
Percutaneous transapical closure of paravalvular leak in bioprosthetic mitral valve without radio-opaque indicators
Introduction
Paravalvular leakage (PVL) after mitral valve replacement surgery is an uncommon but serious complication. Although conventionally surgical repair or replacement has been accepted as a gold standard approach, there is a substantially higher morbidity and mortality rate with reoperation. Since its introduction in 1992, transcatheter techniques have proven to be a safe alternative to surgery with reasonable procedural and clinical success rates, respectively 63–100% and 54–100% (1,2). In some cases, this technique can be quite challenging. In this case report, we wanted to share our experience of percutaneous transapical closure of paravalvular leak in mitral valve bioprosthesis without radio-opaque indicators.
Case presentation
A 76-year-old male was admitted to our department with severe dyspnea (NHYA classes III) and haemolytic anaemia (Hb: 8.4 g/dL, Hct: 24.9%, reticulocyte: 3.8%, LDH: 1,635 U/L) 1 year after coronary artery bypass grafting (LIMA-LAD and Ao-Diagonal SVG) and bioprosthetic mitral valve replacement operation. On physical examination, heart rate is 76/min, and blood pressure is 135/76 mm and an apical 3/6 holosystolic murmur was heard. Two-dimensional echocardiography revealed a severe mitral PVL and systolic pulmonary artery pressure of 55 mmHg. 3D transesophageal echocardiography (TEE) revealed two crescent-shaped paravalvular leaks (PVL) in the posterolateral portion of the mitral valve prosthesis according to the aorta close to the septal side of the prosthetic valve (4 and 5 o’clock) and another small PVL at proximal side (2 o’clock) (Figure 1A). No active endocarditis was present.
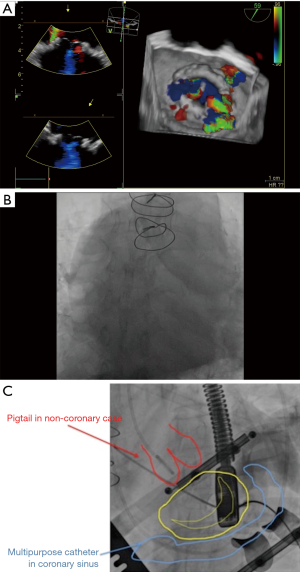
After discussion with the surgical team, re-do surgical option was deemed too risky in view of a high predicted operative mortality (Euroscore II 12%), and the patient was referred to percutaneous PVL closure. The transseptal approach was deemed to be difficult due to the unfavorable location of the PVLs and a decision was made to perform a transapical closure of the mitral PVL. Under general anesthesia, a left mini-thoracotomy at the fifth intercostal space was performed with apical cardiac exposure. After placing pledgets in the standard fashion, the apex was punctured under direct vision with a needle, and a 6-French sheath was placed and fixed. The procedure was performed with 3D-TEE and fluoroscopic guidance. However, mitral bioprosthesis had complete absence of any radio-opaque landmarks that made the procedure extremely challenging (Figure 1B). To describe the mitral valve contours fluoroscopically; a 5 F multipurpose catheter was placed into the coronary sinus, pointing around the mitral valve, via right jugular vein; and a 6F pigtail catheter was placed in the aortic root, pointing to the aortic valve at 12 o’clock, via the femoral artery (Figure 1C).
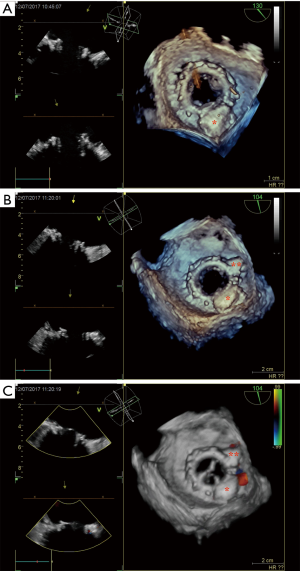
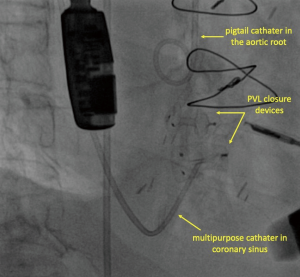
Through the 6-French sheaths, a 0.035 Terumo floppy Glidewire (Terumo Medical Corp, Somerset, NJ) was loaded on a right Judkins 6F catheter and passed through the paravalvular leak localized at 5 o’clock under fluoroscopy and TEE guidance. After the positioning of the catheter inside the left atrium, the Terumo wire was replaced by an Amplatzer stiff wire. Later, a delivery catheter replaced. Through the delivery catheter, a 10×4 mm2 rectangular Waist Occlutech® paravalvular leak device (Occlutech Holding, Switzerland) was successfully deployed across the defect (Figure 2A). However, there still was a mild to moderate leak present. Using the same way and technique, the paravalvular leak localized at 4 o'clock was passed this time and a second 4×4 mm2 square waist Occlutech® paravalvular leak device (Occlutech Holding, Switzerland) was deployed across the other defect (Figure 2B). 2D and 3D TEE showed appropriate position of the both devices, normally functioning bio-prosthesis with normal inflow gradients without leaflet impingement by the closure devices and greatly reduced mitral PVL (Figure 2C). As the point of how these accessory catheters provided guidance, Figure 3 demonstrate the relationship between the aortic root/coronary sinus catheters and the PVL occlusion devices in place. After the removal of the sheath from the left ventricle, LV apex was closed with purse-string sutures. Total procedure time was 115 minutes and fluoroscopic time was 28 minutes. The patient was hemodynamically stable throughout the deployment sequences and no peri-procedural complications were encountered.
Discussion
Classically, transseptal approach is advantageous with the use of a steerable guiding catheter (i.e., Agilis), which simplifies wiring the defect, even if the PVL is medial. However, as stated in the literature, the transapical approach is especially preferred in PVLs with unfavorable anatomy, when transseptal attempt failed, and even when the defect is medial (close to the septum). Because, the transapical approach provides direct access to the mitral valve, allows easy access to defects, particularly close to septum, allows the passage of larger catheters without traversing the aorta or aortic valve, and thereby, reduces procedure times and increases the likelihood of successful mitral PVL closure with less radiation exposure (3). However, sometimes this procedure can be challenging. To guide and facilitate the percutaneous transcatheter closure of PVL in invisible mitral valve bioprosthesis, some radio-opaque indicators might be placed in sinus and aortic non-coronary cusp to describe the mitral valve contours fluoroscopically (of course, besides 3D TEE guidance) (4). To the best of our knowledge, only 2 cases describing the percutaneous closure of the PVL in patients with no radio-opaque mitral bioprosthesis have been reported in the literature until now (5,6).
Although a considerable success rate has been gained with the increasing experience in recent years, the absence of a completely suitable device already manufactured for PVL closure inevitably reduces the success rate. Dedicated rectangular and/or square devices have more favorable waist to disk ratio, which prevents device embolization and has a lower profile, which does not interfere with prosthetic valve function. For this reason, we have used 1 rectangular and 1 square PVL devices with a great success rate.
In conclusion, we believe that our approach in this case can give an inspiration to assist the percutaneous transcatheter closure of PVL of fluoroscopically invisible mitral valve bioprosthesis.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Ruiz CE, Jelnin V, Kronzon I, et al. Clinical outcomes in patients undergoing percutaneous closure of periprosthetic paravalvular leaks. J Am Coll Cardiol 2011;58:2210-7. [Crossref] [PubMed]
- Kim MS, Casserly IP, Garcia JA, et al. Percutaneous transcatheter closure of prosthetic mitral paravalvular leaks: are we there yet? JACC Cardiovasc Interv 2009;2:81-90. [Crossref] [PubMed]
- Fernando R, Briceno DF, Loyalka P, et al. Transapical closure of mitral paravalvular leak over a surgically constructed mitral annulus. Int J Cardiol 2014;171:302-4. [Crossref] [PubMed]
- Kapadia SR, Mentias A, Barakat AF, et al. Relationship of mitral valve annulus plane and circumflex-right coronary artery plane: Implications for Transcatheter Mitral Valve Implantation. Catheter Cardiovasc Interv 2017;89:932-43. [Crossref] [PubMed]
- Cruz-Gonzalez I, Rama-Merchan JC, Rodríguez-Collado J, et al. Percutaneous Paravalvular Leak Closure in "Invisible" Mitral Valve Bioprosthesis Without Radio-Opaque Indicators. Can J Cardiol 2015;31:1205.e7-8. [Crossref] [PubMed]
- Rossi ML, Barbaro C, Pagnotta P, et al. Transapical transcatheter valve-in-valve replacement for deteriorated mitral valve bioprosthesis without radio-opaque indicators: the "invisible" mitral valve bioprosthesis. Heart Lung Circ 2015;24:e19-22. [Crossref] [PubMed]


