Antiplatelet therapy in valvular and structural heart disease interventions
Interventions for valvular and structural heart diseases are rapidly expanding. Currently percutaneous approach represents the standard of care in specific subsets of patients as those with high operative risk for traditional open surgery (1). However, these procedures are burdened by a significant risk of embolic complications, such as stroke and myocardial infarction. Thus, antithrombotic therapy represents the cornerstone of adjunctive pharmacologic therapy although type and doses of antiplatelet agents remain mostly empiric for these indications. Of course, there is also a downside. Patients with structural heart diseases undergoing percutaneous procedures are predisposed to a high bleeding risk, because of specific procedural questions, such as vascular access from larger devices, but also of their co-morbidities and frailty. Thus, in this setting, the delicate balance between thrombotic and bleeding risk is crucial.
Transcatheter aortic valve implantation (TAVI)
Thromboembolic risk
TAVI represents the standard of care in elderly patients with high operative risk and an alternative to surgery in those with severe aortic stenosis at intermediate-to-high risk (1-4). Although this relative new therapeutic strategy has been associated with high procedural success, the occurrence of embolic complications, and in particular cerebrovascular events (CVEs), has emerged as an important concern. In the Placement of Aortic Transcatheter Valves (PARTNER) Trial, the incidence of stroke was higher in the TAVI group compared with patients receiving surgery at 1 year follow-up (5.1% vs. 2.4%, P=0.07) (5). Moreover, several magnetic resonance imaging studies have shown a significant incidence of new, clinically silent, cerebral ischemic lesions after TAVI, ranging from 68% to 86% (6-8). Fortunately, the risk of CVEs has declined over the years with increased operator experience and advancements in valve technology. In a recent report from the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapies Registry, the rate of stroke was 2.5% at 30 days and 4.1% at 1 year (9). Therefore, the all-comers German Aortic Valve Registry reported an incidence of stroke of 1.5% in a cohort of 15,964 patients receiving TAVI from 2011 to 2013 (10). Importantly, CVEs greatly worsen prognosis of patients undergoing TAVI. Major stroke has been demonstrated to be an independent predictor of mortality at 30 days (OR 7.43; 95% CI, 2.45–22.53; P=0.001) and on long-term follow-up (HR 1.75; 95% CI, 1.01–3.04; P=0.043) (11). A meta-analysis of 53 studies confirmed a significantly higher 30-day mortality in patients with post-procedural stroke (25.5%) compared with those without this complication (6.9%) (P=0.001) (12). Considering the relevant adverse clinical impact of CVEs in this setting, it is obvious that their prevention is even more important in younger patients with low-intermediate operative risk that could potentially represent the next beneficiaries of the TAVI strategy.
Thromboembolic complications may occur during or after TAVI, either in the first 48 h or during long-term follow-up, with about 90% within 2 months after valve implantation (13). Different mechanisms are responsible for this temporal pattern. Early events are mainly related to embolization from particulate matter during larger caliber catheters and devices manipulation across the calcified valve leaflets, balloon pre and post-dilation, valve device dislodgement/embolization, and air embolism. All these phenomena may be exacerbated by hemodynamic instability during TAVI procedures that increases hypotension and cerebral hypo-perfusion. This is confirmed by transcranial doppler studies showing that cerebral emboli can occur more frequently during valve prosthesis positioning and implantation (7) and by the evidence of heterogeneous materials captured by embolic protection devices during TAVI (thrombus, fibrin, calcified material, tissue fragments from aortic wall and leaflets) (14). Late CVEs may be associated with the thrombogenicity of implanted valve prosthesis. An anatomopathological study demonstrated that fibrin deposition and inflammatory response developed early after Medtronic CoreValve™ implantation, followed by neointimal coverage and complete device endothelialization at about 3 months (15). Also the damaged native valve leaflets, crimped by the new implanted valve, and the consequent altered rheology in the paravalvular space, may predispose to thrombus formation. Finally, the occurrence of new-onset atrial fibrillation (NOAF) was associated with a higher rate of stroke/ischemic cerebral complications after TAVI (16,17). Nombela-Franco et al. evaluated the predictors of acute (<24 h), subacute (>24 h and <30 days) and late (>30 days) CVEs in 1,061 patients who underwent TAVI (11). According to this analysis, mechanical factors such as post-dilation and valve dislodgement have been demonstrated as predictors of acute events, whereas, NOAF determined mainly the events occurring in the subacute phase. Finally, late events were associated with a history of chronic atrial fibrillation (AF), peripheral vascular disease and prior cerebrovascular disease (11). Recently, a systematic review reported that also female sex, chronic kidney disease and centre experience may predict the occurrence of CVEs at 30-day follow-up after TAVI (18).
Bleeding risk
Bleeding risk remained another important matter to be considered in “frail and vulnerable” patients as those with severe aortic stenosis and high operative risk requiring percutaneous strategy. Généreux et al. reported an incidence of life-threatening and major bleeding after TAVI of 15.6% and 22.3%, respectively, in a weighted meta-analysis of 16 studies using Valve Academic Research Consortium (VARC) definitions (19). Early bleeding complications are frequently related to procedural and technical factors. Major bleedings in patients with transfemoral approach were associated with increased rate of vascular complications and serious adverse events, such as migration or embolization of the prosthesis, requiring hemodynamic support (20,21). Also diabetes mellitus and renal impairment (glomerular filtration rate <30 mL/min) have been demonstrated to be independent clinical predictors of bleeding in this population (22,23). Importantly, in the PARTNER trial major bleedings within 30 days were strongly and independently associated with 1-year mortality in the overall population, both in patients undergoing TAVI than those surgically treated (HR 2.49; 95% CI, 1.85–3.37, P<0.001) (20) and their impact on long term outcome was also confirmed in other TAVI series (21). However, the earliest experience of percutaneous aortic valve replacement was burdened by the use of large first-generation delivery systems (22 or 24 French for transfemoral approach). The negative impact of these large-bore catheters during percutaneous interventions has been confirmed in a retrospective analysis from one of the larger insurance US database (24). According to this analysis, bleeding complications (defined as any transfusion, any hemorrhage or hematoma, or the need for surgical or percutaneous intervention to control the bleeding event) were associated with a higher adjusted risk of in-hospital mortality, longer hospitalization and higher health care costs in patients undergoing TAVI or other procedures requiring their use (24). Recently, considering the ongoing evolution toward lower-profile TAVI devices, the rate of bleeding complications has been reduced. The SOURCE XT Registry reported an incidence of life-threatening and major bleedings of 3.8% and 7.7%, respectively, using the balloon expandable Edwards Sapien XTTM with 18 or 19 French sheaths, confirming the optimal safety profile of new generation TAVI devices (25).
Patients undergoing TAVI have been demonstrated to show also late (>30 days) bleeding events; in this setting, the specific mechanisms are not clear, probably related to the multiple comorbidities and frailty of these patients. An analysis from both cohort A and B of the PARTNER trial demonstrated that major late bleeding complications (between 30 days and 1 year) occurred in about 6% of TAVI patients, with gastrointestinal bleeding as the most frequent type of bleeding on long-term follow-up (26). Indeed, the Heyde’s syndrome is frequently observed in patients with severe aortic valve stenosis, characterized by the presence of bleeding from gastrointestinal angiodysplasia and an acquired von Willebrand factor deficiency (27). Recently, the Bern TAVI Registry confirmed that gastrointestinal disorders accounted for 9.7% of non-cardiovascular hospital readmissions in patients undergoing TAVI (28). In this setting, data regarding proton pump inhibitors (PPI) use and their benefit in reducing gastrointestinal complications in patients on DAPT are limited; however, in a recent randomized trial, all patients undergoing TAVI and suffering gastrointestinal bleedings were receiving a PPI prior to the haemorrhagic event (29). Importantly the occurrence of late bleedings was associated with a more than 3-fold higher risk of mortality (HR 3.83; 95% CI, 2.62–5.61, P<0.001) (26); according to the same PARTNER analysis, moderate or severe aortic paravalvular leaks at 30 days, AF or atrial flutter at baselines or 30 days, greater left ventricular mass and low baseline hemoglobin were identified as independent predictor of major late bleedings after TAVI (26).
Current antithrombotic strategy in patients receiving TAVI
The antithrombotic strategy during and after TAVI was not clearly established in most of the largest randomized trials. In the PARTNER trial, dual antiplatelet therapy (DAPT) was suggested for 6 months after the procedure and a loading dose of clopidogrel was administered in patients not already taking this antiplatelet agent (3). In the Medtronic CoreValve US Pivotal Trial, aspirin (81–325 mg) indefinitely plus clopidogrel (75 mg) for 3 months was administered after TAVI (30). A similar antiplatelet protocol was proposed in the Comparison of Transcatheter Heart Valves in High Risk Patients With Severe Aortic Stenosis: Medtronic CoreValve vs. Edwards SAPIEN XT (CHOICE) trial (31). Finally, several registries about TAVI showed a wide variance in antiplatelet strategy. A loading dose of clopidogrel, 300 or 600 mg, was not always administered and the duration of DAPT was 1–6 months also according patients’ bleeding risk (32).
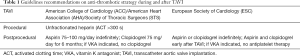
Current European Society of Cardiology (ESC) recommend the use of DAPT with aspirin and clopidogrel for 3–6 months, followed by single antiplatelet therapy with aspirin alone in patients with no other indication for oral anticoagulant therapy (OAC) (Class IIa, C) (Table 1) (1). Moreover, ESC guidelines stated that, single antiplatelet therapy may be considered after TAVI in the case of high bleeding risk (Class IIb, C) (1). Also in a recent focused update of the AHA/ACC guidelines, clopidogrel 75 mg has been considered reasonable for the first 6 months after TAVI in addition to lifelong aspirin (75–100 mg) (Class IIb, C) (Table 1) (2). Interestingly, this document reported that anticoagulation with a vitamin K antagonist (VKA) to achieve an INR of 2.5 might be considered for at least 3 months after TAVI in patients at low risk of bleeding, according to recent observational studies investigating the role of anticoagulation in the occurrence of prosthesis valve thrombosis.
Full table
The use of DAPT after TAVI is empirically based on prior experience of ischemic complications after percutaneous coronary interventions; however, it has not been established if thromboembolic complications following these two different therapeutic strategies are primarily due to similar pathophysiologic mechanisms. Moreover, TAVI is associated with a higher incidence of bleeding and vascular complications compared with coronary procedures. For this reason, several randomized and observational studies have recently questioned the usefulness and safety of the administration of two antiplatelet agents in patients receiving TAVI.
Antiplatelet therapy before and during the procedure
The use of DAPT before TAVI has been associated with significantly higher incidence of bleeding, with no additional benefit in reducing thrombotic events; thus, a routine strategy of pre-treatment with aspirin and clopidogrel is not favorably considered in TAVI patients right now.
The rationale for pre-procedural antiplatelet therapy was based on initial TAVI experience to avoid post-procedural thrombocytopenia, an expected complication of the use of extracorporeal circulation (33). However, recently, Hioki et al. reported data from the OCEAN-TAVI registry enrolling 540 patients who underwent transfemoral TAVI with no pre-procedural antiplatelet therapy, single antiplatelet therapy (SAPT) or DAPT before the procedure (34). Pre-procedural use of antiplatelet agents was usually started 1 week before the procedure (aspirin 100 mg plus clopidogrel 75 mg or aspirin only), whereas, all patients were treated with antiplatelet therapy 1 day after TAVI. The incidence of any bleeding was higher in patients on DAPT at the time of the procedure compared with those with no preprocedural antiplatelet therapy or SAPT (36.5% vs. 21.3% vs. 27.5%, respectively). At the multivariate analysis, DAPT before TAVI significantly increased the risk of bleeding complications (OR 2.30; 95% CI, 1.08–4.90; P=0.031). Notably, on the other hand, the lack of pre-procedural antiplatelet therapy or the use of a single antiplatelet agent did not increase the risk of thrombotic complications compared with DAPT (34). Thus, these findings confirmed that, in the modern practice, the use of clopidogrel preloading should not be recommended before TAVI.
Post-procedural antiplatelet treatment
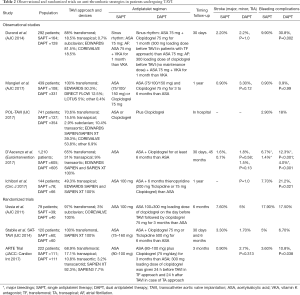
Several observational and randomized trials have recently suggested that the addition of clopidogrel to aspirin did not improve efficacy in preventing thromboembolic complications after TAVI, but significantly reduce the safety of these patients with an increase in bleeding events (Table 2) (29,35-41). Ussia et al. randomized 79 patients to receive DAPT (300 mg loading dose of clopidogrel 1 day before TAVI followed by 3-month maintenance daily dose of 75 mg plus aspirin 100 mg lifelong) or SAPT (aspirin 100 mg) (35). In this study no difference was observed between the two strategy groups in the cumulative incidence of major adverse cardiac and cerebrovascular complications at 30 days and 6 months follow-up. Similar results were observed by Stabile et al. in 120 patients randomized to aspirin alone or DAPT (clopidogrel 75 mg or ticlopidine 500 mg/die for 6 months) (36). DAPT was associated with a significant increase in the rate of vascular complications and with a non-significant higher incidence of bleedings whereas the rates of thrombotic events and cardiovascular death were not different between the two groups. Recently, no difference in the incidence of net adverse clinical events, cardiac mortality and CVEs was reported in an observational retrospective analysis comparing the clinical outcomes of patients discharged in SAPT for clinical reasons (elevated bleeding risk) and those on DAPT (40). A similar rate of valve thrombosis and bleeding complications requiring hospitalization was also observed in both groups (40). Furthermore, a patient-level meta-analysis confirmed these results suggesting no benefit in reducing thrombotic complications associated with DAPT and a trend towards less life-threatening and major bleeding with single antiplatelet therapy (42). Finally, a strategy of single vs. DAPT was compared in the recently published Aspirin vs. Aspirin plus Clopidogrel following Transcatheter Aortic Valve (ARTE) randomized trial (29). Patients were assigned to receive aspirin (80 or 100 mg/day; started at least 24 h before TAVI and continued for at least 6 months) or aspirin plus clopidogrel. Clopidogrel was started within 24 h before the procedure in patients receiving transfemoral approach and 24 h after TAVI in those undergoing other approaches. Of note, a loading dose of clopidogrel was administered in all patients, followed by 75 mg/day, continued for at least 3 months. Single antiplatelet therapy significantly decreased the incidence of life-threatening/major bleeding at 90-day follow-up (3.6% vs. 10.8%, P=0.038), while demonstrating similar benefit in preventing thromboembolic complications. Thus a trend toward a lower incidence of the composite end point (death, ischemic and bleeding complications) was observed in patients on aspirin alone compared with those receiving DAPT.
Full table
On the basis of these findings, the strategy of adding clopidogrel to aspirin after successful TAVI seems to be not superior and beneficial compared with aspirin alone. No data are available on the use of new P2Y12 receptor inhibitors (ticagrelor or prasugrel) in addition to aspirin after TAVI; however, some on-going experimental studies are investigating the efficacy and safety of a single prophylactic therapy with ticagrelor compared with traditional DAPT in the prevention of embolic complications after TAVI (ClinicalTrials.gov; NCT02224066).
Management of patients with pre-existing or new-onset AF
The prevalence of pre-existing AF among patients undergoing TAVI ranges from 16% to 51% (43). Moreover, patients receiving TAVI showed also a significant incidence of NOAF, up to 30% according to several observational studies (16,17). This complication is higher after transapical approach. Of note, both pre-existing and new onset AF have been associated with worse outcome in TAVI patients (18). Despite the relatively common population with pre-existing or new-onset AF, a subset of patients predisposed to both high thrombotic and bleeding risk, the optimal antithrombotic strategy in this field has not been clearly established. At the moment, a combination of VKA and aspirin is generally used, whereas, a triple therapy is prescribed only in patients with a strict indication to DAPT, such as those with recent percutaneous coronary intervention. However, few observational studies have specifically investigated this issue. In an observational study on 621 patients undergoing TAVI with AF, the addition of antiplatelet therapy (aspirin or clopidogrel) conferred no clinical benefit to OAC while potentially may be harmful (44). No difference was observed in the rate of stroke, myocardial infarction and death among patients treated with VKA only vs. those receiving anticoagulant and antiplatelet agents. However, the incidence of major or life-threatening bleedings was significantly higher in this last group (14.8% vs. 5.9%, P=0.02). Interestingly, a further sub-analysis including only patients receiving both anticoagulant and antiplatelet therapy demonstrated a higher risk of major bleedings in the subgroup of patients receiving warfarin and aspirin compared with those treated with warfarin and clopidogrel (44). Currently, few data are available on the use of non-VKA oral anticoagulant (NOACs) in patients undergoing TAVI. Seeger et al. reported the efficacy and safety of apixaban compared with warfarin in patients with AF and TAVI (45). In this study, SAPT plus oral anticoagulation was given for 4 weeks after the procedure (DAPT only after Boston Lotus valve implantation). Patients were anticoagulated with apixaban (141 patients) or VKA (131 patients). There was a significantly lower incidence of 30-day life-threatening and major bleeding in patients treated with apixaban compared with those on warfarin (3.5% vs. 5.3%, P<0.01) and also a tendency toward a reduced stroke rate in this group. Undoubtedly, randomized trials specifically enrolling patients with AF undergoing TAVI are needed to confirm these exploratory results.
Prosthetic valve thrombosis: anticoagulant or antiplatelet therapy after TAVI?
Clinical and subclinical leaflet thrombosis is emerging as an important complication after TAVI, manifesting as reduced leaflet motion and increased mean aortic valve gradient detected by high-resolution CT or echocardiographic follow-up. Chakravarty et al. considered 931 patients who had CT imaging at a median of 83 days after valve implantation from two large registries (46). They observed an incidence of subclinical thrombosis of 13%; of note, it was lower among patients receiving OAC after TAVI (25% of the study population) than among those who were on antiplatelet agents, both DAPT or SAPT. No difference was observed between patients on VKA or NOACs. Interestingly, OAC was associated with restoration of normal leaflet motion in all patients where this strategy was applied, whereas, patients with subclinical leaflet thrombosis treated by antiplatelet therapy or no therapy did not observe any regression (46). Similar findings were also reported by other observational studies (47); Del Trigo et al. confirmed the absence of VKA at discharge as an independent predictor of valve thrombosis (48). Importantly, the rate of transient ischemic attacks was significantly increased in patients with subclinical leaflet thrombosis (46).
The phenomenon of valve thrombosis is not restricted to a short time interval after valve implantation, but may develop later, indicating that TAVI patients are at risk of embolic complications even in an advanced stage (months or years) after the procedure. Moreover, as previously reported, the use of antiplatelet therapy after TAVI, as recommended by guidelines (1,2), may be questionable to prevent this complication. In this setting only the use of anticoagulation has been demonstrated to reduce the incidence of subclinical leaflet thrombosis. Thus, future investigations are needed to establish the usefulness of OAC after TAVI also in patients without AF and, especially, its optimal duration after valve implantation.
Forthcoming trials
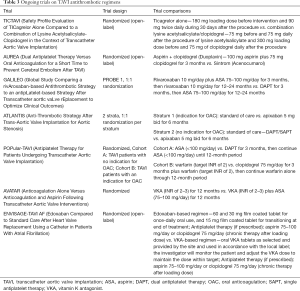
On the basis of this evidence, establishing the optimal antithrombotic strategy after TAVI remains a challenge. Further randomized trials are needed to definitively confirm the uselessness of DAPT compared with single therapy in this setting. Moreover, anticoagulation, but not antiplatelet therapy, has been demonstrated to be effective in the prevention and treatment of prosthesis valve thrombosis. Thus, several studies are currently evaluating the use of VKA and NOACs after TAVI both in patients with and without AF or other indications for anticoagulation therapy. Table 3 reports ongoing trials regarding antithrombotic therapy in patients undergoing TAVI.
Full table
Transcatheter mitral valve repair (TMVR)—the MitraClip
Thromboembolic and bleeding risk
TMVR includes minimally invasive techniques for treatment of symptomatic chronic moderate-severe or severe mitral regurgitation (MR) especially in patients with prohibitive surgical risk (49). While a number of technologies are in clinical development, the Abbott MitraClip NT is currently the only US Food and Drug Administration (FDA) approved device for TMVR. The MitraClip is a percutaneous procedure with its rationale on the surgical Alfieri “edge-to-edge” repair; it utilizes a cobalt chromium clip covered with a polypropylene fabric that grasps both the anterior and posterior mitral valve leaflets suturing together the middle segments of both leaflets, thereby creating a “double orifice” MR area (50).
Percutaneous MitraClip procedure involves use of potentially thrombogenic materials through the venous system, trans-septal advancement of large-bore catheter devices and beating-heart maneuvering of the clip within complex anatomy of the mitral valve and subvalvular apparatus (51). This procedure is associated with an overall complication rate of 15% to 19% at 30 days (49,52). Complications include access site bleeding, partial clip detachment, rarely device embolization or thrombosis, stroke and development of mitral stenosis (52).
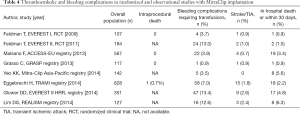
In the Endovascular Valve Edge-to-Edge Repair Study (EVEREST) II trial, 13% of patients randomized to TMVR required transfusion of ≥2 units of blood, although this percentage was lower compared with 45% observed in the surgical group (49). However, in the latest registries regarding MitraClip, the rate of transfusions indicating bleeding complications has been significantly reduced, ranging from 0.9% to 3.9%, due to the increased operators’ experience and improved devices (53,54). On the other hand, comparing with other percutaneous structural procedures, stroke is a rare complication after TMVR; only an incidence of 0.9% of ischemic strokes was documented on 30 days follow-up in the EVEREST RCT trial (49), 2.6% in the EVEREST-HRR (52) and 2.4% in the EVEREST-REALISM registries (55) (Table 4). Moreover, device thrombosis was recently reported in two cases immediately post-MitralClip placement despite DAPT administration (56,57).
Full table
In this complex scenario, dealing with periprocedural antiplatelet and anticoagulant drugs for patients receiving MitraClip implantation is crucial for reducing the risk of stroke, systemic embolism, device thrombosis and to achieve successful procedural and clinical outcomes. However, currently, no clear evidence based guidelines exist on choice or duration of antiplatelet and anticoagulant regimens and the strategies are generally left to operators’ discretion. Moreover, most of the patients undergoing TMVR have also an increased risk of adverse events “per se” due to their cardiovascular comorbidities requiring in some cases antithrombotic therapy and potentially predisposing to bleeding and ischemic accidents.
Antiplatelet therapy before and during the procedure
A standard regimen of antiplatelet therapy and anticoagulation prior to MitralClip placement has not yet been established. Conventionally, patients who are already being treated with aspirin or clopidogrel are continued on aspirin and clopidogrel without interruption before the procedure (58). Patients on oral anticoagulation for any reason should be managed in the same way as any percutaneous intervention; thus, anticoagulation should be interrupted to minimize bleeding complications during the procedure. Antiplatelet naïve patients should be started on aspirin and clopidogrel immediately after the procedure (59). The role of loading patients with antiplatelet agents just before the clip implantation is unknown and needs to be investigated.
Post-procedural antiplatelet treatment
The manufacturer (Abbott Vascular) did not make strict recommendations on the choice or the duration of antiplatelet therapy after the procedure due to lack of specific investigations comparing various agents or protocols. The regimen was extrapolated from those previously being used after septal occluder device implantation for atrial and septal defects with the purpose of allowing for complete endothelialization of the device. In the EVEREST I (59) trial, EVEREST II study protocol (60), EVEREST II RCT (49,60) and the EVEREST II high risk registry (HRR) (61), a regimen of aspirin at a dose of 325 mg daily for 6 months to 1 year was used associated with clopidogrel at a dose of 75 mg daily for 1 month. In Europe, a regimen composed of aspirin (100 mg/day) for 3 months and clopidogrel (75 mg/day, without a loading dose) for 4 weeks is more commonly used (53). However, these regimens have not been evaluated in controlled randomized trials. Moreover, thrombotic complications are rare while bleeding events seem to be more frequent; thus, it could be hypothesized that a single antiplatelet/anticoagulant agent may be enough post-operatively. These findings underline the need of further research to evaluate the impact of other peri-/postprocedural strategies including also the use of newer antiplatelet medications (prasugrel and ticagrelor). Moreover, OAC after device placement is not routinely recommended, whereas, recent studies suggest that interventional left atrial appendage occlusion may have a role in patients after MitraClip implantation (62).
Management of patients with AF
Patients undergoing MitraClip implantation have a high prevalence of AF requiring anticoagulation (33.9% in EVEREST II; 67.7% in ACCESS-EU), which increases the risk of bleeding from sources apart from the vascular access site (i.e., gastro-intestinal) (49,53). After MitraClip implantation, for patients with AF or other indications for OAC, the optimal anticoagulation/antiplatelet regimen is unknown. Triple therapy should be avoided in order not to increase the risk of bleeding. On the other hand, thrombus formation within the left atrium but also left ventricle has been observed after MitraClip implantation despite DAPT (56).
Patent foramen ovale (PFO)/atrial septal defects (ASD) transcatheter closure
Transcatheter closure of heart defects has become a largely alternative to surgery for a variety of congenital pathologies. The ASD and the PFO represent the most common congenital heart diseases (63,64). With the development of new occlusion devices and improvement of implantation techniques, the implementation of these procedures has risen over the years. In consequence, today’s indication for the use of percutaneous occlusion devices ranges from the relevant left-to-right shunt in patients with ASD to the prevention of recurrent paradoxical embolism in those with diagnosis of PFO (65). Different peri-procedural complications after ASD and PFO closure have been described, such as vascular complications, air embolism, atrial wall perforation with pericardial effusion and device embolization (63,66). Later adverse events are generally associated with late device embolization, device arm fracture and atrial wall erosion. Thrombus formation may be up to 10% of all cases and related to the implantation of large devices in low flow cavities such as right and left atria (67,68). Case histories have indicated that thrombus formation may be a potential and serious complication after device implantation requiring surgical removal in some cases. Moreover, thrombus deposition typically occurs on the metallic structures of the occlusion devices and develops early after implantation, within the first 4 weeks, caused by lack of endothelialization in this initial period (69). Actually, animal experiments have shown that about 4 weeks after implantation only half of the occluder surface was covered by neointima whereas complete endothelialization was achieved in 3 months (70). In the largest reported study on 1,000 patients receiving ASD or PFO occluders, Krumsdorf et al. reported a rate of thrombosis of 2% using a transesophageal echocardiography (TEE) follow-up. Interestingly, independent predictors for thrombus formation included post-procedural AF and persistent atrial septal aneurysm (69).
Based on the better understanding of the thrombosis mechanisms, platelet-induced thrombus growth is now considered to be more important than plasmatic hemostasis in this setting (71). Thus, antithrombotic prophylaxis in patients undergoing percutaneous closure of ASD or PFO represents an important issue especially considering that in most cases the occlusion is indicated to prevent recurrent embolic events. However, peri- and post-interventional antiplatelet management still remains controversial. Generally, ASA and clopidogrel are started 24 h before the procedure in naïve patients with a loading dose respectively of 300 and 300/600 mg. Additional mid-term antithrombotic therapy is variable. To date no randomized studies have been published to assess the optimal strategy after device implantation. In the REDUCE trial 664 patients with previous cryptogenic stroke were randomized in a 2:1 ratio to undergo PFO closure (Gore Occluder) plus antiplatelet therapy or antiplatelet therapy alone. Antiplatelet therapy could consist of ASA alone (75 to 325 mg once daily), a combination of aspirin (50 to 100 mg daily) and dipyridamole (225 to 400 mg daily) or clopidogrel (75 mg once daily). All patients continued the antiplatelet therapy for the median follow-up of 3.2 years. Device related thrombosis occurred in 2 patients and the risk of recurrent stroke was significantly lower with PFO closure plus antiplatelet therapy than with antiplatelet therapy alone (1.4% vs. 5.4%, P=0.002) (72). In the RESPECT trial, 980 patients with previous cryptogenic ischemic stroke were randomly assigned to undergo PFO closure (Amplatz PFO occluder) or receive medical therapy for a median follow-up of 5.9 years. Patient undergoing PFO closure received 81 to 325 mg of ASA plus clopidogrel 75 mg daily for 1 month, followed by ASA 81 mg for 5 months. In the medical-therapy group four regimens were allowed: ASA 81 mg daily, clopidogrel 75 mg daily, warfarin with a goal INR of 2–3, ASA plus dipyridamole (225 to 400 mg daily). There were 2 cases of device thrombosis treated successfully with intravenous heparin and no significant differences in terms of bleedings between the 2 groups. On long-term, closure of PFO was associated with a lower rate of recurrent ischemic stroke than medical therapy (3.6% vs. 5.8%, P=0.007) (73). Finally, in the CLOSE trial, 663 patients with a recent stroke attributed to PFO were assigned in a 1:1:1 ratio to PFO closure (11 different devices were used) plus long term antiplatelet therapy, oral anticoagulation alone or antiplatelet therapy alone. Patients who underwent PFO closure received DAPT (75 mg of ASA plus 75 mg of clopidogrel) for 3 months, followed by SAPT for a median follow-up of 5.5 years. Among patients assigned to oral anticoagulation, 93% received VKA and 7% NOACs. In the antiplatelet therapy group, 87% received ASA 75 mg, 10% Clopidogrel 75 mg and 3% ASA 75 mg plus dipyridamole (225 to 400 mg daily) throughout the study period. One device related thrombosis occurred and no significant differences in terms of major bleeding were described. The rate of recurrent stroke was significantly lower with PFO closure plus long-term antiplatelet therapy with ASA than with antiplatelet or anticoagulant therapy only (respectively 0% vs. 5.9% vs. 1.6%; P<0.001) (74).
In conclusion there are no randomized studies available to assess the effectiveness of any of these antiplatelet strategies against the others, but it seems reassuring that 70% of the patients presenting with device thrombosis in the review of Sherman et al. were successfully managed medically by systemic anticoagulation with heparin or warfarin (75). Moreover, inherited thrombophilic disorders such as factor V Leiden or prothrombin mutations have to be excluded before device implantation in order to adapt post-procedural antithrombotic prophylaxis (combination of antiplatelet and anticoagulant therapy).
Finally, in routine clinical practice, most experienced centers recommend a post-procedural regimen using ASA (81 to 325 mg) for 3 to 12 months or a combination of ASA (81 to 100 mg) plus clopidogrel 75 mg for 6 to 8 weeks followed by ASA only (81 to 100 mg) for additional 4 to 8 months.
Left atrial appendage closure (LAAC)
Interventional LAAC is an established therapeutic option for AF patients at risk for thromboembolic complications and with contraindications to long-term OAC (class II b, level of evidence B) (76). The aim of the procedure is to completely seal the LAA in order to eliminate the major source of cardiac emboli and avoid the necessity for any long-life antithrombotic medication, thus minimizing the patients’ bleeding risk. However, after the LAAC, clots may form on the surface of the device and preventive measures need to be applied until complete occluder endothelialization has occurred.
Uncertainty exists about the optimal post-interventional drug regimen as well as treatment duration. Although no randomized clinical trials have been specifically performed to date, various observational studies were conducted and published. Moreover, trends in antithrombotic management have switched from early aggressive treatments following procedures with specific devices such as Boston Scientific Watchman (6 weeks of anticoagulation and aspirin followed by DAPT until 6 months after LAAC) to more conservative approaches, mainly because the vast majority of implants in ‘real life’ are performed in patients with contraindications to oral anticoagulation after serious bleeding events.
In the PLAATO prospective non-randomized study, 64 patients with relative contraindication to OAC were treated with DAPT consisting of clopidogrel 75 mg and ASA 325 mg for 4–6 weeks after LAAC, followed by lifelong ASA therapy. During TEE follow-up, no thrombi were detected on the device at 1 and 6 months (77). The ASAP prospective study confirmed the feasibility of DAPT for 6 months in patients with contraindication for OAC (78). In this study, patients with non-valvular AF and mean CHA2DS2-VASc of 4.4±1.7 were enrolled. Device thrombosis was discovered in 4% of patients at 6 months follow-up, causing stroke in one patient. On the other hand, five patients experienced a bleeding complication translating in an estimated annual bleeding rate of 6.6% (78). On the basis of these findings, the last European Heart Rhythm Association consensus recommends using DAPT for up to 6 months after a LAA closing device implantation in patients with contraindications to OAC (79).
However, as stated above, these observational experiences also reported that antiplatelet therapy is associated with an increased incidence of bleedings, importantly, in a subset of patients already at high bleeding risk. Indeed, aspirin therapy in elderly patients with medically managed AF has been associated with a significant increase in bleeding rate without any benefit in the reduction of thromboembolic complications (80). Moreover, ASA demonstrated similar bleeding rates to patients on apixaban (81). According to these data, short DAPT or use of SAPT after device occlusion appear to be reasonable alternatives after LAAC, particularly in elderly fragile patients where the risk for bleeding exceeds the risk of thromboembolic complications. Weise et al. reported data on 6 weeks short-term DAPT in a large cohort of patient undergoing LAAC using different devices (82). A total of 298 patients (CHA2DS2-VASc 4.3±1.5; HASBLED 3.5±1.0) were included. DAPT was administered for 6 weeks and then decreased to SAPT; the mean follow-up was 2.2 years. At 45±10 days after LAAC, a device related thrombosis was detected in 2.3% of patients on DAPT. Throughout long-term follow-up, therapy regimens consisted of no antithrombotic medication (9%), SAPT (75%) and DAPT (5%). Importantly, early DAPT cessation did not lead to a higher incidence of thromboembolic events; in fact, the observed annual stroke rate was 1.7%, with an overall risk reduction of 78.2% compared with the expected stroke rate of 7.8%. Conversely, the annual major bleeding incidence was 3.9%; given the expected rate of 8.7%, this reflects a reduction of 55.2% in overall bleeding events. Of note, age >75 years and impaired renal function were identified as independent predictors for bleeding events using DAPT after LAAC (82). Finally, a recent observational study reported the results of a French two-centers experience with Amplatzer Cardiac Plug device followed by SAPT for at least 12 months (83). Device thrombosis was observed at 3 months in 6.8% of patients who remained asymptomatic. After a mean follow-up of 13 months, the rates of death, stroke and major bleeding were 2.6%, 4.0% and 1.3%, respectively. Embolic and bleeding events were less frequent than expected from enrolled population CHA2DS2-VASc and HAS-BLED risk scores, suggesting that a SAPT could be safe and effective in high-risk patients receiving LAAC. In conclusion, in patients with contraindications to OAC, a short-term (6 weeks) treatment with DAPT appears to be effective and safe in preventing on-device thrombus. It should be the final goal to eliminate any antithrombotic therapy after LAA closure, but the feasibility of stopping ASA remains to be tested in clinical trials.
Conclusions
The effectiveness of antiplatelet therapy and its optimal regimen before and after percutaneous valvular and structural disease interventions are still a subject of research and debate. Current guidelines recommend the use of DAPT with aspirin and clopidogrel for 3–6 months in patients undergoing TAVI. However, no clear benefit of DAPT has emerged compared with SAPT in observational and randomized trials until now, against a harmful increase in bleeding complications. Given this background, it seems reasonable to propose outright a SAPT strategy except for patients undergoing concomitant coronary interventions and/or with AF. Furthermore, the clinical impact of valve thrombosis and the role of oral anticoagulation, especially of novel oral anticoagulant agents, have to be clarified in “ad hoc” investigations.
Even less evidence is available on antithrombotic therapy in patients undergoing TMVR. The choice and duration of antiplatelet and anticoagulant therapies in this setting are generally left to operators’ experience. The most commonly used strategy includes aspirin for 3–6 months and clopidogrel for 1 month after Mitraclip, even if, also in this field it could be hypothesized that a single antiplatelet agent may be enough post-operatively.
In patients undergoing ASD/PFO closure, it is recommended a post-procedural regimen using a combination of aspirin plus clopidogrel for 1–3 months followed by ASA only for additional 3–5 months. Finally, uncertainty exists about the optimal regimen as well as treatment duration in patients undergoing LAA closure, a truly frail subset of patients because of contraindications to oral anticoagulation after serious bleeding events. In those patients, a short period of DAPT after implantation (1–3 months) is generally administered followed by long-term ASA, when tolerated; otherwise, in those with very high bleeding risk, a single antiplatelet therapy should be reasonable.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017;38:2739-91. [Crossref] [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2017;70:252-89. [Crossref] [PubMed]
- Leon MB, Smith CR, Mack MJ, et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med 2016;374:1609-20. [Crossref] [PubMed]
- Reardon MJ, Van Mieghem NM, Popma JJ, et al. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med 2017;376:1321-31. [Crossref] [PubMed]
- Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011;364:2187-98. [Crossref] [PubMed]
- Rodés-Cabau J, Dumont E, Boone RH, et al. Cerebral embolism following transcatheter aortic valve implantation: comparison of transfemoral and transapical approaches. J Am Coll Cardiol 2011;57:18-28. [Crossref] [PubMed]
- Kahlert P, Knipp SC, Schlamann M, et al. Silent and apparent cerebral ischemia after percutaneous transfemoral aortic valve implantation: a diffusion-weighted magnetic resonance imaging study. Circulation 2010;121:870-8. [Crossref] [PubMed]
- Van Mieghem NM, El Faquir N, Rahhab Z, et al. Incidence and predictors of debris embolizing to the brain during transcatheter aortic valve implantation. JACC Cardiovasc Interv 2015;8:718-24. [Crossref] [PubMed]
- Holmes DR Jr, Brennan JM, Rumsfeld JS, et al. Clinical outcomes at 1 year following transcatheter aortic valve replacement. JAMA 2015;313:1019-28. [Crossref] [PubMed]
- Walther T, Hamm CW, Schuler G, et al. Perioperative Results and Complications in 15,964 Transcatheter Aortic Valve Replacements: Prospective Data From the GARY Registry. J Am Coll Cardiol 2015;65:2173-80. [Crossref] [PubMed]
- Nombela-Franco L, Webb JG, de Jaegere PP, et al. Timing, predictive factors, and prognostic value of cerebrovascular events in a large cohort of patients undergoing transcatheter aortic valve implantation. Circulation 2012;126:3041-53. [Crossref] [PubMed]
- Eggebrecht H, Schmermund A, Voigtländer T, et al. Risk of stroke after transcatheter aortic valve implantation (TAVI): a meta-analysis of 10,037 published patients. EuroIntervention 2012;8:129-38. [Crossref] [PubMed]
- Tay EL, Gurvitch R, Wijesinghe N, et al. A high-risk period for cerebrovascular events exists after transcatheter aortic valve implantation. JACC Cardiovasc Interv 2011;4:1290-7. [Crossref] [PubMed]
- Van Mieghem NM, Schipper ME, Ladich E, et al. Histopathology of embolic debris captured during transcatheter aortic valve replacement. Circulation 2013;127:2194-201. [Crossref] [PubMed]
- Noble S, Asgar A, Cartier R, et al. Anatomo-pathological analysis after CoreValve Revalving system implantation. EuroIntervention 2009;5:78-85. [Crossref] [PubMed]
- Amat-Santos IJ, Rodés-Cabau J, Urena M, et al. Incidence, predictive factors, and prognostic value of new-onset atrial fibrillation following transcatheter aortic valve implantation. J Am Coll Cardiol 2012;59:178-88. [Crossref] [PubMed]
- Fanning JP, Walters DL, Platts DG, et al. Characterization of neurological injury in transcatheter aortic valve implantation: how clear is the picture? Circulation 2014;129:504-15. [Crossref] [PubMed]
- Auffret V, Regueiro A, Del Trigo M, et al. Predictors of Early Cerebrovascular Events in Patients With Aortic Stenosis Undergoing Transcatheter Aortic Valve Replacement. J Am Coll Cardiol 2016;68:673-84. [Crossref] [PubMed]
- Généreux P, Head SJ, Van Mieghem NM, et al. Clinical outcomes after transcatheter aortic valve replacement using valve academic research consortium definitions: a weighted meta-analysis of 3,519 patients from 16 studies. J Am Coll Cardiol 2012;59:2317-26. [Crossref] [PubMed]
- Généreux P, Cohen DJ, Williams MR, et al. Bleeding complications after surgical aortic valve replacement compared with transcatheter aortic valve replacement: insights from the PARTNER I Trial (Placement of Aortic Transcatheter Valve). J Am Coll Cardiol 2014;63:1100-9. [Crossref] [PubMed]
- Gurvitch R, Toggweiler S, Willson AB, et al. Outcomes and complications of transcatheter aortic valve replacement using a balloon expandable valve according to the Valve Academic Research Consortium (VARC) guidelines. EuroIntervention 2011;7:41-8. [Crossref] [PubMed]
- Pilgrim T, Stortecky S, Luterbacher F. Transcatheter aortic valve implantation and bleeding: incidence, predictors and prognosis. J Thromb Thrombolysis 2013;35:456-62. [Crossref] [PubMed]
- Nuis RJ, Rodés-Cabau J, Sinning JM, et al. Blood transfusion and the risk of acute kidney injury after transcatheter aortic valve implantation. Circ Cardiovasc Interv 2012;5:680-8. [Crossref] [PubMed]
- Redfors B, Watson BM, McAndrew T, et al. Mortality, Length of Stay, and Cost Implications of Procedural Bleeding After Percutaneous Interventions Using Large-Bore Catheters. JAMA Cardiol 2017;2:798-802. [Crossref] [PubMed]
- Schymik G, Lefèvre T, Bartorelli AL, et al. European experience with the second-generation Edwards SAPIEN XT transcatheter heart valve in patients with severe aortic stenosis: 1-year outcomes from the SOURCE XT Registry. JACC Cardiovasc Interv 2015;8:657-69. [Crossref] [PubMed]
- Généreux P, Cohen DJ, Mack M, et al. Incidence, predictors, and prognostic impact of late bleeding complications after transcatheter aortic valve replacement. J Am Coll Cardiol 2014;64:2605-15. [Crossref] [PubMed]
- Vincentelli A, Susen S, Le Tourneau T, et al. Acquired von Willebrand syndrome in aortic stenosis. N Engl J Med 2003;349:343-9. [Crossref] [PubMed]
- Franzone A, Pilgrim T, Arnold N, et al. Rates and predictors of hospital readmission after transcatheter aortic valve implantation. Eur Heart J 2017;38:2211-7. [Crossref] [PubMed]
- Rodés-Cabau J, Masson JB, Welsh RC, et al. Aspirin Versus Aspirin Plus Clopidogrel as Antithrombotic Treatment Following Transcatheter Aortic Valve Replacement With a Balloon-Expandable Valve: The ARTE (Aspirin Versus Aspirin + Clopidogrel Following Transcatheter Aortic Valve Implantation) Randomized Clinical Trial. JACC Cardiovasc Interv 2017;10:1357-65. [Crossref] [PubMed]
- Adams DH, Popma JJ, Reardon MJ, et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med 2014;370:1790-8. [Crossref] [PubMed]
- Abdel-Wahab M, Mehilli J, Frerker C, et al. Comparison of balloon-expandable vs self-expandable valves in patients undergoing transcatheter aortic valve replacement: the CHOICE randomized clinical trial. JAMA 2014;311:1503-14. [Crossref] [PubMed]
- Gilard M, Eltchaninoff H, Iung B, et al. Registry of transcatheter aortic-valve implantation in high-risk patients. N Engl J Med 2012;366:1705-15. [Crossref] [PubMed]
- Grube E, Laborde JC, Gerckens U, et al. Percutaneous implantation of the CoreValve self-expanding valve prosthesis in high-risk patients with aortic valve disease: the Siegburg first-in-man study. Circulation 2006;114:1616-24. [Crossref] [PubMed]
- Hioki H, Watanabe Y, Kozuma K, et al. Pre-procedural dual antiplatelet therapy in patients undergoing transcatheter aortic valve implantation increases risk of bleeding. Heart 2017;103:361-7. [Crossref] [PubMed]
- Ussia GP, Scarabelli M, Mulè M, et al. Dual antiplatelet therapy versus aspirin alone in patients undergoing transcatheter aortic valve implantation. Am J Cardiol 2011;108:1772-6. [Crossref] [PubMed]
- Stabile E, Pucciarelli A, Cota L, et al. SAT-TAVI (single antiplatelet therapy for TAVI) study: a pilot randomized study comparing double to single antiplatelet therapy for transcatheter aortic valve implantation. Int J Cardiol 2014;174:624-7. [Crossref] [PubMed]
- Durand E, Blanchard D, Chassaing S, et al. Comparison of two antiplatelet therapy strategies in patients undergoing transcatheter aortic valve implantation. Am J Cardiol 2014;113:355-60. [Crossref] [PubMed]
- Czerwińska-Jelonkiewicz K, Zembala M, Dabrowski M, et al. Can TAVI patients receive aspirin monotherapy as patients after surgical aortic bioprosthesis implantation? Data from the Polish Registry - POL-TAVI. Int J Cardiol 2017;227:305-11. [Crossref] [PubMed]
- Ichibori Y, Mizote I, Maeda K, et al. Clinical Outcomes and Bioprosthetic Valve Function After Transcatheter Aortic Valve Implantation Under Dual Antiplatelet Therapy vs. Aspirin Alone. Circ J 2017;81:397-404. [Crossref] [PubMed]
- Mangieri A, Jabbour RJ, Montalto C, et al. Single-Antiplatelet Therapy in Patients with Contraindication to Dual-Antiplatelet Therapy After Transcatheter Aortic Valve Implantation. Am J Cardiol 2017;119:1088-93. [Crossref] [PubMed]
- D'Ascenzo F, Benedetto U, Bianco M, et al. Which is the best antiaggregant or anticoagulant therapy after TAVI? A propensity-matched analysis from the ITER registry. The management of DAPT after TAVI. EuroIntervention 2017;13:e1392-400. [Crossref] [PubMed]
- Hassell ME, Hildick-Smith D, Durand E, et al. Antiplatelet therapy following transcatheter aortic valve implantation. Heart 2015;101:1118-25. [Crossref] [PubMed]
- Tarantini G, Mojoli M, Urena M, et al. Atrial fibrillation in patients undergoing transcatheter aortic valve implantation: epidemiology, timing, predictors, and outcome. Eur Heart J 2017;38:1285-93. [PubMed]
- Abdul-Jawad Altisent O, Durand E, Muñoz-García AJ, et al. Warfarin and Antiplatelet Therapy Versus Warfarin Alone for Treating Patients With Atrial Fibrillation Undergoing Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv 2016;9:1706-17. [Crossref] [PubMed]
- Seeger J, Gonska B, Rodewald C, et al. Apixaban in Patients With Atrial Fibrillation After Transfemoral Aortic Valve Replacement. JACC Cardiovasc Interv 2017;10:66-74. [Crossref] [PubMed]
- Chakravarty T, Søndergaard L, Friedman J, et al. Subclinical leaflet thrombosis in surgical and transcatheter bioprosthetic aortic valves: an observational study. Lancet 2017;389:2383-92. [Crossref] [PubMed]
- Sondergaard L, De Backer O, Kofoed KF, et al. Natural history of subclinical leaflet thrombosis affecting motion in bioprosthetic aortic valves. Eur Heart J 2017;38:2201-7. [Crossref] [PubMed]
- Del Trigo M, Muñoz-Garcia AJ, Wijeysundera HC, et al. Incidence, Timing, and Predictors of Valve Hemodynamic Deterioration After Transcatheter Aortic Valve Replacement: Multicenter Registry. J Am Coll Cardiol 2016;67:644-55. [Crossref] [PubMed]
- Feldman T, Foster E, Glower DD, et al. Percutaneous repair or surgery for mitral regurgitation. N Engl J Med 2011;364:1395-406. [Crossref] [PubMed]
- Maisano F, La Canna G, Colombo A, et al. The evolution from surgery to percutaneous mitral valve interventions: the role of the edge-to-edge technique. J Am Coll Cardiol 2011;58:2174-82. [Crossref] [PubMed]
- O'Gara PT, Calhoon JH, Moon MR, et al. Transcatheter therapies for mitral regurgitation: a professional society overview from the American College of Cardiology, the American Association for Thoracic Surgery, Society for Cardiovascular Angiography and Interventions Foundation, and the Society of Thoracic Surgeons. J Am Coll Cardiol 2014;63:840-52. [Crossref] [PubMed]
- Glower DD, Kar S, Trento A, et al. Percutaneous mitral valve repair for mitral regurgitation in high-risk patients: results of the EVEREST II study. J Am Coll Cardiol 2014;64:172-81. [Crossref] [PubMed]
- Maisano F, Franzen O, Baldus S, et al. Percutaneous mitral valve interventions in the real world: early and 1-year results from the ACCESS-EU, a prospective, multicenter, nonrandomized post-approval study of the MitraClip therapy in Europe. J Am Coll Cardiol 2013;62:1052-61. [Crossref] [PubMed]
- Attizzani GF, Ohno Y, Capodanno D, et al. Extended use of percutaneous edge-to-edge mitral valve repair beyond EVEREST (Endovascular Valve Edge-to-Edge Repair) criteria: 30- day and 12-month clinical and echocardiographic outcomes from the GRASP (Getting Reduction of Mitral Insufficiency by Percutaneous Clip Implantation) registry. JACC Cardiovasc Interv 2015;8:74-82. [Crossref] [PubMed]
- Lim DS, Reynolds MR, Feldman T, et al. Improved functional status and quality of life in prohibitive surgical risk patients with degenerative mitral regurgitation after transcatheter mitral valve repair. J Am Coll Cardiol 2014;64:182-92. [Crossref] [PubMed]
- Bekeredjian R, Mereles D, Pleger S, et al. Large atrial thrombus formation after MitraClip implantation: is anticoagulation mandatory? J Heart Valve Dis 2011;20:146-8. [PubMed]
- Hamm K, Barth S, Diegeler A, et al. Stroke and thrombus formation appending to the MitraClip: what is the appropriate anticoagulation regimen? J Heart Valve Dis 2013;22:713-5. [PubMed]
- Guyatt GH, Akl EA, Crowther M, et al. American College of Chest Physicians. Introduction to the ninth edition: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2012;141:48S-52S.
- Feldman T, Wasserman HS, Herrmann HC, et al. Percutaneous mitral valve repair using the edge-to-edge technique: six- month results of the EVEREST phase I clinical trial. J Am Coll Cardiol 2005;46:2134-40. [Crossref] [PubMed]
- Mauri L, Garg P, Massaro JM, et al. The EVEREST II trial: design and rationale for a randomized study of the evalve mitraclip system compared with mitral valve surgery for mitral regurgitation. Am Heart J 2010;160:23-9. [Crossref] [PubMed]
- Whitlow PL, Feldman T, Pedersen WR, et al. Acute and 12-month results with catheter-based mitral valve leaflet repair. The EVEREST II (Endovascular Valve Edge-to-Edge Repair) high risk study. J Am Coll Cardiol 2012;59:130-9. [Crossref] [PubMed]
- Schade A, Kerber S, Hamm K. Two in a single procedure: Combined approach for MitraClip implantation and left atrial appendage occlusion using the watchman device. J Invasive Cardiol 2014;26:E32-4. [PubMed]
- Harper RW, Mottram PM, McGaw DJ. Closure of secundum atrial septal defects with the Amplatzer septal occluder device: techniques and problems. Catheter Cardiovasc Interv 2002;57:508-24. [Crossref] [PubMed]
- Windecker S, Wahl A, Chatterjee T, et al. Percutaneous closure of patent foramen ovale in pa- tients with paradoxical embolism: long-term risk of recurrent thromboembolic events. Circulation 2000;101:893-8. [Crossref] [PubMed]
- ESC Guidelines for the management of grown-up congenital heart disease: The Task Force on the Management of Grown-up Congenital Heart Disease of the European Society of Cardiology (ESC). Eur Heart J 2010;31:2915-57. [Crossref]
- Braun M, Gliech V, Boscheri A, et al. Transcatheter closure of patent foramen ovale (PFO) in pa-tients with paradoxical embolism. Periprocedural safety and mid- term follow-up results of three different device occluder systems. Eur Heart J 2004;25:424-30. [Crossref] [PubMed]
- Brandt RR, Neumann T, Neuzner J, et al. Transcatheter closure of atrial septal defect and patent foramen ovale in adult patients using the Amplatzer occlusion device: no evidence for thrombus deposition with antiplatelet agents. J Am Soc Echocardiogr 2002;15:1094-8. [Crossref] [PubMed]
- Rickers C, Hamm C, Stern H, et al. Percutaneous closure of secundum atrial septal defect with a new self centering device ("angel wings"). Heart 1998;80:517-21. [Crossref] [PubMed]
- Krumsdorf U, Ostermayer S, Billinger K, et al. Incidence and clinical course of thrombus forma- tion on atrial septal defect and patient foramen ovale closure de- vices in 1,000 consecutive patients. J Am Coll Cardiol 2004;43:302-9. [Crossref] [PubMed]
- Kuhn MA, Latson LA, Cheatham JP, et al. Biological response to Bard Clamshell Septal Occluders in the canine heart. Circulation 1996;93:1459-63. [Crossref] [PubMed]
- Polzin A, Dannenberg L, Sophia Popp V, et al. Antiplatelet effects of clopidogrel and aspirin after interventional patent foramen ovale/atrium septum defect closure. Platelets 2016;27:317-21. [Crossref] [PubMed]
- Søndergaard L, Kasner SE, Rhodes JF, et al. Patent Foramen Ovale Closure or Antiplatelet Therapy for Cryptogenic Stroke. N Engl J Med 2017;377:1033-42. [Crossref] [PubMed]
- Saver JL, Carroll JD, Thaler DE, et al. Long-Term Outcomes of Patent Foramen Ovale Closure or Medical Therapy after Stroke. N Engl J Med 2017;377:1022-32. [Crossref] [PubMed]
- Mas JL, Derumeaux G, Guillon B, et al. Patent Foramen Ovale Closure or Anticoagulation vs. Antiplatelets after Stroke. N Engl J Med 2017;377:1011-21. [Crossref] [PubMed]
- Sherman JM, Hagler DJ, Cetta F. Thrombosis after septal closure device placement: A review of the current literature. Catheter Cardiovasc Interv 2004;63:486-9. [Crossref] [PubMed]
- Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016;37:2893-962. [Crossref] [PubMed]
- Block PC, Burstein S, Casale PN, et al. Percutaneous left atrial appendage occlusion for patients in atrial fibrillation suboptimal for warfarin therapy: 5-year results of the PLAATO (Percutaneous Left Atrial Appendage Transcatheter Occlusion) Study. JACC Cardiovasc Interv 2009;2:594-600. [Crossref] [PubMed]
- Reddy VY, Möbius-Winkler S, Miller MA, et al. Left atrial appendage closure with the Watchman device in patients with a contraindication for oral anticoagulation: the ASAP study (ASA Plavix Feasibility Study With Watchman Left Atrial Appendage Closure Technology). J Am Coll Cardiol 2013;61:2551-6. [Crossref] [PubMed]
- Pison L, Potpara TS, Chen J, et al. Left atrial appendage closure-indications, techniques, and outcomes: results of the EuropeanHeart Rhythm Association Survey. Europace 2015;17:642-6. [Crossref] [PubMed]
- Mant J, Hobbs FD, Fletcher K, et al. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trial. Lancet 2007;370:493-503. [Crossref] [PubMed]
- Connolly SJ, Eikelboom J, Joyner C, et al. Apixaban in patients with atrial fibrillation. N Engl J Med 2011;364:806-17. [Crossref] [PubMed]
- Weise FK, Bordignon S, Perrotta L, et al. Short-term dual antiplatelet therapy after interventional left atrial appendage closure with different devices. EuroIntervention 2018;13:e2138-46. [Crossref] [PubMed]
- Jalal Z, Dinet ML, Combes N, et al. Percutaneous left atrial appendage closure followed by single antiplatelet therapy: Short- and mid-term outcomes. Arch Cardiovasc Dis 2017;110:242-9. [Crossref] [PubMed]


