Antiplatelet agents in uncertain clinical scenarios—a bleeding nightmare
Introduction
Antiplatelet therapy has been one of the most widely researched areas of medicine since the introduction of Aspirin in the 1960s (1,2) and Ticlopidine in the 1970s (3). Despite over 30 years of experience of using dual antiplatelet therapy (DAPT), significant uncertainties remain in how best to manage various distinct clinical scenarios. While studies have helped to provide many answers, virtually all of these same studies have raised further questions through a lack of uniformity in study design and definitions (4-6). These uncertainties have been magnified by an ever-growing, aging population, who invariably have never been definitively studied, tend to have complex coronary disease, and have many other co-morbidities which invariably influence our clinical decision making (7).
In particular, the aging population has led to an increased number of cases of patients with AF requiring PCI (8). The optimal regime for this challenging group of patients remains elusive, and is made even more complicated when one considers the expanding list of newer anticoagulant choices aside from Warfarin. Additionally, each of these new anticoagulants require tailoring to the individual based on such characteristics as renal function, age and weight (9).
In this review, we will focus mainly on the importance of bleeding in patients undergoing PCI, since it is largely our concerns with the risk of bleeding that guides the prescription of antiplatelet therapy. We will discuss the time at which bleeding tends to occur following PCI, the location of bleeding, the lack of standardisation of bleeding classification, the issue of bleeding in specific groups, especially the elderly and those taking concomitant oral anticoagulation, the failure of platelet function testing to help guide clinical decision making, and what potential measures could be considered to modify risk.
The ‘Catch 22’ scenario
Patients who are at high risk of bleeding that require DAPT post PCI represent a ‘Catch-22’ scenario. A ‘Catch 22’ scenario is defined as a dilemma or difficult circumstance(s) from which there is no escape because of mutually conflicting or dependent conditions. Not infrequently, the reaction to bleeding is to modify DAPT, thereby reducing the risk of bleeding whilst increasing the risk of thrombosis, thus increasing the risk of major adverse cardiovascular events (MACE) (10,11). This is every cardiologist’s nightmare. The difficulty faced by clinicians is that they, currently, do not have any robust barometer to safely guide modification of antiplatelet or anticoagulant therapy, such that the appropriate balance between bleeding and thrombosis is achieved.
The significance and classification of bleeding—‘the lack of standard’
It is only in recent years that the implications of bleeding post PCI have been fully recognized by clinicians and the regulatory authorities (12-14). In fact, early PCI studies did not appear to fully appreciate the issue at all (15-20). Some clinicians may have presumed that if someone bled this could simply be remedied by giving a blood transfusion. However, we now know that this is not the case as even moderate bleeding translates into a worse outcome, and indeed the simple act of transfusing blood itself is associated with a significantly worse outcome (13,21,22). Even minor bleeding can result in the interruption of DAPT (10) which can have prognostic significance; with previous evidence suggesting that there is a 90-fold increase in stent thrombosis (ST) for patients following premature antiplatelet discontinuation (11).
Before considering PCI, it is crucial to reassess a patient’s symptoms to ensure that their symptoms do indeed sound ischemic and that these symptoms are likely to be alleviated by the combination of a ‘stent for life’ and DAPT. The latter will undoubtedly increase bleeding risk for 1, 6, 12 months or indeed 3 years depending on the chosen duration. As clinicians, it is essential to thoroughly assess bleeding risk in every case and if the risk is unacceptably high, PCI may need to be reconsidered.
It may be difficult to resist the temptation of stent implantation especially for enthusiastic fellows in training when an apparently angiographically significant lesion is identified. PCI has become increasingly accessible, and has delivered such favorable, well-publicised outcomes, that the medical community may have forgotten the potentially devastating consequences of bleeding. It is therefore mandatory that cardiologists use every tool available such as non-invasive ischaemic assessment, fractional flow reserve, instantaneous wave-free ratio and intravascular modalities to validate the need for PCI. If bleeding risk is high, objective evidence should be sought beforehand to justify PCI.
Simply put, bleeding is bad, and clinicians are therefore obliged to be fully aware of the features that increase the risk of bleeding (21-24). Special attention must be given to the aging population as they are considered high risk and the proportion of patients over the age of 75, undergoing PCI, appears to be steadily increasing (25). This elderly population has not been definitively studied in the context of DAPT strategies, nor indeed in general (26-31). Some might say that this is ironic since elderly patients are the majority users of healthcare. This will be discussed in detail later.
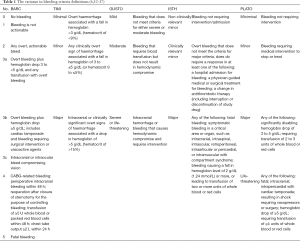
There are multiple bleeding classifications such as TIMI, GUSTO, ISTH, PLATO and BARC (32-37) to name but a few. The variance in these classifications, highlighted in Table 1, makes it virtually impossible for clinicians to compare studies. Such is the lack of agreement between classifications that in one study it is possible to classify the same type of bleeding for an individual as either major or minor, depending on which classification is used. Therefore, it is extremely difficult to implement any approach to avoid or reduce rates of bleeding without agreed criteria. Ideally all future research should agree on a single bleeding classification(s) at the study design phase to bring consistency to this ever-puzzling area.
Predicting who is more likely to bleed
This is again a “Catch-22”, as those at risk of bleeding are also at risk of MACE. Table 2 illustrates the shared features that we see in many patients. How should clinicians decide which guideline to follow or what to do for patients who have equally high bleeding and ischemic risk, such as the elderly diabetic with renal dysfunction? This is certainly not a rare case in current clinical practice.

Full table
Risk prediction calculators, such as the PRECISE-DAPT or PARIS risk scores (45) may be useful for clinicians to predict bleeding post PCI. Ideally a risk prediction score would not only aid clinicians to identify high-risk patients, but should also help to determine the DAPT regime and duration. There is a paucity of prospective data evaluating the clinical utility of these scores (46). Recent data, however, suggests that these scores have moderate predicative power at best, with the PARIS score out-performing the PRECISE-DAPT score (47). While there appears to be promise from the PARIS risk score more work is needed to find better risk prediction models.
In the ACS population, early bleeding may be reduced by minimizing the duration of low molecular weight heparin (LMWH). Providing more expedient access to PCI following NSTEMI may reduce the exposure to LMWH thereby decreasing rates of early bleeding. The use of glycoprotein IIB/IIIA inhibitors (GPI) has fallen in certain parts (48). One might speculate that GPI usage is less relevant now with the advent of Prasugrel and Ticagrelor and that avoidance of GPI may be associated with lower risk of bleeding.
In the elective population it appears that the bleeding rates are lower compared to the ACS population particularly in the first 30 days (48). Previous data shows that pre-treatment of elective patients with DAPT reduces MACE, however the duration of pre-treatment is short in most studies being significantly less than 30 days (49-51). Anecdotally, DAPT does tend in cases to unmask occult malignancies and this may be an unexpected benefit for the patient if it leads to early detection and rapid, effective treatment of a malignancy. One might speculate that a longer duration of pre-treatment with DAPT may not only be efficacious, but may unmask the potential for patients to bleed; even those not perceived to be high-risk, thus avoiding PCI and exposure to DAPT. In summary, identifying the time at which most bleeding occurs may help to implement strategies that will reduce bleeding risk. Once a stent is deployed in a coronary artery, clinicians are committed to a course of antiplatelet therapy and this may be too late.
In both the elective and ACS population chronic kidney disease is an area of particular interest as these patients are also at a higher risk of both ischemic and bleeding events, although the data is conflicting as recent studies suggest it has less of an impact than previously reported (52,53). Post-hoc analysis of PLATO suggests that Ticagrelor may be more effective than Clopidogrel in patients with ACS and concomitant renal dysfunction (54).
The timing of bleeding—when does bleeding occur?
Virtually every study that has examined rates of bleeding has used a wide variety of pre-determined time-points at which bleeding is reported. Studies have evaluated bleeding events at 30 days, 3 months, 6 months, 1 year, and indeed 3 years depending on the study (4-6,30,55,56). Similar to the issue mentioned above about bleeding classification, the visible lack of standardization of time-points across such studies again makes it challenging to compare studies. It is however important to review, where possible, when bleeding actually occurs.
A recent systematic review, of over half a million participants post-PCI, found that major bleeding increased overall mortality by 3-fold, whilst also increasing the risk of MACE by a similar degree (12). This same review looked at a very large number of patients mainly drawn from a registry of 280,390 participants (24). One potential criticism of this review is that the follow-up period for over half of these patients was less than 30 days, which is surprisingly short. However, this did highlight that many events and indeed fatal events occur in the first 30 days following PCI, with an adjusted risk of mortality odds ratio of 2.91 (95% CI, 2.77–3.05) if major bleeding occurs (24). Similarly several randomized trials also report that the vast majority of bleeding occurs in the first 30 days, with rates of major bleeding as high as 8% by 30 days in patients with ACS (5,57-60). While the rate of bleeding beyond 30 days increased to 12%, the rate of increase was much less marked (6). Landmark analysis of more recent data from PLATO shows that almost half of all major bleeds occurred within the first 30 days, with both Ticagrelor and Clopidogrel having high early major bleeding rates (13,61). Given that a very significant amount of bleeding occurs in the first 30 days following PCI and that the shortest possible duration of antiplatelet therapy post PCI is 1 month, it may be impossible to significantly impact on bleeding in the first 30 days. Maybe the site of the bleed is more important to focus on as bleeding risk is modifiable depending on the site.
What sites do patients bleed from?
While there is a lack of standardization amongst the multitude of bleeding classifications each one does agree that intracranial haemorrhage (ICH) is life-threatening, with an incidence ranging from 0.1% to 0.34% with DAPT (4-6). The rest of the bleeding that occurs seems to be split between gastrointestinal (GI) and non-GI bleeding; the latter split between epistaxis, genitourinary bleeding, vascular access, and indeterminate. It is generally accepted that, while it is devastating, ICH is relatively uncommon and virtually impossible to predict. Reducing the risk of ICH remains a challenge aside from identifying those with previous history of ICH and carefully assessing need for PCI and therefore DAPT.
In contrast, bleeding that arises from vascular access is clearly modifiable (62-67). Historically, vascular access site has been a major driver of bleeding in many studies, although this influence has decreased for several reasons. Measures such as reduction in the sheath size, use of slender technologies, greater attention to the femoral puncture (68-70) and, ultimately, the switch by many operators to the transradial approach, has significantly reduced the rate of bleeding (66). The transradial approach has arguably been the greatest driver in the crusade to reduce bleeding rates, and subsequently mortality (62-67). Furthermore the benefit of transradial PCI may be even more striking in the most high-risk patients, such as patients with a STEMI, those with cardiogenic shock, with renal failure, and in the elderly (66).
The most common bleeding site in patients drawn from the GRACE Registry receiving DAPT was GI at 31.5% followed by vascular access site (23.8%), although this was before the shift to the transradial approach (71). In contrast, the PLATO trial found that vascular access bleeds were the least common (0.2%), although again GI bleeds were astonishingly high representing 33.7% of the overall bleeding, followed by epistaxis (17.6%) (6,61). Interestingly in the PLATO trial, Clopidogrel had a higher rate of GI bleeding than Ticagrelor (36.9% vs. 31.5%), and this was more likely to be fatal (6). On average, GI bleeding accounts for the majority of major bleeds for patients post PCI (61). This shift in the site of bleeding means our focus must now change to focus on how to prevent GI bleeds, especially as GI with other non-access site bleeds account for a 3.94-fold increase in 1 year mortality (95% CI, 3.07–5.15, P<0.0001) (72). The issue of GI bleeding becomes even more striking in the elderly discussed below.
Antiplatelet therapy in the elderly population—a double edged sword?
Undoubtedly, the most challenging population to treat are the elderly given their co-morbidities and frailty. GI bleeding in under 75 years old patients is remarkable, but in the over the 75 years old population who have undergone PCI, it is even more notable, rising to 53.8% (73). Strikingly, major GI bleeds, in patients over 75 years on DAPT are frequently fatal or disabling [based on the modified Rankin score (74)] (73). Recent work shows that the numbers needed to treat with a proton pump inhibitor to prevent one major upper GI bleed at 5 years in patients aged 75 years or older is 23 (73). These data provide a strong rationale for recommending the routine use of proton pump inhibitor (PPI) in the over 75 years old population requiring DAPT.
Antiplatelet therapy and anticoagulation—the standard of care for ACS and AF—are most commonly prescribed together in older patients (75-79) further increasing bleeding risk. With increasing life expectancy and improvements in healthcare, older patients represent a growing population among those who undergo PCI (25).
Lack of evidence for DAPT in the elderly
While improvement in stent technology and optimal antithrombotic therapies have further improved clinical outcomes, the management of DAPT in advanced age remains challenging. This is primarily due to the scarcity of evidence to guide clinical decisions. Although over 75 years old patients represent one-third of patients admitted with MI, and two-thirds of all patients that die from MI (80), they are significantly under-represented in ACS trials (81).
Moreover, extrapolating findings from current randomized trials to this elderly population is extremely challenging, as they have the most undesirable trait of having an increased risk of both ischemia and bleeding. This is due to different pharmacokinetics and pharmacodynamics as compared with younger patients (25). Other contributing factors include the presence of multiple comorbidities, frailty, concomitant use of anticoagulation and non-steroidal anti-inflammatory drugs (NSAIDs), age-related reduction in renal and hepatic function, reduced mobility, poly-pharmacy, and higher peri-procedural bleeding complications (82,83). In the Oxford Vascular Study (84), almost 50% of patients who bled were aged >75 years, with advancing age associated with increased risk of fatal bleeding [hazard ratio (HR) 5.53; 95% CI, 2.65–11.54, P<0.0001], major bleeding (HR 3.10; 95% CI, 2.27–4.24, P<0.0001), and major upper GI bleeding (HR 4.13; 95% CI, 2.60–6.57, P<0.0001). As mentioned, bleeding events have important prognostic implications and, in many cases, lead to death (85).
Bleeding complications are usually perceived to be higher in these fragile patients, which may lead to sub-optimal treatment, with a reduced threshold by clinicians to prematurely discontinue DAPT even in the case of minor bleeding. Furthermore elderly patients are less likely to receive drug eluting stents due to concerns about prolonged DAPT (10,86-88). The complex, calcified lesions in elderly patients are technically more challenging to treat with PCI, and have a higher peri-procedural complication rate (89). In addition, advanced age is independently associated with a reduced effectiveness of adenosine diphosphate (ADP) antagonists and a higher rate of high residual on-treatment platelet reactivity in patients receiving DAPT (90).
Do antiplatelets work in an elderly population?
In CURE (4), the combination of Aspirin and Clopidogrel for 12 months reduced the composite endpoints of cardiovascular death, MI, or stroke as compared to Aspirin alone [relative risk (RR) 0.80; 95% CI, 0.72–0.90, P<0.001] but in the over 65 population major bleeding was increased (RR 1.38; 95% CI, 1.13–1.60, P<0.001). In the PLATO trial, 18,624 ACS patients received either Ticagrelor or Clopidogrel with Aspirin for up to 12 months. Overall, the ischemic events and total mortality were lower with Ticagrelor (6). This was consistent among older patients, with numerically greater absolute mortality reduction in patients aged >75 years. There was no significant increase in major bleeding rates in the older subgroup. The PLATO study did not find a significant difference in age-matched cohorts (6), however the study was not powered to answer this. TRITON-TIMI 38 randomized 13,608 ACS patients undergoing PCI to either Prasugrel or Clopidogrel in combination with Aspirin for a median of 14.5 months. In 1,769 (13%) patients aged >75 years, there was 6% RR reduction in MACE at a cost of increased non-CABG related TIMI major bleeding with Prasugrel (HR 1.32; 95% CI, 1.03–1.6, P<0.03) especially in patients ≥75 years. Patients ≥75 years had no net benefit (death, MI, stroke, or non-CABG-related TIMI major bleeding; HR 0.99; 95% CI, 0.81–1.21) (5). Data like this highlights the need for further work in elderly patients.
Recent guidelines recommend Clopidogrel on top of Aspirin for up to 1 year after elective PCI in the elderly (91). They also recommend that Clopidogrel, should be considered if bleeding risk is high in patients with ACS, though Ticagrelor is still recommended in most elderly ACS patients. In light of the above data, the European Society of Cardiology (ESC) suggests that the use of Prasugrel is cautioned in patients’ ≥75 years and is contraindicated for those with prior stroke/TIA.
The CRUSADE Registry looked at the rates of bleeding, MACE and all-cause mortality at 1, 12 and 30 months in patients post NSTEMI who underwent PCI and found a significant rise in MACE in patients ≥65 years (22). A recent subgroup analysis of a multicentre, prospective registry of PCI patients highlighted that the risk of the combined bleeding and MACE at 30 days in patients ≥80 years of age was significantly higher if the patients were taking Ticagrelor and Aspirin, compared to Clopidogrel and Aspirin (12.9% vs. 7.2%; P=0.02) (92,93). Clearly, the optimal regime for the elderly remains unknown.
DAPT duration and stent choice in the elderly
The recent After Eighty RCT looked at treatment strategy in the elderly, comparing PCI with optimum medical therapy (OMT) vs. OMT alone in ACS patients ≥80 years old. It found a significant reduction in the composite end-point of all-cause mortality and MACE in patients undergoing PCI. However, After Eighty was limited by the small number of patients they were able to recruit (n=457).
The LEADERS-FREE trial, which compared the use of BioFreedom DES vs. bare metal stent (BMS) in high-risk patients (mean age of 75.7), found a significant reduction in MACE with BioFreedom and only 1 month of DAPT (26). The SENIOR study, which compared the use of a SYNERGY DES vs. a BMS and shortened DAPT regimens, in 1,200 patients aged over 75 years, found a significant reduction in MACE at 1 year in those treated with a DES (RR 0.71; 95% CI, 0.52–0.94, P=0.02), with no difference in bleeding (27). SENIOR had BARC 3–5 bleeding rates of 3.3% at 1 year, which is much lower than the 13.7% seen in LEADERS-FREE (26). This may be because LEADERS-FREE had a higher rate of anticoagulation, lower rate of transradial access and deliberately targeted a high bleeding risk population. In SENIOR, patients were also treated with shortened lengths of DAPT; 1 month for stable presentations and 6 months for ACS, with the authors concluding that a shortened regimen, along with a DES, was an attractive strategy for elderly patients (27).
A meta-analysis has looked at the impact of age in RCTs where they compared 3–6 months DAPT vs. 12-month DAPT after PCI with DES implantation (30). It concluded that shorter DAPT was non-inferior for ischemic composite endpoints of MI, ST, or stroke in elderly patients, but was safer than 12-month DAPT due to reduced bleeding. As highlighted, almost all patients received Clopidogrel, limiting the generalization of these findings to Ticagrelor or Prasugrel, and most used Zotarolimus- and Everolimus-DES (94). These results may not be applicable to high-risk bleeding or high-risk ACS populations.
The above seems to suggest that that Biofreedom or Synergy may be useful to facilitate shorter DAPT in the elderly.
Thrombocytopenia
A low platelet count defined as <150×109 /L is more commonly seen in patients undergoing ACS with figures as high as 5% and higher in the elderly (95). Thrombocytopenia was excluded in most of the major studies (5,6). It does appear that the approach to these patients undergoing PCI is inconsistent and poorly understood due to a lack of guidance. There is a belief that low platelets and DAPT means increased risk of bleeding, but this is not always the case as it depends on severity.
Depending on the etiology of the thrombocytopenia, platelet populations may co-exist which may be pro-thrombotic to compensate for decreased numbers as evidenced by the presence of immature platelets known as reticulo-platelets. The presence of these types of platelets may be underappreciated and so thrombocytopenic patients may indeed be undertreated. Looking to platelet function testing may again be ineffective, as the commercially available tests appear to have little application in the presence of low platelet counts. This is an area that requires exploration.
Platelet function testing to guide DAPT—the promise unrealised
Clinicians hoped that by using platelet function tests, they might be able to personalise DAPT for an individual to prevent thrombotic events, however this approach disappointingly failed (95-100). In 2009, Cuisset et al. (101) suggested that platelet function testing could also be used to identify patients at higher risk of bleeding by identifying hyper-responsiveness to the effects of Clopidogrel. Unfortunately, the findings of ANTARCTIC have further stifled the approach to personalize antiplatelet therapy (102). The only guidance as to how best to manage high bleeding risk comes largely from consensus of opinion (103,104). While this is helpful it is arguably not ideal when considering the complexity and significance of this particular clinical decision.
The adverse effects of thrombosis in relation to DAPT has long been recognized, but there is a growing body of literature to say that bleeding has a very significant impact on a patient’s morbidity and mortality (12-14). It is, therefore, crucial for us to re-examine the issue of bleeding in certain clinical scenarios in light of the expanding literature and to examine whether it is possible to reduce bleeding risk where possible.
Bleeding avoidance strategies
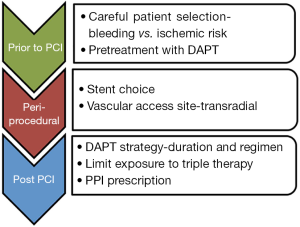
Figure 1 represents the bleeding avoidance strategies we would recommend. Recent trials of newer stents, such as LEADERS-FREE and SENIOR, provide promising evidence for the use of biodegradable polymers with shorter DAPT duration, in high risk individuals (26,27). Concerns still exist for bleeding from intracranial, genitourinary and indeterminate sites. RCTs into antiplatelet continuation vs. interruption in bleeding patients are critically needed but may be impossible to conduct.
Guidance on active bleeding and DAPT interruption
Patients who bleed on DAPT are possibly the most uncertain group of all. There are guidelines and statements in relation to this (103-105), however, there is a paucity of randomized data or otherwise to support any recommendations. This is of major concern when one considers the potentially devastating outcomes from interruption of DAPT.
The recent ESC guidelines and consensus group document recognize the difficulty in balancing ischaemic and bleeding risk, thus a flow chart is provided making recommendations to clinicians which vary depending on the severity of bleeding (103,104). Trials are notably lacking in terms of continuation vs. interruption, duration of interruption, and choice of single antiplatelet agent to use following a bleed. A Cochrane systematic review is currently underway for non-cardiac surgery patients (106), although another meta-analysis has admitted the paucity of evidence in these patients (107). Clearly, robust evidence is required to determine the best approach, ideally with the creation of a better scoring calculator to help physicians in their decision-making.
Antiplatelet therapy and concurrent anticoagulation
Almost 10% of patients undergoing PCI have an indication for oral anticoagulation, most commonly AF (108-110). There is a 2- to 3-fold increase in bleeding rate with the addition of DAPT to an anticoagulant, referred to as triple therapy (TT) (111), yet this is the current gold standard in the European and American guidelines (47,103). Patients on TT have a 5% to 15% rate of major bleeding at 1 year, and major bleeding is associated with a 2- to 8-fold rise in the risk of death and MACE (23). AF rates are expected to increase with the epidemic of aging, because in those aged 75–84, the prevalence of AF is as high as 12%, while in those aged over 85 it is as high as 33% (8,112). The Framingham heart study found a lifetime risk of AF in those aged 70 of 23–24.3%, and that MI was an independent risk factor (113). Decisions in this area are difficult balancing acts, weighing up the risks of thrombosis with those of bleeding (114). We must note the different pathogenic processes of coronary/ST compared with thromboembolism formation in AF, though recognizing the shared influence of thrombin (115,116).
This is an evolving area of research given the emergence of direct oral anticoagulants (DOACs), previously referred to as novel oral anticoagulants (NOACs) for non-valvular atrial fibrillation (NVAF), beginning with the WOEST trial from 2013 (117), continuing with the recently published PIONEER AF-PCI and RE-DUAL PCI papers (118,119), and awaiting the AUGUSTUS and ENTRUST-AF PCI trials (NCT02415400, NCT02866175).
Given the myriad of trials, and the heterogeneity of their definitions, criteria and regimens, it is again challenging to draw comparisons to enable us to best treat our patients. However, comparisons must be drawn; in particular in how the trials defined bleeding, what proportion of PPIs were used, and the different drug regimens used. Each trial uses bleeding as its primary endpoint, and MACE as a secondary endpoint. It is worth summarizing the data to date.
The WOEST trial was a Prospective Randomized, Open-label, Blinded Endpoint (PROBE) study of 573 patients who were randomized to TT with Warfarin, Aspirin and Clopidogrel compared to double therapy (DT) with Warfarin and Clopidogrel. The TT group had significantly more bleeding than the DT group, without a rise in MACE in the latter (117). Importantly, this study included metallic heart valves, unlike all others. A landmark analysis of bleeding in the TT group showed that 26.1% of patients bled within the first 30 days, while MACE, including all-cause mortality, occurred in 4.2% during that time. This showed that over half of those that bled on TT did so in the first 30 days. Meta-analysis has also reinforced the efficacy and safety of DT (120).
It is therefore noteworthy that the current ESC guidelines suggest that TT should be prescribed in the first month after PCI in the face of significant 30-day bleeding rates using the TT strategy. Surely there is merit in initially prescribing TT with the view to stopping Aspirin as soon as the patient’s international normalised ratio (INR) is therapeutic. This is likely to further reduce the exposure to TT in these patients .
PIONEER AF-PCI was a larger PROBE trial of 2,124 participants with three arms consisting of (I) Rivaroxaban and a P2Y12 inhibitor, (II) low-dose, twice daily Rivaroxaban with DAPT and (III) TT with Warfarin. It reported a lower bleeding rate in both Rivaroxaban arms over TT, without a rise in MACE (118).
The RE-DUAL PCI trial was another PROBE study of 2,725 patients with three regimens (I) low- or high-dose Dabigatran with a P2Y12 inhibitor, and (II) TT using Warfarin. Again, it found a lower incidence of bleeding in the Dabigatran arms, with no rise in MACE. Of note, it had a higher rate of PPI usage than other trials (60.2% vs. 36.5–38.1%) (119).
Twelve months of TT has consistently been shown to significantly increase bleeding rate without an improvement in mortality or MACE. However, the dual therapy DOAC regimens using Rivaroxaban and Dabigatran are arguably unfair in that they were compared to TT, when DT using Warfarin and a P2Y12 inhibitor is clearly safer. The latest ESC antiplatelet guidelines also do not advise longer than 6 months of TT (103), though a year of TT was used in some patients in PIONEER AF-PCI. The PIONEER AF-PCI trial is comparing Rivaroxaban to a TT regimen that is no longer used in clinical practice, and it also reflected the WOEST finding of a higher bleeding rate in the first 30 days in patients receiving Warfarin plus DAPT. Furthermore, it found a significant rise in stroke events in those prescribed low-dose Rivaroxaban and DAPT at 6 months (P=0.02), and there were multiple subgroups within the Rivaroxaban arms depending on the length of DAPT (118). As such, its application to real-world practice appears limited.
Additionally, RE-DUAL PCI suffers from significant limitations, most importantly that it used Warfarin TT. The original RE-LY trial, which was powered for MACE, also showed a significant rise in MIs in patients taking 150 mg Dabigatran vs. those on Warfarin alone (0.74% vs. 0.53%; P=0.048) (119,121).
The first meta-analysis of the above three trials plus ISAR-TRIPLE, which had the goal of evaluating the optimal length of TT with Warfarin (122), was published this year. Six thousand and thirty-six patients were included, with AF being the commonest indication (95.4%). It found that DT had a significantly less major and minor bleeding, without a rise in MACE. It concluded that DT using a P2Y12 inhibitor and a DOAC, particularly in high bleeding risk cases, is the preferred treatment strategy (122). However, the newer anticoagulants do not yet have the real-world registry data as seen with Warfarin (123,124). In an effort to further reduce the bleeding it may be that Clopidogrel should be used in any DT regime in preference to Prasugrel or Ticagrelor. The AUGUSTUS and ENTRUST AF-PCI studies do involve a Warfarin and P2Y12 DT arm, which should shine some light on this area.
Table 3 provides a comparison of the published trials, while Table 4 represents what is known about those that are, currently, unpublished. It highlights the similarities and differences in these studies, allowing us to see the heterogeneity amongst the three published studies, particularly in bleeding definition, regime and PPI usage. The ISTH bleeding definition is being used in AUGUSTUS and ENTRUST AF-PCI, which may enable comparison between them and RE-DUAL PCI. AUGUSTUS is attractive given its larger size, placebo-containing arms and crossover design, allowing better comparison of the impact of Aspirin.

Full table
Given that the first 30 days is the period in which the number of bleeds is highest, how do the guidelines justify a recommendation that TT should be used routinely in these patients? Surely, if the patient were on Warfarin it would be advantageous to discontinue Aspirin as soon as the patient’s INR is therapeutic. Indeed, if the patient is already on therapeutic Warfarin, and is going to have a PCI performed through trans-radial access, then the question must be asked if there is a need for any Aspirin at all, particularly when one consider the signals coming from older anticoagulation studies, showed that rates of MI were numerically lower (121,125-127). Clinicians need to be mindful that thrombin is a powerful platelet aggregator, and that antagonism of this is extremely effective at reducing arterial thrombosis (128).
Once the AUGUSTUS and ENTRUST AF-PCI results are published, practice will need to be reviewed once again. However, they will not answer all questions, particularly for those with indications for anticoagulation other than NVAF, such as mechanical valve replacements, chronic thromboembolic disease and AF with mitral stenosis. There are at least five trials involving DOACs and either trans-aortic valve replacements or biological prosthesis (NCT03284827, NCT03183843, NCT02833948, NCT02664649 and NCT02247128). To our knowledge there are no studies exploring combinations in patients with metallic valves, and clearly research is needed but this will be more challenging. In addition, the WOEST 2 Registry data (NCT02635230) is a prospective, multi-centre cohort study intending to provide data on 2,200 patients requiring anticoagulation and coronary revascularization.
Assessment of the patient’s ischemic and bleeding risks is recommended along with early discontinuation of Aspirin once the INR is therapeutic (i.e., 2–3 for NVAF) to avoid any prolonged duration TT. Achieving a therapeutic INR should be done using a slow induction protocol to avoid high INRs (70). Recommendations to tightly control the INR between 2.0–2.5 are notoriously difficult to achieve in the real-world setting most likely requiring more frequent monitoring. One must bear in mind that the time within the therapeutic range (TTR), i.e., an INR of between 2.0 and 3.0 was only achieved in approximately 60% of the cases for the DOAC studies (121,125-127), making it improbable to achieve an even more narrow therapeutic window of 2.0–2.5. One year after PCI the patient should remain on life long Warfarin which can be switched to DOAC if good Warfarin control of >65% in the TTR cannot be achieved.
If antiplatelet therapy in combination with Warfarin was not suitable, currently available evidence would favor the use of Dabigatran 110 mg BD with Clopidogrel alone in the NVAF patients, although a subsequent meta-analysis did suggest that the higher dose (Dabigatran 150 mg BD) should be given in light of the non-significant trend toward ST in the low dose group (122). The significant rise in MIs in the 150 mg Dabigatran cohort in the original RE-LY trial, which was powered for MACE is notable and difficult to explain fully (121). Nevertheless concomitant PPI therapy seems very reasonable with any combined antiplatelet and anticoagulation regime.
Conclusions
The risk vs. benefit exercise still troubles clinicians and it is becoming even more challenging with an increasingly complex, older patient. While bleeding was once seen as a minor reversible problem, we now know that it carries significant mortality and morbidity. Efforts to reduce bleeding risk, such as transradial access, PPI usage, and optimal stent/medication strategies must be used, along with reacting appropriately to those who do bleed. Our increasingly elderly, frail and complex population are, paradoxically, not a group at all, but individuals who require a personalised approach to manage their risk to achieve best outcomes. They are less well studied than other cohorts, though this is a recognized issue and hopefully, will become clearer soon. Concurrent anticoagulation with DAPT provides the greatest risk for bleeding, and with the introduction of DOACs it has become more difficult for the clinician to select the best option. Hopefully the days of extended TT are numbered as this is clearly an undesirable strategy. Further work in all these areas is desperately needed if we are to make informed decisions with our patients for their benefit.
Acknowledgements
None.
Footnote
Conflicts of Interest: A Peace has received honoraria from Bayer, Pfizer, BMS, Astra-Zeneca, Boehringer, Elli Lily, Medtronic, Boston Scientic, Biosensors, Abbott, St Jude. The other authors have no conflicts of interest to declare.
References
- O’Brien JR. An in-vivo trial of an anti-adhesive drug. Thromb Diath Haemorrh 1963;9:120-5. [Crossref]
- O’Brien JR. Aspirin and platelet aggregation. Lancet 1968;1:779-83. [Crossref] [PubMed]
- Thebault JJ, Blatrix CE, Blanchard JF, et al. Effects of ticlopidine, a new platelet aggregation inhibitor in man. Clin Pharmacol Ther 1975;18:485-90. [Crossref] [PubMed]
- Yusuf S, Zhao F, Mehta SR, et al. Effects of Clopidogrel in Addition to Aspirin in Patients with Acute Coronary Syndromes without ST-Segment Elevation. N Engl J Med 2001;345:494-502. [Crossref] [PubMed]
- Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus Clopidogrel in Patients with Acute Coronary Syndromes. N Engl J Med 2007;357:2001-15. [Crossref] [PubMed]
- Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus Clopidogrel in Patients with Acute Coronary Syndromes. N Engl J Med 2009;361:1045-57. [Crossref] [PubMed]
- Sambola A, Mutuberría M, García Del Blanco B, et al. Impact of Triple Therapy in Elderly Patients with Atrial Fibrillation Undergoing Percutaneous Coronary Intervention. PLoS One 2016;11. [Crossref] [PubMed]
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation. J Am Coll Cardiol 2014;64:e1-76. [Crossref] [PubMed]
- Webster E, Gil M. Advances in anticoagulation therapy. JAAPA 2018;31:30-5. [Crossref] [PubMed]
- Choi JH, Seo JM, Lee DH. Clinical utility of new bleeding criteria: A prospective study of evaluation for the Bleeding Academic Research Consortium definition of bleeding in patients undergoing percutaneous coronary intervention. J Cardiol 2015;65:324-9. [Crossref] [PubMed]
- Iakovou I, Schmidt T, Bonizzoni E, et al. Incidence, Predictors, and Outcome of Thrombosis After Successful Implantation of Drug-Eluting Stents. JAMA 2005;293:2126. [Crossref] [PubMed]
- Kwok CS, Rao SV, Myint PK, et al. Major bleeding after percutaneous coronary intervention and risk of subsequent mortality: a systematic review and meta-analysis. Open Heart 2014;1. [Crossref] [PubMed]
- Ducrocq G, Schulte PJ, Becker RC, et al. Association of spontaneous and procedure-related bleeds with short- and long-term mortality after acute coronary syndromes: An analysis from the PLATO trial. EuroIntervention 2015;11:737-45. [Crossref] [PubMed]
- Ducrocq G, Schulte PJ, Budaj A, et al. Balancing the risk of spontaneous ischemic and major bleeding events in acute coronary syndromes. Am Heart J 2017;186:91-9. [Crossref] [PubMed]
- Serruys PW, Morice MC, Kappetein AP, et al. Percutaneous Coronary Intervention versus Coronary-Artery Bypass Grafting for Severe Coronary Artery Disease. N Engl J Med 2009;360:961-72. [Crossref] [PubMed]
- Serruys PW, de Jaegere P, Kiemeneij F, et al. A Comparison of Balloon-Expandable-Stent Implantation with Balloon Angioplasty in Patients with Coronary Artery Disease. N Engl J Med 1994;331:489-95. [Crossref] [PubMed]
- Stone GW, Ellis SG, Cannon L, et al. Comparison of a Polymer-Based Paclitaxel-Eluting Stent With a Bare Metal Stent in Patients With Complex Coronary Artery Disease. JAMA 2005;294:1215. [Crossref] [PubMed]
- Stone GW, Ellis SG, Cox DA, et al. A Polymer-Based, Paclitaxel-Eluting Stent in Patients with Coronary Artery Disease. N Engl J Med 2004;350:221-31. [Crossref] [PubMed]
- Colombo A, Drzewiecki J, Banning A, et al. Randomized Study to Assess the Effectiveness of Slow- and Moderate-Release Polymer-Based Paclitaxel-Eluting Stents for Coronary Artery Lesions. Circulation 2003;108:788-94. [Crossref] [PubMed]
- Holmes DR, Leon MB, Moses JW, et al. Analysis of 1-Year Clinical Outcomes in the SIRIUS Trial: A Randomized Trial of a Sirolimus-Eluting Stent Versus a Standard Stent in Patients at High Risk for Coronary Restenosis. Circulation 2004;109:634-40. [Crossref] [PubMed]
- Doyle BJ, Rihal CS, Gastineau DA, et al. Bleeding, Blood Transfusion, and Increased Mortality After Percutaneous Coronary Intervention. J Am Coll Cardiol 2009;53:2019-27. [Crossref] [PubMed]
- Lopes RD, Subherwal S, Holmes DN, et al. The association of in-hospital major bleeding with short-, intermediate-, and long-term mortality among older patients with non-ST-segment elevation myocardial infarction. Eur Heart J 2012;33:2044-53. [Crossref] [PubMed]
- Angiolillo DJ, Goodman SG, Bhatt DL, et al. Antithrombotic Therapy in Patients with Atrial Fibrillation Undergoing Percutaneous Coronary Intervention: A North American Perspective-2016 Update. Circ Cardiovasc Interv 2016;9:1-27. [Crossref] [PubMed]
- Chhatriwalla AK, Amin AP, Kennedy KF, et al. Association Between Bleeding Events and In-hospital Mortality After Percutaneous Coronary Intervention. JAMA 2013;309:1022. [Crossref] [PubMed]
- Waltenberger J, Vainer J. PCI comes to age as age increasingly comes to PCI. Neth Heart J 2008;16:115-6. [Crossref] [PubMed]
- Urban P, Meredith IT, Abizaid A, et al. Polymer-free Drug-Coated Coronary Stents in Patients at High Bleeding Risk. N Engl J Med 2015;373:2038-47. [Crossref] [PubMed]
- Varenne O, Cook S, Sideris G, et al. Drug-eluting stents in elderly patients with coronary artery disease (SENIOR): A randomised single-blind trial. Lancet 2018;391:41-50. [Crossref] [PubMed]
- Tegn N, Abdelnoor M, Aaberge L, et al. Invasive versus conservative strategy in patients aged 80 years or older with non-ST-elevation myocardial infarction or unstable angina pectoris (After Eighty study): an open-label randomised controlled trial. Lancet 2016;387:1057-65. [Crossref] [PubMed]
- Alnasser SM, Bagai A, Jolly SS, et al. Transradial approach for coronary angiography and intervention in the elderly: A meta-analysis of 777,841 patients. Int J Cardiol 2017;228:45-51. [Crossref] [PubMed]
- Lee SY, Hong MK, Palmerini T, et al. Short-Term Versus Long-Term Dual Antiplatelet Therapy After Drug-Eluting Stent Implantation in Elderly Patients. JACC Cardiovasc Interv 2018;11:435-43. [Crossref] [PubMed]
- Office for National Statistics. National Population Projections: 2014-based statistical bulletin. 2015 (cited 2018 Mar 2). Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationprojections/bulletins/nationalpopulationprojections/2015-10-29
- Ndrepepa G, Schuster T, Hadamitzky M, et al. Validation of the bleeding academic research consortium definition of bleeding in patients with coronary artery disease undergoing percutaneous coronary intervention. Circulation 2012;125:1424-31. [Crossref] [PubMed]
- Schulman S, Angerås U, Bergqvist D, et al. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in surgical patients. J Thromb Haemost 2010;8:202-4. [Crossref] [PubMed]
- Schulman S, Kearon C. Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005;3:692-4. [Crossref] [PubMed]
- Rao SV, O’Grady K, Pieper KS, et al. A Comparison of the Clinical Impact of Bleeding Measured by Two Different Classifications Among Patients With Acute Coronary Syndromes. J Am Coll Cardiol 2006;47:809-16. [Crossref] [PubMed]
- Mehran R, Rao SV, Bhatt DL, et al. Standardized Bleeding Definitions for Cardiovascular Clinical Trials: A Consensus Report From the Bleeding Academic Research Consortium. Circulation 2011;123:2736-47. [Crossref] [PubMed]
- Bovill EG, Terrin ML, Stump DC, et al. Hemorrhagic events during therapy with recombinant tissue-type plasminogen activator, heparin, and aspirin for acute myocardial infarction. Results of the Thrombolysis in Myocardial Infarction (TIMI), Phase II Trial. Ann Intern Med 1991;115:256-65. [Crossref] [PubMed]
- Hart RG, Pearce LA, Rothbart RM, et al. Stroke with intermittent atrial fibrillation: incidence and predictors during aspirin therapy. Stroke Prevention in Atrial Fibrillation Investigators. J Am Coll Cardiol 2000;35:183-7. [Crossref] [PubMed]
- Vanassche T, Lauw MN, Eikelboom JW, et al. Risk of ischaemic stroke according to pattern of atrial fibrillation: analysis of 6563 aspirin-treated patients in ACTIVE-A and AVERROES. Eur Heart J 2015;36:281-7a. [Crossref] [PubMed]
- Healey JS, Connolly SJ, Gold MR, et al. Subclinical Atrial Fibrillation and the Risk of Stroke. N Engl J Med 2012;366:120-9. [Crossref] [PubMed]
- Gage BF, Waterman AD, Shannon W, et al. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA 2001;285:2864-70. [Crossref] [PubMed]
- Lip GY, Frison L, Halperin JL, et al. Identifying Patients at High Risk for Stroke Despite Anticoagulation: A Comparison of Contemporary Stroke Risk Stratification Schemes in an Anticoagulated Atrial Fibrillation Cohort. Stroke 2010;41:2731-8. [Crossref] [PubMed]
- Chao TF, Liu CJ, Wang KL, et al. Should Atrial Fibrillation Patients With 1 Additional Risk Factor of the CHA2DS2-VASc Score (Beyond Sex) Receive Oral Anticoagulation? J Am Coll Cardiol 2015;65:635-42. [Crossref] [PubMed]
- Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with Non-ST-Elevation acute coronary syndromes: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;64:e139-228. [Crossref] [PubMed]
- Baber U, Mehran R, Giustino G, et al. Coronary Thrombosis and Major Bleeding after PCI with Drug-Eluting Stents Risk Scores from Paris. J Am Coll Cardiol 2016;67:2224-34. [Crossref] [PubMed]
- Costa F, van Klaveren D, James S, et al. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score: a pooled analysis of individual-patient datasets from clinical trials. Lancet 2017;389:1025-34. [Crossref] [PubMed]
- Abu-Assi E, Raposeiras-Roubin S, Cobas-Paz R, et al. Assessing the performance of the PRECISE-DAPT and PARIS risk scores for predicting 1-year out-of-hospital bleeding in acute coronary syndrome patients. EuroIntervention 2018;13:1914-22. [Crossref] [PubMed]
- British Cardiovascular Intervention Society. National Audit of Percutaneous Coronary Interventions Annual Report 2015. 2015 (cited 2018 Mar 13). Available online: http://www.ucl.ac.uk/nicor/audits/adultpercutaneous/documents/Report-Dec2015V5.pdf
- De Luca L, Danchin N, Valgimigli M, et al. Effectiveness of Pretreatment With Dual Oral Antiplatelet Therapy. Am J Cardiol 2015;116:660-8. [Crossref] [PubMed]
- Mehta SR, Yusuf S, Peters RJ, et al. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet 2001;358:527-33. [Crossref] [PubMed]
- Bellemain-Appaix A, O’Connor SA, Silvain J, et al. Association of Clopidogrel Pretreatment With Mortality, Cardiovascular Events, and Major Bleeding Among Patients Undergoing Percutaneous Coronary Intervention. JAMA 2012;308:2507. [Crossref] [PubMed]
- Hwang D, Park KW, Lee JM, et al. Efficacy and safety of dual antiplatelet therapy after coronary stenting in patients with chronic kidney disease. Am Heart J 2018;197:103-12. [Crossref] [PubMed]
- Gargiulo G, Santucci A, Piccolo R, et al. Impact of chronic kidney disease on 2-year clinical outcomes in patients treated with 6-month or 24-month DAPT duration: An analysis from the PRODIGY trial. Catheter Cardiovasc Interv 2017;90:E73-84. [Crossref] [PubMed]
- James S, Budaj A, Aylward P, et al. Ticagrelor versus clopidogrel in acute coronary syndromes in relation to renal function: Results from the platelet inhibition and patient outcomes (PLATO) trial. Circulation 2010;122:1056-67. [Crossref] [PubMed]
- Bonaca MP, Bhatt DL, Cohen M, et al. Long-Term Use of Ticagrelor in Patients with Prior Myocardial Infarction. N Engl J Med 2015;372:1791-800. [Crossref] [PubMed]
- Palmerini T, Della Riva D, Benedetto U, et al. Three, six, or twelve months of dual antiplatelet therapy after DES implantation in patients with or without acute coronary syndromes: an individual patient data pairwise and network meta-analysis of six randomized trials and 11 473 patients. Eur Heart J 2017;38:1034-43. [PubMed]
- Budaj A, Eikelboom JW, Mehta SR, et al. Improving clinical outcomes by reducing bleeding in patients with non-ST-elevation acute coronary syndromes. Eur Heart J 2009;30:655-61. [Crossref] [PubMed]
- Stone GW, McLaurin BT, Cox DA, et al. Bivalirudin for Patients with Acute Coronary Syndromes. N Engl J Med 2006;355:2203-16. [Crossref] [PubMed]
- Shahzad A, Kemp I, Mars C, et al. Unfractionated heparin versus bivalirudin in primary percutaneous coronary intervention (HEAT-PPCI): an open-label, single centre, randomised controlled trial. Lancet 2014;384:1849-58. [Crossref] [PubMed]
- Mehta SR, Bassand J-P, Chrolavicius S, et al. Dose Comparisons of Clopidogrel and Aspirin in Acute Coronary Syndromes. N Engl J Med 2010;363:930-42. [Crossref] [PubMed]
- Becker RC, Bassand JP, Budaj A, et al. Bleeding complications with the P2Y12 receptor antagonists clopidogrel and ticagrelor in the PLATelet inhibition and patient Outcomes (PLATO) trial. Eur Heart J 2011;32:2933-44. [Crossref] [PubMed]
- Romagnoli E, Biondi-Zoccai G, Sciahbasi A, et al. Radial Versus Femoral Randomized Investigation in ST-Segment Elevation Acute Coronary Syndrome. J Am Coll Cardiol 2012;60:2481-9. [Crossref] [PubMed]
- Roffi M, Patrono C, Collet JP, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 2016;37:267-315. [Crossref] [PubMed]
- Andò G, Capodanno D. Radial Access Reduces Mortality in Patients With Acute Coronary Syndromes. JACC Cardiovasc Interv 2016;9:660-70. [Crossref] [PubMed]
- Valgimigli M, Gagnor A, Calabró P, et al. Radial versus femoral access in patients with acute coronary syndromes undergoing invasive management: A randomised multicentre trial. Lancet 2015;385:2465-76. [Crossref] [PubMed]
- Mamas MA, Ratib K, Routledge H, et al. Influence of access site selection on PCI-related adverse events in patients with STEMI: meta-analysis of randomised controlled trials. Heart 2012;98:303-11. [Crossref] [PubMed]
- Jolly SS, Yusuf S, Cairns J, et al. Radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes (RIVAL): A randomised, parallel group, multicentre trial. Lancet 2011;377:1409-20. [Crossref] [PubMed]
- Seto AH, Abu-Fadel MS, Sparling JM, et al. Real-Time Ultrasound Guidance Facilitates Femoral Arterial Access and Reduces Vascular Complications. JACC Cardiovasc Interv 2010;3:751-8. [Crossref] [PubMed]
- Ambrose JA, Lardizabal J, Mouanoutoua M, et al. Femoral Micropuncture or Routine Introducer Study (FEMORIS). Cardiology 2014;129:39-43. [Crossref] [PubMed]
- Aminian A, Saito S, Takahashi A, et al. Comparison of a new slender 6 Fr sheath with a standard 5 Fr sheath for transradial coronary angiography and intervention: RAP and BEAT (Radial Artery Patency and Bleeding, Efficacy, Adverse evenT), a randomised multicentre trial. EuroIntervention 2017;13:e549-56. [Crossref] [PubMed]
- Moscucci M, Fox KA, Cannon CP, et al. Predictors of major bleeding in acute coronary syndromes: the Global Registry of Acute Coronary Events (GRACE). Eur Heart J 2003;24:1815-23. [Crossref] [PubMed]
- Verheugt FW, Steinhubl SR, Hamon M, et al. Incidence, prognostic impact, and influence of antithrombotic therapy on access and nonaccess site bleeding in percutaneous coronary intervention. JACC Cardiovasc Interv 2011;4:191-7. [Crossref] [PubMed]
- Li L, Geraghty OC, Mehta Z, et al. Age-specific risks, severity, time course, and outcome of bleeding on long-term antiplatelet treatment after vascular events: a population-based cohort study. Lancet 2017;390:490-9. [Crossref] [PubMed]
- van Swieten JC, Koudstaal PJ, Visser MC, et al. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988;19:604-7. [Crossref] [PubMed]
- Taggar JS, Lip GY. Anticoagulation for elderly patients with atrial fibrillation: not to be neglected. Europace 2008;10:1-2. [Crossref] [PubMed]
- Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009;373:1849-60. [Crossref] [PubMed]
- Nichols M, Townsend N, Scarborough P, et al. Cardiovascular disease in Europe: Epidemiological update. Eur Heart J 2013;34:3028-34. [Crossref] [PubMed]
- Avezum A, Makdisse M, Spencer F, et al. Impact of age on management and outcome of acute coronary syndrome: observations from the Global Registry of Acute Coronary Events (GRACE). Am Heart J 2005;149:67-73. [Crossref] [PubMed]
- Stegemann S, Ecker F, Maio M, et al. Geriatric drug therapy: Neglecting the inevitable majority. Ageing Res Rev 2010;9:384-98. [Crossref] [PubMed]
- Aronow WS. Approach to symptomatic coronary disease in the elderly: TIME to change? Lancet 2001;358:945-6. [Crossref] [PubMed]
- Rich MW, Chyun DA, Skolnick AH, et al. Knowledge Gaps in Cardiovascular Care of Older Adults: A Scientific Statement from the American Heart Association, American College of Cardiology, and American Geriatrics Society: Executive Summary. J Am Geriatr Soc 2016;64:2185-92. [Crossref] [PubMed]
- Ndrepepa G, Neumann FJ, Schulz S, et al. Incidence and prognostic value of bleeding after percutaneous coronary intervention in patients older than 75 years of age. Catheter Cardiovasc Interv 2014;83:182-9. [Crossref] [PubMed]
- Murali-Krishnan R, Iqbal J, Rowe R, et al. Impact of frailty on outcomes after percutaneous coronary intervention: a prospective cohort study. Open Heart 2015;2. [Crossref] [PubMed]
- Rothwell PM, Coull AJ, Silver LE, et al. Population-based study of event-rate, incidence, case fatality, and mortality for all acute vascular events in all arterial territories (Oxford Vascular Study). Lancet 2005;366:1773-83. [Crossref] [PubMed]
- Belardi J, Manoharan G, Albertal M, et al. The influence of age on clinical outcomes in patients treated with the resolute zotarolimus-eluting stent. Catheter Cardiovasc Interv 2016;87:253-61. [Crossref] [PubMed]
- Thompson RC, Holmes DRJ, Gersh BJ, et al. Percutaneous transluminal coronary angioplasty in the elderly: Early and long-term results. J Am Coll Cardiol 1991;17:1245-50. [Crossref] [PubMed]
- Thompson RC, Holmes DR, Grill DE, et al. Changing outcome of angioplasty in the elderly. J Am Coll Cardiol 1996;27:8-14. [Crossref] [PubMed]
- Batchelor WB, Anstrom KJ, Muhlbaier LH, et al. Contemporary outcome trends in the elderly undergoing percutaneous coronary interventions: Results in 7,472 octogenarians. J Am Coll Cardiol 2000;36:723-30. [Crossref] [PubMed]
- Means G, End C, Kaul P. Management of Percutaneous Coronary Intervention Complications. Curr Treat Options Cardiovasc Med 2017;19:25. [Crossref] [PubMed]
- Verdoia M, Pergolini P, Rolla R, et al. Advanced age and high-residual platelet reactivity in patients receiving dual antiplatelet therapy with clopidogrel or ticagrelor. J Thromb Haemost 2016;14:57-64. [Crossref] [PubMed]
- Andreotti F, Rocca B, Husted S, et al. Antithrombotic therapy in the elderly: expert position paper of the European Society of Cardiology Working Group on Thrombosis. Eur Heart J 2015;36:3238-49. [PubMed]
- De Luca L, De Servi S, Musumeci G, et al. Is ticagrelor safe in octogenarian patients with non-ST elevation acute coronary syndromes? Eur Heart J Cardiovasc Pharmacother 2018;4:12-4. [Crossref] [PubMed]
- De Luca L, D’Ascenzo F, Musumeci G, et al. Incidence and outcome of switching of oral platelet P2Y12 receptor inhibitors in patients with acute coronary syndromes undergoing percutaneous coronary intervention: The SCOPE registry. EuroIntervention 2017;13:459-66. [Crossref] [PubMed]
- Gargiulo G, Valgimigli M. Long-term dual antiplatelet therapy and concomitant optimal medical therapy following percutaneous coronary intervention. Cardiovasc Diagn Ther 2017;7:S102-6. [Crossref] [PubMed]
- McCarthy CP, Steg G, Bhatt DL. The management of antiplatelet therapy in acute coronary syndrome patients with thrombocytopenia: a clinical conundrum. Eur Heart J 2017;38:3488-92. [Crossref] [PubMed]
- Trenk D, Stone GW, Gawaz M, et al. A Randomized Trial of Prasugrel Versus Clopidogrel in Patients With High Platelet Reactivity on Clopidogrel After Elective Percutaneous Coronary Intervention With Implantation of Drug-Eluting Stents. J Am Coll Cardiol 2012;59:2159-64. [Crossref] [PubMed]
- Legrand V, Cuisset T, Chenu P, et al. Platelet reactivity and cardiovascular events after percutaneous coronary intervention in patients with stable coronary artery disease: the Stent Thrombosis In Belgium (STIB) trial. EuroIntervention 2014;10:204-11. [Crossref] [PubMed]
- Brar SS, ten Berg J, Marcucci R, et al. Impact of Platelet Reactivity on Clinical Outcomes After Percutaneous Coronary Intervention. J Am Coll Cardiol 2011;58:1945-54. [Crossref] [PubMed]
- Price MJ, Berger PB, Teirstein PS, et al. Standard- vs High-Dose Clopidogrel Based on Platelet Function Testing After Percutaneous Coronary Intervention. JAMA 2011;305:1097-105. [Crossref] [PubMed]
- Collet JP, Cuisset T, Rangé G, et al. Bedside Monitoring to Adjust Antiplatelet Therapy for Coronary Stenting. N Engl J Med 2012;367:2100-9. [Crossref] [PubMed]
- Cuisset T, Cayla G, Frere C, et al. Predictive value of post-treatment platelet reactivity for occurrence of post-discharge bleeding after non-ST elevation acute coronary syndrome. Shifting from antiplatelet resistance to bleeding risk assessment? EuroIntervention 2009;5:325-9. [Crossref] [PubMed]
- Cayla G, Cuisset T, Silvain J, et al. Platelet function monitoring to adjust antiplatelet therapy in elderly patients stented for an acute coronary syndrome (ANTARCTIC): an open-label, blinded-endpoint, randomised controlled superiority trial. Lancet 2016;388:2015-22. [Crossref] [PubMed]
- Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS. Eur Heart J 2018;39:213-60. [Crossref] [PubMed]
- Halvorsen S, Storey RF, Rocca B, et al. Management of antithrombotic therapy after bleeding in patients with coronary artery disease and/or atrial fibrillation: Expert consensus paper of the European Society of cardiology working group on thrombosis. Eur Heart J 2017;38:1455-62. [PubMed]
- Yasuda H. Treatment and prevention of gastrointestinal bleeding in patients receiving antiplatelet therapy. World J Crit Care Med 2015;4:40. [Crossref] [PubMed]
- Lewis SR, Alderson P, Smith AF. Continuation versus discontinuation of antiplatelet therapy for bleeding and ischaemic events in adults undergoing non-cardiac surgery. Cochrane Database Syst Rev 2018;7. [PubMed]
- Isted A, Cooper L, Colville RJ. Bleeding on the cutting edge: A systematic review of anticoagulant and antiplatelet continuation in minor cutaneous surgery. J Plast Reconstr Aesthet Surg 2018;71:455-67. [Crossref] [PubMed]
- Rubboli A, Colletta M, Herzfeld J, et al. Periprocedural and medium-term antithrombotic strategies in patients with an indication for long-term anticoagulation undergoing coronary angiography and intervention. Coron Artery Dis 2007;18:193-9. [Crossref] [PubMed]
- Wang TY, Robinson LA, Ou FS, et al. Discharge antithrombotic strategies among patients with acute coronary syndrome previously on warfarin anticoagulation: Physician practice in the CRUSADE registry. Am Heart J 2008;155:361-8. [Crossref] [PubMed]
- Pérez-Gómez F, Alegría E, Berjón J, et al. Comparative effects of antiplatelet, anticoagulant, or combined therapy in patients with valvular and nonvalvular atrial fibrillation. J Am Coll Cardiol 2004;44:1557-66. [Crossref] [PubMed]
- Paikin JS, Wright DS, Crowther MA, et al. Triple antithrombotic therapy in patients with atrial fibrillation and coronary artery stents. Circulation 2010;121:2067-70. [Crossref] [PubMed]
- Fitzmaurice DA, Hobbs FD, Jowett S, et al. Screening versus routine practice in detection of atrial fibrillation in patients aged 65 or over: cluster randomised controlled trial. BMJ 2007;335:383. [Crossref] [PubMed]
- Lloyd-Jones DM. Lifetime Risk for Development of Atrial Fibrillation: The Framingham Heart Study. Circulation 2004;110:1042-6. [Crossref] [PubMed]
- Steg PG, Bhatt DL. Viewpoint: a proposal for a simple algorithm for managing oral anticoagulation and antiplatelet therapy in patients with non-valvular atrial fibrillation and coronary stents. Eur Heart J Acute Cardiovasc Care 2017;6:93-7. [Crossref] [PubMed]
- Lau DH, Schotten U, Mahajan R, et al. Novel mechanisms in the pathogenesis of atrial fibrillation: practical applications. Eur Heart J 2016;37:1573-81. [Crossref] [PubMed]
- Singh RB, Mengi SA, Xu YJ, et al. Pathogenesis of atherosclerosis: A multifactorial process. Exp Clin Cardiol 2002;7:40-53. [PubMed]
- Dewilde WJ, Oirbans T, Verheugt FW, et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: An open-label, randomised, controlled trial. Lancet 2013;381:1107-15. [Crossref] [PubMed]
- Gibson CM, Mehran R, Bode C, et al. Prevention of Bleeding in Patients with Atrial Fibrillation Undergoing PCI. N Engl J Med 2016;375:2423-34. [Crossref] [PubMed]
- Cannon CP, Bhatt DL, Oldgren J, et al. Dual Antithrombotic Therapy with Dabigatran after PCI in Atrial Fibrillation. N Engl J Med 2017;377:1513-24. [Crossref] [PubMed]
- Chen CF, Chen B, Zhu J, et al. Antithrombotic therapy after percutaneous coronary intervention in patients requiring oral anticoagulant treatment. Herz 2015;40:1070-83. [Crossref] [PubMed]
- Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus Warfarin in Patients with Atrial Fibrillation. N Engl J Med 2009;361:1139-51. [Crossref] [PubMed]
- Cavallari I, Patti G. Meta-Analysis Comparing the Safety and Efficacy of Dual Versus Triple Antithrombotic Therapy in Patients With Atrial Fibrillation Undergoing Percutaneous Coronary Intervention. Am J Cardiol 2018;121:718-24. [Crossref] [PubMed]
- D’Ascenzo F, Taha S, Moretti C, et al. Meta-Analysis of Randomized Controlled Trials and Adjusted Observational Results of Use of Clopidogrel, Aspirin, and Oral Anticoagulants in Patients Undergoing Percutaneous Coronary Intervention. Am J Cardiol 2015;115:1185-93. [Crossref] [PubMed]
- Lamberts M, Gislason GH, Olesen JB, et al. Oral Anticoagulation and Antiplatelets in Atrial Fibrillation Patients After Myocardial Infarction and Coronary Intervention. J Am Coll Cardiol 2013;62:981-9. [Crossref] [PubMed]
- Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus Warfarin in Patients with Atrial Fibrillation. N Engl J Med 2013;369:2093-104. [Crossref] [PubMed]
- Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus Warfarin in Patients with Atrial Fibrillation. N Engl J Med 2011;365:981-92. [Crossref] [PubMed]
- Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus Warfarin in Nonvalvular Atrial Fibrillation. N Engl J Med 2011;365:883-91. [Crossref] [PubMed]
- Jensen MS, Larsen OH, Christiansen K, et al. Platelet activation and aggregation: the importance of thrombin activity-A laboratory model. Haemophilia 2013;19:403-8. [Crossref] [PubMed]