cells: implications for reperfusion in acute myocardial infarction. Circulation 1997;96:1809-18. [Crossref] [PubMed]
- Bombeli T, Schwartz BR, Harlan JM. Adhesion of activated platelets to endothelial cells: evidence for a GPIIbIIIa-dependent bridging mechanism and novel roles for endothelial intercellular adhesion molecule 1 (ICAM-1), alphavbeta3 integrin, and GPIbalpha. J Exp Med 1998;187:329-39. [Crossref] [PubMed]
- Langer H, May AE, Bultmann A, et al. ADAM 15 is an adhesion receptor for platelet GPIIb-IIIa and induces plateletactivation. Thromb Haemost 2005;94:555-61. [Crossref] [PubMed]
- Weber C. Platelets and chemokines in atherosclerosis: partners in crime. Circ Res 2005;96:612-6. [Crossref] [PubMed]
- Boehlen F, Clemetson KJ. Platelet chemokines and their receptors: what is their relevance to platelet storage and transfusion practice? Transfus Med 2001;11:403-17. [Crossref] [PubMed]
- Kraaijeveld AO, de Jager SC, de Jager WJ, et al. CC chemokine ligand-5 (CCL5/ RANTES) and CC chemokine ligand-18 (CCL18/PARC) are specific markers of refractory unstable angina pectoris and are transiently raised during severe ischemic symptom. Circulation 2007;116:1931-41. [Crossref] [PubMed]
- Otterdal K, Smith C, Oie E, et al. Platelet-derived LIGHT induces inflammatory responses in endothelial cells and monocytes. Blood 2006;108:928-35. [Crossref] [PubMed]
- Scheuerer B, Ernst M, Dürrbaum-Landmann I, et al. The CXC-chemokine platelet factor 4 promotes monocyte survival and induces monocyte differentiation into macrophages. Blood 2000;95:1158-66. [PubMed]
- Sachais BS, Kuo A, Nassar T, et al. Platelet factor 4 binds to low-density lipoprotein receptors and disrupts the endocytic machinery, resulting in retention of low-density lipoprotein on the cell surface. Blood 2002;99:3613-22. [Crossref] [PubMed]
- Nassar T, Sachais BS, Akkawi S, et al. Platelet factor 4 enhances the binding of oxidized low-density lipoprotein to vascular wall cells. J Biol Chem 2003;278:6187-93. [Crossref] [PubMed]
- von Hundelshausen P, Weber KS, Huo Y, et al. RANTES deposition by platelets triggers monocyte arrest on inflamed and atherosclerotic endothelium. Circulation 2001;103:1772-7. [Crossref] [PubMed]
- Schober A, Manka D, von Hundelshausen P, et al. Deposition of platelet RANTES triggering monocyte recruitment requires P-selectin and is involved in neointima formation after arterial injury. Circulation 2002;106:1523-9. [Crossref] [PubMed]
- von Hundelshausen P, Koenen RR, Sack M, et al. Heterophilic interactions of platelet factor 4 and RANTES promote monocyte arrest on endothelium. Blood 2005;105:924-30. [Crossref] [PubMed]
- Lu B, Rutledge BJ, Gu L, et al. Abnormalities in monocyte recruitment and cytokine expression in monocyte chemoattractant protein 1-deficient mice. J Exp Med 1998;187:601-8. [Crossref] [PubMed]
- Hawrylowicz CM, Howells GL, Feldmann M. Platelet-derived interleukin 1 induces human endothelial adhesion molecule expression and cytokine production. J Exp Med 1991;174:785-90. [Crossref] [PubMed]
- Henn V, Slupsky JR, Gräfe M, et al. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature 1998;391:591-4. [Crossref] [PubMed]
- Heeschen C, Dimmeler S, Hamm CW, et al. Soluble CD40 ligand in acute coronary syndromes. N Engl J Med 2003;348:1104-11. [Crossref] [PubMed]
- Celik S, Langer H, Stellos K, et al. Platelet-associated LIGHT (TNFSF14) mediates adhesion of platelets to human vascular endothelium. Thromb Haemost 2007;98:798-805. [Crossref] [PubMed]
- McEver RP. Adhesive interactions of leukocytes, platelets, and the vessel wall during hemostasis and inflammation. Thromb Haemost 2001;86:746-56. [Crossref] [PubMed]
- van Gils JM, Zwaginga JJ, Hordijk PL. Molecular and functional interactions among monocytes, platelets, and endothelial cells and their relevance for cardiovascular diseases. J Leukoc Biol 2009;85:195-204. [Crossref] [PubMed]
- Totani L, Evangelista V. Platelet-leukocyte interactions in cardiovascular disease and beyond. Arterioscler Thromb Vasc Biol 2010;30:2357-61. [Crossref] [PubMed]
- Raz O, Lev DL, Battler A, et al. Pathways Mediating the Interaction between Endothelial Progenitor Cells (EPCs) and Platelets. PLoS One 2014;9. [Crossref] [PubMed]
- Zucker TP, Bönisch D, Muck S, et al. Thromboxane A2 potentiates thrombin-induced proliferation of coronary artery smooth muscle cells. Adv Exp Med Biol 1997;433:387-90. [Crossref] [PubMed]
- Kobayashi T, Tahara Y, Matsumoto M, et al. Roles of thromboxane A(2) and prostacyclin in the development of atherosclerosis in apoE-deficient mice. J Clin Invest 2004;114:784-94. [Crossref] [PubMed]
- Hohlfeld T, Schrör K. Antiinflammatory effects of aspirin in ACS: relevant to its cardiocoronary actions? Thromb Haemost 2015;114:469-77. [Crossref] [PubMed]
- Williams PC, Coffey MJ, Coles B, et al. In vivo aspirin supplementation inhibits nitric oxide consumption by human platelets. Blood 2005;106:2737-43. [Crossref] [PubMed]
- Husain S, Andrews NP, David Mulcahy D, et al. Aspirin Improves Endothelial Dysfunction in Atherosclerosis. Circulation 1998;97:716-20. [Crossref] [PubMed]
- Monobe H, Yamanari H, Nakamura K, et al. Effects of low-dose aspirin on endothelial function in hypertensive patients. Clin Cardiol 2001;24:705-9. [Crossref] [PubMed]
- Heitzer T, Ollmann I, Köke K, et al. Platelet Glycoprotein IIb/IIIa Receptor Blockade Improves Vascular Nitric Oxide Bioavailability in Patients with Coronary Artery Disease. Circulation 2003;108:536-41. [Crossref] [PubMed]
- Warnholtz A, Ostad AM, Heitzer T, et al. Effect of Tirofiban on Percutaneous Coronary Intervention-Induced Endothelial Dysfunction in Patients with Stable Coronary Artery Disease. Am J Cardiol 2005;95:20-3. [Crossref] [PubMed]
- Nylander S, Schulz R. Effects of P2Y12 receptor antagonists beyond platelet inhibition--comparison of ticagrelor with thienopyridines. Br J Pharmacol 2016;173:1163-78. [Crossref] [PubMed]
- Heitzer T, Rudolph V, Schwedhelm E, et al. Clopidogrel improves systemic endothelial nitric oxide bioavailability in patients with coronary artery disease: evidence for antioxidant and antiinflammatory effects. Arterioscler Thromb Vasc Biol 2006;26:1648-52. [Crossref] [PubMed]
- Warnholtz A, Ostad MA, Velich N, et al. A single loading dose of clopidogrel causes dose-dependent improvement of endothelial dysfunction in patients with stable coronary artery disease: results of a double-blind, randomized study. Atherosclerosis 2008;196:689-95. [Crossref] [PubMed]
- Patti G, Grieco D, Dicuonzo G, et al. High versus standard clopidogrel maintenance dose after percutaneous coronary intervention and effects on platelet inhibition, endothelial function, and inflammation results of the ARMYDA-150 mg (antiplatelet therapy for reduction of myocardial damage during angioplasty) randomized study. J Am Coll Cardiol 2011;57:771-8. [Crossref] [PubMed]
- Bonello L, Harhouri K, Sabatier F, et al. Level of adenosine diphosphate receptor P2Y12 blockade during percutaneous coronary intervention predicts the extent of endothelial injury, assessed by circulating endothelial cell measurement. J Am Coll Cardiol 2010;56:1024-31. [Crossref] [PubMed]
- Muller O, Hamilos M, Bartunek J, et al. Relation of endothelial function to residual platelet reactivity after clopidogrel in patients with stable angina pectoris undergoing percutaneous coronary intervention. Am J Cardiol 2010;105:333-8. [Crossref] [PubMed]
- Hamilos M, Muller O, Ntalianis A, et al. Relationship between peripheral arterial reactive hyperemia and residual platelet reactivity after 600 mg clopidogrel. J Thromb Thrombolysis 2011;32:64-71. [Crossref] [PubMed]
- Ostad MA, Nick E, Paixao-Gatinho V, et al. Lack of evidence for pleiotropic effects of clopidogrel on endothelial function and inflammation in patients with stable coronary artery disease: results of the double-blind, randomized CASSANDRA study. Clin Res Cardiol 2011;100:29-36. [Crossref] [PubMed]
- Fujisue K, Sugiyama S, Ono T, et al. Effects of endothelial dysfunction on residual platelet aggregability after dual antiplatelet therapy with aspirin and clopidogrel in patients with stable coronary artery disease. Circ Cardiovasc Interv 2013;6:452-9. [Crossref] [PubMed]
- Haynes A, Linden MD, Robey E, et al. Relationship between monocyte-platelet aggregation and endothelial function in middle-aged and elderly adults. Physiol Rep 2017.5. [PubMed]
- Rudolph TK, Fuchs A, Klinke A, et al. Prasugrel as opposed to clopidogrel improves endothelial nitric oxide bioavailability and reduces platelet-leukocyte interaction in patients with unstable angina pectoris: A randomized controlled trial. Int J Cardiol 2017;248:7-13. [Crossref] [PubMed]
- Armstrong D, Summers C, Ewart L, et al. Characterization of the adenosine pharmacology of ticagrelor reveals therapeutically relevant inhibition of equilibrative nucleoside transporter 1. J Cardiovasc Pharmacol Ther 2014;19:209-19. [Crossref] [PubMed]
- van Giezen JJ, Sidaway J, Glaves P. Ticagrelor inhibits adenosine uptake in vitro and enhances adenosine-mediated hyperemia responses in a canine model. J Cardiovasc Pharmacol Ther 2012;17:164-72. [Crossref] [PubMed]
- Ohman J, Kudira R, Albinsson S, et al. Ticagrelor inducesadenosine triphosphate release from human red blood cells. Biochem Biophys Res Commun 2012;418:754-8. [Crossref] [PubMed]
- Mangiacapra F, Panaioli E, Colaiori I, et al. Clopidogrel Versus Ticagrelor for Antiplatelet Maintenance in Diabetic Patients Treated with Percutaneous Coronary Intervention: Results of the CLOTILDIA Study (Clopidogrel High Dose Versus Ticagrelor for Antiplatelet Maintenance in Diabetic Patients). Circulation 2016;134:835-7. [Crossref] [PubMed]
- Torngren K, Ohman J, Salmi H, et al. Ticagrelor improves peripheral arterial function in patients with a previous acute coronary syndrome. Cardiology 2013;124:252-8. [Crossref] [PubMed]
- Rusnak J, Mogler C, Buttler A, et al. Ticagrelor promotes atherosclerotic lesion stabilization in advanced lesions of Apolipoprotein E deficient mice. Clin Res Cardiol 2014.103.
- Patil SB, Jackman LE, Francis SE, et al. Ticagrelor effectively and reversibly blocks murine plateletP2Y12-mediated thrombosis and demonstrates a requirement for sustained P2Y12 inhibition to prevent subsequent neointima. Arterioscler Thromb Vasc Biol 2010;30:2385-91. [Crossref] [PubMed]
- Sürer S, Toktas F, Ay D, et al. Effect of the P2Y12 antagonist Ticagrelor on neointimal hyperplasia in a rabbit carotid anastomosis model. Interact Cardiovasc Thorac Surg 2014;19:198-204. [Crossref] [PubMed]
- Bonello L, Frere C, Cointe S, et al. Ticagrelor increases endothelial progenitor cell level compared to clopidogrel in acute coronary syndromes: A prospective randomized study. Int J Cardiol 2015;187:502-7. [Crossref] [PubMed]
- Reiner MF, Akhmedov A, Stivala S, et al. Ticagrelor, but not clopidogrel, reduces arterial thrombosis via endothelial tissue factor suppression. Cardiovasc Res 2017;113:61-9. [Crossref] [PubMed]
- Kim HK, Jeong MH, Lim KS, et al. Effects of ticagrelor on neointimal hyperplasia and endothelial function, compared with clopidogrel and prasugrel, in a porcine coronary stent restenosis model. Int J Cardiol 2017;240:326-31. [Crossref] [PubMed]
- Robinson JG, Farnier M, Krempf M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med 2015;372:1489-99. [Crossref] [PubMed]
- Navarese EP, Kolodziejczak M, Kereiakes DJ, et al. Proprotein Convertase Subtilisin/Kexin Type 9 Monoclonal Antibodies for Acute Coronary Syndrome: A Narrative Review. Ann Intern Med 2016;164:600-7. [Crossref] [PubMed]
- Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N Engl J Med 2017;376:1713-22. [Crossref] [PubMed]


