Left ventricular noncompaction cardiomyopathy
Background
The diagnosis of adults with Left Ventricular Noncompaction (LVNC) has been reported with increasing frequency in the literature. The diagnosis of this disorder depends largely on findings obtained in advanced cardiac imaging and further requires special considerations when treatment is pursued. A systematic review of the literature follows describing the rationale to management.
Introduction
LVNC, also known as spongy myocardium, is a distinct form of cardiomyopathy occurring in-utero when segments of spongy myocardium fail to transform into compact, mature musculature resulting in prominent myocardial trabeculae, deep intra-trabecular recesses, and decreased cardiac function (1). It has been proposed that LVNC results from arrest of the compaction process during the second month of embryological development. Prior to the development of the coronary arteries, there are prominent myocardial trabeculations, termed sinusoids, in the myocardium that allow for increased surface area for the diffusion of oxygen. After the coronary vasculature develops the sinusoids disappear and a transformation of the spongy myocardium into compact musculature typically takes place. For unknown reasons, in persons with LVNC this transition does not take place leading to the development of a thickened, non-compacted endomyocardial layer with prominent myocardial trabeculations that are continuous with the LV cavity without communication with the epicardial circulation, and a thin compacted epicardial layer (2). In this regard, LVNC differs from other patterns of “persisting sinusoids” often seen in association with congenital heart diseases, such as pulmonary atresia and its variants, in which the cardiac chamber communicates with the epicardial circulation (2,3). The changes associated with LVNC are most pronounced in the apex and lateral walls of the heart and occur in the absence of any coexisting congenital lesion (3-6).
Epidemiology
LVNC was initially described by Chin and colleagues in 1990 in a pediatric series, but the largest series’ to date involve adult patients (7-11). The true prevalence and incidence of adult LVNC among the general population is not known, and unlike in pediatric populations occurs more sporadically with less familial occurrences, 12-18%. LVNC in the pediatric populations can frequently co-exist with anatomical abnormalities although relationships and characterized syndromes are not identified (12-14).
Clinical presentation
The clinical presentations of LVNC include heart failure, arrhythmias, and propensity for thromboembolism (21-24%). Heart failure remains the most common presentation with patients often developing progressive symptoms of exertional dyspnea, orthopnea and lower extremity edema. Furthermore, patients with LVNC are particularly at increased risk of developing cardiac arrhythmias, both atrial (5-29%) and ventricular (18-47%) (8,15,16). Additionally, the myocardial trabeculations in LVNC predispose the formation of ventricular thrombi and arrhythmias (Figure 1). The areas of noncompaction often display wall motion abnormalities on imaging. Additionally, abnormal vasodynamic responses within the native vessels are believed to lead to progressive micro-ischemia and progressive dilatation of the ventricle (2,13,16).
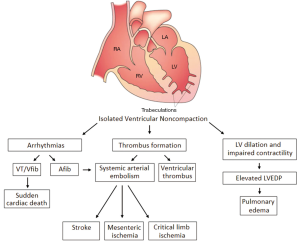
The increased risk for thromboembolism is believed to be multi-factorial; and is due to the combination of impaired LV function, perhaps simultaneous occurrence of atrial fibrillation, and the presence of abnormal myocardial trabeculae. Systemic arterial emboli occur with the greatest frequency, whereas pulmonary embolism and isolated RV thrombi (6-9%) are less likely. LVNC associated paradoxical emboli have never been described (7-9,15,18).
Diagnostic cardiovascular imaging
The diagnosis of LVNC may be pursued once the more common etiologies for heart failure are excluded. The work up for new onset LV dysfunction is traditionally achieved upon further evaluation by echocardiography, cardiac catheterization, and cardiac MRI (CMR). Echocardiography often provides the first clue to diagnose LVNC and is used with other modalities in concert including contrast ventriculography, Cardiac Computed Tomography and CMR. Imaging features typically include LV systolic dysfunction with depressed ejection <40% fraction and wall motion abnormalities involving the regions of noncompaction.
Echocardiography criteria
LVNC on echocardiogram typically is characterized as a two-layered myocardial structure with a thin, compacted outer (epicardial) band and a much thicker, non-compacted inner (endomyocardial) layer and deep myocardial trabeculae, particularly in the apex and free wall of the left ventricle (Figure 2) (11). Color Doppler imaging demonstrates at least 3 deep intra-trabecular recesses that communicate with the ventricular cavity (15,19). Finally, the 2-layered structure of the myocardium will have an increased noncompacted (NC) endomyocardial layer depth compared to the compacted (C) epicardial layer. A NC/C ration >2.0 in adults when measured in systole is considered diagnostic for LVNC (9). Three-dimensional echocardiography may play a role in the future however its role currently remains anecdotal (20).
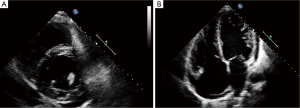
LVNC is often difficult to differentiate from severe forms of left ventricular hypertrophy and hypertrophic or dilated cardiomyopathy (HCM, DCM). However, in hearts with prominent myocardial trabeculae from other causes, the thickness ratios between trabeculated and normal myocardium do not reach a ratio >2. In addition the trabeculated regions associated with LVNC tend to be segmental rather than diffuse as might occur in LVH. The cardiac segments (Table 1), most affected in LVNC are the LV cavity involving the apex, lateral, inferior wall (91-100%), mid-cavity (78%) while the basal segments were least affected (21%) (7-11,15,18,21). The differences in segmental prevalence suggest an interruption in normal progression of myocardial compaction from the basal-septal to the apical-lateral segments during early development.

Full Table
Cardiac MRI criteria
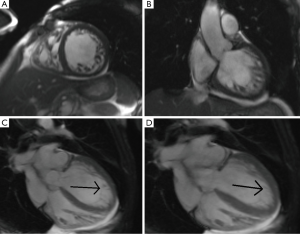
CMR is particularly useful in patients in whom the apex is difficult to visualize with echocardiography. CMR provides greater spatial resolution by imaging in all planes. The specifications for CMR are outlined by the American Heart Association. Their recommendations include a minimum magnet strength of 1.5 Tesla in order to provide visualization of both long and short axis views in approximately 17 segments (22,23). To diagnose LVNC on CMR it is recommended to take three diastolic long axis views and choose the myocardial segment with the most prominent myocardial trabeculations to measure the NC/C ratio (22,24) (Figure 3). In contrast to echocardiography, the NC/C ratio on CMR is higher, NC/C >2.3, and the measurements are taken at the end of diastole (Table 2) (25). The diagnostic value from CMR is highly sensitive (86%) and specific (99%) for the diagnosis of LVNC. Like color doppler echocardiography, CMR black blood imaging may detect increased signal intensity, an indication of stagnant blood flow, within the myocardial trabeculae and therefore support the diagnosis of LVNC (26-28). An unexplored area of investigation is whether determining the degree of noncompaction, as defined by CMR, offers added prognostic and therapeutic insights. CMR faces some barriers for longitudinal use in prognostication, namely the contraindication after implanted devices.

Full Table
Genetic basis
A substantial proportion of LVNC has a defined genetic basis. The earliest reports are with mutations in genes encoding taffazin, LIM domain binding protein 3/ZASP (LDB3), and alpha-dystrobrevin (29). Mutations have been reported in three genes; β-myosin heavy chain (MYH7), α-cardiac actin (ACTC), and cardiac troponin T (TNNT2). MYH7 and ACTC are associated with familial occurrence but sporadic mutations of MYH7 as well as TNNT2 have also been identified in LVNC (30). Individuals carrying identical sarcomeric gene mutations display a phenotypic spectrum that may span from those with early-onset lethal disease, to those without clinical symptoms. Variations in expressivity of these genes may give insight into differences in disease severity as well finding a common link for LVNC, HCM, and DCM (31).
Controversies
Recently the current diagnostic criteria for LVNC have been revisited. Several studies have re-examined the echocardiography and CMR based diagnostic criteria of LVNC (32,33). The current echocardiographic classification maybe affected by subjectivity due to user inter-variability and may contribute to inappropriate diagnosis. In a cross-sectional analysis, the echocardiography definitions for LVNC were seen to correlate poorly with only 29.8% having clinical disease. Likewise, NC/C ratio >2.3 were seen in 43% patients in one study without cardiac disease or hypertension. On the other hand however, slightly higher NC/C ratios were present in patients with elevated LVEDV (left ventricular end-diastolic volume) which could represent subclinical cardiomyopathy. Future considerations may call for revisions to the current guidelines. Incorporating new CMR approaches, such as automated segmentation of the myocardial trabeculae, may provide promise and perhaps be applied to the diagnosis of LVNC (34,35).
Clinical course and management
The largest series to date for adults with LVNC are small but include the most information pertaining to the natural history of disease (7-11,15). The clinical course of LVNC is variable but reported mortality from case series is high ranging from 35-47% over a 42- to 72-month follow-up period from diagnosis (8,15). Those affected succumb to end-stage heart failure, sudden cardiac death from arrhythmias, and complications associated with thromboembolism.
The current recommendations for treatment follow the international guidelines of heart failure management. Treatment usually requires the combination of beta-blockers, ACE inhibitors/ARB, diuretics and aldosterone antagonists. Implanted automated defibrillators are recommended in those with EF<35% or those with previously life threatening arrhythmias (3-6). Furthermore, lifelong systemic anticoagulation is indicated to obviate risk for thromboembolism. Lastly, 12% of patients with LVNC may go on to develop end stage heart failure and require orthotopic heart transplantation (5,8,9,15,36).
Conclusions
LVNC is a rare cardiomyopathy leading to progressive heart failure, arrhythmias, and thromboembolic events. The myocardial apical-lateral segments are affected more commonly whereas basal-septal segments are least affected. This suggests that an interruption in the normal progression of myocardial compaction from the basal-septal to the apical-lateral segments during embryologic development.
The cardiovascular imaging criteria reported from clinical series can be applied in making the diagnosis. Treatment strategies usually require combination guideline driven treatment for heart failure, recurrent arrhythmia as well as the risk for thromboembolism.
The genetic basis for LVNC is well characterized and genetic testing may be offered. The spectrum of phenotypic variations in LVNC offers exciting insights regarding the pathophysiology of various cardiomyopathies. Genetic counseling can further provide family members with the risk for developing inherited LVNC as well as understanding the condition and management for future family planning. LVNC may be difficult to distinguish from other cardiac conditions characterized by prominent myocardial trabeculae including LVH, HCM, and DCM.
Recently, the current classification guidelines for LVNC have been brought into question. Over diagnosis is one of the cited criticisms under the current LVNC classification. In recent studies surveying both the accuracy of echocardiography and CMR, weaker correlations were demonstrated with the current NC/C ratios for the diagnosis of LVNC. Although, LVNC is a clinically well described entity, a reevaluation of the diagnostic imaging criteria is currently under investigation.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Reynen K, Bachmann K, Singer H. Spongy myocardium. Cardiology 1997;88:601-2. [PubMed]
- Borges AC, Kivelitz D, Baumann G. Isolated left ventricular non-compaction: cardiomyopathy with homogeneous transmural and heterogeneous segmental perfusion. Heart 2003;89:e21. [PubMed]
- Weiford BC, Subbarao VD, Mulhern KM. Noncompaction of the ventricular myocardium. Circulation 2004;109:2965-71. [PubMed]
- Rosa LV, Salemi VM, Alexandre LM, et al. Noncompaction cardiomyopathy: a current view. Arq Bras Cardiol 2011;97:e13-9. [PubMed]
- Roberts WC, Karia SJ, Ko JM, et al. Examination of isolated ventricular noncompaction (hypertrabeculation) as a distinct entity in adults. Am J Cardiol 2011;108:747-52. [PubMed]
- Murphy RT, Thaman R, Blanes JG, et al. Natural history and familial characteristics of isolated left ventricular non-compaction. Eur Heart J 2005;26:187-92. [PubMed]
- Stöllberger C, Finsterer J, Blazek G. Left ventricular hypertrabeculation/noncompaction and association with additional cardiac abnormalities and neuromuscular disorders. Am J Cardiol 2002;90:899-902. [PubMed]
- Oechslin EN, Attenhofer Jost CH, Rojas JR, et al. Long-term follow-up of 34 adults with isolated left ventricular noncompaction: a distinct cardiomyopathy with poor prognosis. J Am Coll Cardiol 2000;36:493-500. [PubMed]
- Jenni R, Oechslin E, Schneider J, et al. Echocardiographic and pathoanatomical characteristics of isolated left ventricular non-compaction: a step towards classification as a distinct cardiomyopathy. Heart 2001;86:666-71. [PubMed]
- Ichida F, Hamamichi Y, Miyawaki T, et al. Clinical features of isolated noncompaction of the ventricular myocardium: long-term clinical course, hemodynamic properties, and genetic background. J Am Coll Cardiol 1999;34:233-40. [PubMed]
- Chin TK, Perloff JK, Williams RG, et al. Isolated noncompaction of left ventricular myocardium. A study of eight cases. Circulation 1990;82:507-13. [PubMed]
- Yiginer O, Uz O, Kardesoglu E, et al. Noncompaction of the myocardium coexistent with vertebral hemangiomas. Tex Heart Inst J 2011;38:212-3. [PubMed]
- Gomathi SB, Makadia N, Ajit SM. An unusual case of isolated non-compacted right ventricular myocardium. Eur J Echocardiogr 2008;9:424-5. [PubMed]
- Errando CL, Tatay J, Serrano-Romero A, et al. Splenic rupture and haemoperitoneum in a patient with non-compaction of the left ventricular myocardium. Br J Anaesth 2005;95:358-61. [PubMed]
- Ritter M, Oechslin E, Sütsch G, et al. Isolated noncompaction of the myocardium in adults. Mayo Clin Proc 1997;72:26-31. [PubMed]
- Kato Y, Horigome H, Takahashi-Igari M, et al. Isolation of pulmonary vein and superior vena cava for paroxysmal atrial fibrillation in a young adult with left ventricular non-compaction. Europace 2010;12:1040-1. [PubMed]
- Towbin JA, Bowles NE. The failing heart. Nature 2002;415:227-33. [PubMed]
- Bax JJ, Lamb HJ, Poldermans D, et al. Non-compaction cardiomyopathy-echocardiographic diagnosis. Eur J Echocardiogr 2002;3:301-2. [PubMed]
- Koo BK, Choi D, Ha JW, et al. Isolated noncompaction of the ventricular myocardium: contrast echocardiographic findings and review of the literature. Echocardiography 2002;19:153-6. [PubMed]
- Gopalamurugan AB, Kapetanakis S, Monaghan MJ, et al. Images in cardiology: Left ventricular non-compaction diagnosed by real time three dimensional echocardiography. Heart 2005;91:1274. [PubMed]
- Boyd MT, Seward JB, Tajik AJ, et al. Frequency and location of prominent left ventricular trabeculations at autopsy in 474 normal human hearts: implications for evaluation of mural thrombi by two-dimensional echocardiography. J Am Coll Cardiol 1987;9:323-6. [PubMed]
- Petersen SE, Selvanayagam JB, Wiesmann F, et al. Left ventricular non-compaction: insights from cardiovascular magnetic resonance imaging. J Am Coll Cardiol 2005;46:101-5. [PubMed]
- Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 2002;105:539-42. [PubMed]
- Daimon Y, Watanabe S, Takeda S, et al. Two-layered appearance of noncompaction of the ventricular myocardium on magnetic resonance imaging. Circ J 2002;66:619-21. [PubMed]
- Weiss F, Habermann CR, Lilje C, et al. MRI in the diagnosis of non-compacted ventricular myocardium (NCVM) compared to echocardiography. Rofo 2003;175:1214-9. [PubMed]
- Moon JC, Fisher NG, McKenna WJ, et al. Detection of apical hypertrophic cardiomyopathy by cardiovascular magnetic resonance in patients with non-diagnostic echocardiography. Heart 2004;90:645-9. [PubMed]
- Dodd JD, Holmvang G, Hoffmann U, et al. Quantification of left ventricular noncompaction and trabecular delayed hyperenhancement with cardiac MRI: correlation with clinical severity. AJR Am J Roentgenol 2007;189:974-80. [PubMed]
- Amir O, Delgado RM 3rd, Kar B, et al. The value of cardiac magnetic resonance imaging in the diagnosis of isolated non-compaction of the left ventricle. Isr Med Assoc J 2009;11:313-4. [PubMed]
- Vatta M, Mohapatra B, Jimenez S, et al. Mutations in Cypher/ZASP in patients with dilated cardiomyopathy and left ventricular non-compaction. J Am Coll Cardiol 2003;42:2014-27. [PubMed]
- Klaassen S, Probst S, Oechslin E, et al. Mutations in sarcomere protein genes in left ventricular noncompaction. Circulation 2008;117:2893-901. [PubMed]
- Dellefave L, McNally EM. Sarcomere mutations in cardiomyopathy, noncompaction, and the developing heart. Circulation 2008;117:2847-9. [PubMed]
- Kohli SK, Pantazis AA, Shah JS, et al. Diagnosis of left-ventricular non-compaction in patients with left-ventricular systolic dysfunction: time for a reappraisal of diagnostic criteria? Eur Heart J 2008;29:89-95. [PubMed]
- Kawel N, Nacif M, Arai AE, et al. Trabeculated (noncompacted) and compact myocardium in adults: the multi-ethnic study of atherosclerosis. Circ Cardiovasc Imaging 2012;5:357-66. [PubMed]
- Freling HG, van Wijk K, Jaspers K, et al. Impact of right ventricular endocardial trabeculae on volumes and function assessed by CMR in patients with tetralogy of Fallot. Int J Cardiovasc Imaging 2013;29:625-31. [PubMed]
- Jaspers K, Freling HG, van Wijk K, et al. Improving the reproducibility of MR-derived left ventricular volume and function measurements with a semi-automatic threshold-based segmentation algorithm. Int J Cardiovasc Imaging 2013;29:617-23. [PubMed]
- Fan KY, Chan CW, Cheng LC, et al. Isolated left ventricular non-compaction: an unusual indication for heart transplantation. Hong Kong Med J 2009;15:378-80. [PubMed]


