Monitoring platelet function: what have we learned from randomized clinical trials?
Introduction
Oral antiplatelet therapy including aspirin and P2Y12 inhibitors are widely used with proven benefit for the prevention of recurrent ischemic events after acute coronary syndrome (ACS) and percutaneous coronary intervention (PCI) for stable angina (1,2). Current guidelines recommend the use of dual antiplatelet therapy (DAPT) since Clopidogrel in Unstable Angina to Prevent Recurrent Events (CURE) results in 2001 (3-5). Clopidogrel has been considered as the gold standard therapy (1,2) before newer P2Y12 blockers (i.e., prasugrel and ticagrelor) demonstrated their clinical benefit in large randomized controlled trials (6,7). Both drugs have proved significant reduction of ischemic events after ACS and an increased hazard of bleeding. Those three antiplatelet agents act as platelet aggregation inhibitor by targeting the P2Y12 platelet receptor. Adenosine diphosphate (ADP)—mediated activation of platelet P2Y12 receptor represents a critical pathway, that results in arterial thrombosis and leads to tissue anoxia and inflammatory response. Binding of ADP to P2Y12 receptor amplifies platelet activation and aggregation and also increases granule secretion and platelet procoagulant activity (8,9). Therefore, the potency of P2Y12 inhibitor biological effect can be evaluated by platelet function testing (PFT) (10,11).
Variability of response to antiplatelet drugs: the emergence of platelet testing
Following ACS, in spite of DAPT with aspirin and clopidogrel, up to 15% of patients experienced recurrent ischemic events (1,2). Low response to clopidogrel has been proposed as one of the responsible factors. Indeed, many biological studies showed a broad interindividual variability of clopidogrel response assessed by PFT (12-14). Clopidogrel is an inactive prodrug that is converted into its active form by several cytochrome P450 enzymes (CYP). Variations in CYP activity contribute to insufficient active metabolite generation, leading to resistance. Common loss of function polymorphisms of CYP2C19 have been associated with biological reduction of drug potency and with worse outcomes (15). Carriers of the CYP2C19*2 allele have a 2.4 times higher cardiovascular event rate compared with non-carriers (16,17). On the other hand, carriers of CYP2C19*17 allele have excessive platelet inhibition on thienopyridines and develop more often bleeding complications (18-22). Additionally, various clinical and environmental factors can influence clopidogrel metabolism and modulate its biological effect, including diabetes, age, smoking, weight and drug interactions (23,24). This highly variable metabolism explain the high rates of high on treatment platelet reactivity (HTPR) (up to 40%) observed on clopidogrel treated patients after an ACS (12-15).
A consensus document defined HTPR according to platelet function assessment (25). Several PFT are available and have been evaluated (25). Nowadays, the most widely used assays (VerifyNow P2Y12 assay, vasodilator stimulated phosphoprotein phosphorylation (VASP) assay and Multiplate analyzer) have overcome many of the technical and methodological limitations of previous assays and are the first choice in clinical practice. Based on those tests, HTPR defines insufficient platelet inhibition while, low on treatment platelet reactivity (LTPR) corresponds to an excessive platelet inhibition (18-22,25,26).
Both status has been associated with clinical outcomes in both post ACS and post PCI patients: HTPR has been repetitively associated with higher incidence of ischemic recurrence (15,27,28), while LTPR is correlated to the occurrence of bleeding events on DAPT (20-22). Indeed, evidence from multiple studies involving over 20,000 patients demonstrated a strong association between biological resistance to clopidogrel (HTPR) and post-PCI ischemic events, especially stent thrombosis (ST) in the setting of ACS (28-30). In the large Assessment of Dual Antiplatelet Therapy with Drug-Eluting Stents (ADAPT-DES) trial, HTPR was independently associated with ST and myocardial infarction (MI) [hazard ratio (HR) 2.49, 95% confidence interval (95% CI) 1.43–4.31, P=0.001; HR 1.42, 95% CI, 1.09–1.86, P=0.01, respectively] (30). Moreover, HTPR have a 1.5-fold higher risk for mortality compared with those with optimal platelet reactivity following PCI (31). Therefore, resistance to clopidogrel after an ACS has been identified as a risk factor for recurrent cardiac events. Whether HTPR is a marker of higher risk or a potentially modifiable risk factor for adverse events remained unanswered and randomized clinical trials were needed to test this hypothesis.
Meanwhile, newer P2Y12 blockers have been developed. Prasugrel induces a more predictable response than clopidogrel due to less competing metabolic pathway to inactive metabolites, less drug-drug interaction and CYP2C19 genetic effect (6,18,32). Ticagrelor is a direct and reversible antiplatelet P2Y12 receptor blocker, which does not need activation metabolism (7,33,34). Both are characterized by a stronger platelet inhibition and superiority on clinical outcomes in comparison with clopidogrel (32,34). The Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel-Thrombolysis in Myocardial Infarction 38 (TRITON-TIMI 38) randomized 13,608 ACS patients to prasugrel or standard dose clopidogrel, and showed that prasugrel was associated with significantly reduced rates of ischemic events, including ST, but with an increased risk of major bleeding (6). In a sub-study of the TRITON study, two PFT were performed: VASP assay and LTA. Mean VASP was significantly lower in prasugrel-treated subjects than in clopidogrel-treated subjects at both 1 and 2 h post loading dose (LD) (51.8±5.1 vs. 78.8±2.5, P=0.001) and during maintenance phase (30 day) (33.6±2.9 vs. 47.9±2.7, P=0.001) (32). This study was not powered to correlate biological findings with clinical outcomes but confirmed the stronger and more reliable antiplatelet effect of prasugrel (32). In the randomized PLATO (PLATelet inhibition and patient Outcomes) study, ticagrelor also reduced the incidence of the primary end point of cardiovascular death, myocardial infarction, and stroke compared with clopidogrel (7). In this study, LTA, Verifynow P2Y12 assay and VASP were performed in a dedicated sub-study (34). Platelet reactivity was lower in the ticagrelor compared with the clopidogrel group, both before the next maintenance dose (MD) (trough) and 2 to 4 h post-dose (peak). Clopidogrel achieved overall moderate inhibition of platelet aggregation induced by ADP, with marked interindividual variation, whereas ticagrelor achieved marked inhibition by 1 h post-dose with consistency over time (34).
The stronger platelet inhibition conferred by either prasugrel or ticagrelor over clopidogrel has been confirmed in several trials (31,35,36). Of note, a trend in favor of more potent inhibition on ticagrelor than on prasugrel was observed (37).
Evaluation of the benefit of tailored antiplatelet therapy based on platelet function testing
Recurrence of ischemic events despite a DAPT with aspirin plus clopidogrel led to platelet testing development and routine assessment of clopidogrel response in many centers. Those testing identified various factors implicated in poor antiplatelet inhibition on clopidogrel, which is frequently observed after ACS. Newer P2Y12 blockers have been developed and their superiority over clopidogrel is at least partially related to stronger and faster platelet inhibition. A strong relation between inappropriate platelet inhibition (HTPR or LTPR) and higher risk of events has been proved. Therefore, randomized clinical trials have been designed to adjust the value of antiplatelet regimen based on antiplatelet drugs monitoring. Strategies to target a therapeutic window for antiplatelet therapy, with intermediate degree of platelet inhibition (non-HTPR and non-LTPR), that would allow prevention of both ischemic and bleeding events, have been developed (38,39).
The Gauging Responsiveness with a VerifyNow assay, Impact on Thrombosis and Safety (GRAVITAS) study, was the first randomized trial to assess high-dose clopidogrel in patients with HTPR (40). Patients with 12 to 24 hours post-PCI HTPR using the VerifyNow™ assay, were randomized to either high-dose (LD of 600 mg followed by 150 mg) or standard dose (placebo LD followed by 75 mg) clopidogrel. Of note, stable angina was the indication for PCI in 60% of cases. Out of 5,429 patients, 2,214 (40.8%) were defined as HTPR. Compared with standard-dose clopidogrel, high-dose clopidogrel provided a 22% absolute reduction in the rate of HTPR at 30 days (P<0.001) and 24% at 6 months. At 6 months, the primary end point [cardiovascular (CV) death, non-fatal MI and ST] had occurred in 25 of 1,109 patients (2.3%) receiving high-dose clopidogrel compared with 25 of 1,105 patients (2.3%) receiving standard-dose clopidogrel (HR 1.01; 95% CI, 0.58–1.76; P=0.97). The low event rates and modest sample size reduced the power of the study to identify statistically significant differences on clinical outcomes. The relative benefit of high-dose clopidogrel may also have been diluted by the decrease in the frequency of HTPR in both randomized groups over the initial 30 days after PCI. HTPR measured 12 to 24 hours after PCI resolved at the 30-day follow-up in 38% of the patients randomly assigned to standard-dose clopidogrel (40).
The Testing Platelet Reactivity In Patients Undergoing Elective Stent Placement on Clopidogrel to Guide Alternative Therapy With Prasugrel (TRIGGER PCI) study aimed to randomize elective-PCI patients with HTPR on clopidogrel to prasugrel or continuation of standard dose of clopidogrel (41). In this study, HTPR was defined using the VerifyNow™ assay. After screening 3,525 patients, 423 patients with HPR on clopidogrel were randomized. Finally, 273 patents completed the study, which was stopped prematurely. A substantial decrease in PRU in the prasugrel arm (P<0.001), but only a small, albeit statistically significant (P=0.001), decrease in the clopidogrel arm. No difference was shown in terms of clinical outcomes with only 1 primary efficacy endpoint and 4 bleeding.
The premature termination of this study does not allow any conclusion regarding the benefit of switching to prasugrel the patients defined HTPR on clopidogrel.
Main limitations of both GRAVITAS and TRIGGER were inclusion of very low risk population (mainly elective PCI) and adjustment of antiplatelet strategy after PCI, likely missing the high-risk period for periprocedural MI and acute ST (40,41). Therefore, the ARCTIC study has been designed, including ACS patients with pre-PCI PFT-based treatment adjustment.
Indeed, The Assessment by a Double Randomization of a Conventional Antiplatelet Strategy versus a Monitoring-guided Strategy for Drug-Eluting Stent Implantation and of Treatment Interruption versus Continuation One Year after Stenting (ARCTIC) trial aimed to evaluate a randomized strategy of adjusted treatment based on platelet function monitoring in patients with a poor response to aspirin, thienopyridine (clopidogrel or prasugrel), or both, as compared with a conventional approach in which similar treatment was given, without platelet-function assessment (42). In the monitoring arm, platelet function was monitored using the VerifyNow™ both before PCI and during the maintenance phase (14 days to one month after PCI). In case of HTPR identification, drug regimen was intensified by either high dose clopidogrel (600 mg LD and 150 mg MD) or prasugrel (60 mg LD and 10 mg MD). Interestingly, at 14 to 30 days visit, patients with LTPR were switched to clopidogrel if on prasugrel or the dosage of clopidogrel was decreased to 75 mg if 150 mg. For patients with adequate biological response (therapeutic window) no treatment change was made; 2,440 patients were randomized of whom 1,213 were assigned to monitoring strategy. An ACS was reported in 27% of the patients. At randomization, 34.5% of patients in the monitoring group showed HTPR with clopidogrel, and had their drug treatment adjusted. When response to aspirin was tested before stent implantation, HTPR was rare (7.6%) and led to the administration of an additional bolus of intravenous aspirin in four of five patients. In the monitoring group, at the time of discharge, 9.3% of patients were being treated with prasugrel, 47.8% of those who were being treated with clopidogrel were receiving a MD of 150 mg or more, and 37.1% of those who were being treated with aspirin were receiving a dose higher than recommended (>100 mg). At follow up visit, there was a reduction of approximately 50% in the percentage of patients who had a poor response to P2Y12 inhibitors (15.6% vs. 34.5% at the time of the procedure; P<0.001). At 1 year of follow-up, the primary end point (death, MI, ST, urgent revascularization, stroke) had occurred in 34.6% of patients in the monitoring group and 31.1% of those in the conventional treatment group (P=0.10). In this trial, the biological effectiveness of antiplatelet adjustment did not translate in improved outcomes (42).
Those three studies, testing benefit of tailored therapy based on PFT, were mainly focused on prevention of ischemic events by optimizing platelet inhibition in “so-called” low-responders. Therefore, the Platelet function monitoring to adjust antiplatelet therapy in elderly patients stented for an acute coronary syndrome (ANTARCTIC) study has been designed to assess in a high-risk population (ACS patients >75-year-old) the value of de-escalating P2Y12 inhibition based on PFT on the bleeding risk compared to standard approach without PFT (43). This study included 877 patients who were randomized to prasugrel 5 mg daily with a potential dose or drug adjustment in case of inadequate response (monitoring group) or prasugrel 5 mg daily with no monitoring or treatment adjustment (conventional group). PFT (VerifyNow™) was done 14 days after randomization and repeated 14 days after treatment adjustment in patients in the monitoring group. When the second test was done 66% of the patients had reached the prespecified target of platelet inhibition. 39% of the patients had been switched from prasugrel to clopidogrel for LTPR. Prasugrel 5 mg was up-adjusted to 10 mg in only 4% of patients with HTPR. 55% patients in the monitoring group remained on prasugrel 5 mg. The primary endpoint (CV death, MI, stroke, ST, urgent revascularization, bleeding BARC ≥2) occurred in 120 (28%) patients in the monitoring group compared with 123 (28%) patients in the conventional group (HR 1.003, 95% CI, 0.78–1.29; P=0.98). Rates of bleeding and ischemic events did not differ significantly between groups.
Taken together, those results do not support a routine use of PFT-guided adaptation of thienopyridine treatment after an ACS to target a therapeutic window of platelet inhibition, as compared to standard antiplatelet therapy without monitoring (Table 1). Adjusting antiplatelet regimen according to PFT did improve the biological effectiveness of P2Y12 blocker, while it did not translate into clinical benefit.
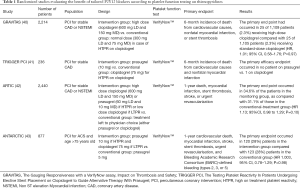
Full table
Following those results European guidelines recommend newer P2Y12 blocker in first intention after an ACS, while clopidogrel is reserved to contra indication to those treatment based on clinical judgment and not PFT (IA). Indeed, routine PFT to adjust antiplatelet therapy before or after elective stenting is not recommended (class IIIA) (3).
Residual and innovative roles of platelet function testing: PFT is still alive!
Since platelet reactivity is higher in the early phases of ACS and generally decreases quickly within days (40), strategies based on strong antiplatelet treatment in the acute phase of ACS followed by a de-escalation to less potent antiplatelet drug in the maintenance phase have been evaluated in recent studies. This hypothesis is also supported by post hoc analysis of PLATO and TRITON-TIMI 38 where arguments for a greatest ischemic benefit of potent antiplatelet drugs over the less potent clopidogrel occur early, while bleeding events arise mostly during the maintenance phase (44,45).
Testing Responsiveness to Platelet Inhibition on Chronic Antiplatelet Treatment for Acute Coronary Syndromes (TROPICAL-ACS) study has been recently published (46). In this study, 2,619 patients with PCI for ACS were randomized to either standard treatment with prasugrel for 12 months (1,306 patients, control group) or a stepdown regimen (1 week prasugrel followed by 1 week clopidogrel and platelet function-guided maintenance therapy with clopidogrel or prasugrel from day 14 after hospital discharge; 1,304 patients, guided de-escalation group). In this de-escalation group, patients with HTPR were switched back to prasugrel (39%), while in the absence of HTPR they were maintained on clopidogrel for 1 year. At 1 year, the combined primary endpoint (CV death, MI, stroke, bleeding BARC ≥2) occurred in 95 patients (7%) in the guided de-escalation group and in 118 patients (9%) in the control group (P non-inferiority =0.0004; HR 0.81; 95% CI, 0.62–1.06, P superiority =0.12). The ischaemic components of the primary endpoint occurred in 32 patients (3%) in the guided de-escalation group and in 42 patients (3%) in the control group (HR 0.77 95% CI, 0.48–1.21; P=0.25), indicating that early de-escalation did not result in an increased risk of ischemic events (P non-inferiority =0.0115). The incidence of the key secondary endpoint of BARC 2 or higher bleedings was 5% (64 events) in the guided de-escalation group versus 6% (79 events) in the control group (HR 0.82; 95% CI, 0.59–1.13; P=0.23).
The timing of platelet inhibition after acute coronary syndrome (TOPIC) study is a monocentric trial randomizing patients one month after a stented ACS to either continuation of DAPT with aspirin plus newer P2Y12 blocker, or de-escalation to aspirin plus clopidogrel (47). The main study results showed that the de-escalation strategy did reduce the incidence of bleeding BARC ≥2 (4.0% vs. 14.9%, HR 0.30; 95% CI, 0.18–0.50, P<0.01) while the ischemic events (9.3% vs. 11.5%, HR 0.80; 95% CI, 0.50–1.29, P=0.36) were not different between the two groups. All patients underwent a PFT using VASP assay at randomization (48). At this time, 47% of the patients were classified as LTPR on newer P2Y12 blocker. Patients defined as LTPR and randomized to unchanged DAPT were at highest risk of primary endpoint (CV death, stroke, urgent revascularisation, bleeding BARC ≥2) occurrence (P<0.01). Conversely, in de-escalation arm, initially LTPR patients had no significant difference in primary outcome incidence compared with no LTPR patients (HR 0.78; 95% CI, 0.40–1.49, P=0.45). De-escalation strategy was associated with important reduction in primary endpoint incidence in LTPR patients (HR 0.29; 95% CI, 0.17–0.51, P<0.01), and only numerically lower incidence in no LTPR (HR 0.79; 95% CI, 0.46–1.35, P=0.39). Those result support the fact that patients defined as LTPR on newer P2Y12 blockers are at higher risk of events after an ACS and mainly increased bleeding risk with benefit of a de-escalation strategy to clopidogrel was greater in this cohort (48).
Overall, TROPICAL ACS and TOPIC showed that de-escalation from newer P2Y12 blocker to clopidogrel after an ACS may be guided by platelet function and is feasible without any excess in terms of ischemic or bleeding events. After an ACS, de-escalation could be used for several reasons, whatever medical or socio economic. This should take into account clinical factors and could be guided by PFT as an additional argument (49). Most recent guidelines suggest that an approach of DAPT de-escalation guided by PFT may be considered in ACS patients as an alternative to 12 months potent platelet inhibition, especially for patients deemed unsuitable for maintained potent platelet inhibition (class IIb level B) (3).
The ischemic and bleeding risks balance is central after an ACS regarding duration and type of antiplatelet regimen. Added to several clinical (age, previous bleeding…) and biological (hemoglobin, creatinine…) factors, platelet reactivity could still have a place in complex clinical situations.
Standard treatment should be a newer P2Y12 blocker after ACS, as recommended by European guidelines (3). However, DAPT should be adjusted to clinical evaluation. A possible scenario could be a patient on oral anticoagulant developing an ACS. Per se those patients are at high risk of bleeding because of the need for triple therapy and clopidogrel remains the rule associated with aspirin (3). However, in case of very high thrombotic risk (i.e., ST under oral anticoagulation and clopidogrel), identification of HTPR on clopidogrel could motivate an escalation to newer P2Y12 blocker. Accordingly, European guidelines recommend that PFT or genetic testing may be considered in specific high-risk situations (e.g., history of stent thrombosis; compliance issue; suspicion of resistance; high bleeding risk) (class IIb C) (5).
Other utility of PFT could be the assessment of patient adherence to treatment after an ACS (50). Indeed, patient adherence remains a major issue with more than 10% of post ACS patients being non-adherent to DAPT with direct clinical consequences (51). It has been shown that antiplatelet resistance to aspirin is very rare in patients taking their medication and therefore it could be considered in post ACS patients as a marker of compliance with potential use during follow up (50). In such situations, physician could be guided by platelet testing to reinforce patient education and develop strategies to optimize compliance (fixed dose combination, low side-effect molecules…). This strategy tested by our group with aspirin could be used for drugs providing potent and predictable platelet inhibition such as newer P2Y12 blockers, while it would be more challenging with clopidogrel to distinguish resistance and non-adherence (47,50,52).
Conclusions
In conclusion, the clinical benefit of a tailored antiplatelet therapy based on platelet function is still unproved. The efforts to correct HTPR on clopidogrel, based on PFT after PCI, do not translate in improved outcomes in randomized studies. However, we learned from use of PFT that platelet inhibition should be optimized in high risk patients such as ACS. Accordingly, newer P2Y12 blockers, providing stronger and predictable platelet inhibition, are the gold standard therapy after ACS, and therefore limit the room for testing clopidogrel response in this setting. However, the side effects observed with prolonged use of newer P2Y12 blockers, led to emergence of de-escalation strategy. De-escalation of antiplatelet regimen after an ACS should take into account several clinical factors and could be assisted by platelet testing as suggested by recent studies. Patients with hyper response to newer P2Y12 blockers may be the ideal candidate for a de-escalation to clopidogrel; while those with adequate inhibition could remain on same treatment. Dedicated randomized trials are still needed to evaluate the potential utility of PFT.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Yusuf S, Zhao F, Mehta SR, et al. Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001;345:494-502. [Crossref] [PubMed]
- Mehta SR, Yusuf S, Peters RJ, et al. Clopidogrel in Unstable angina to prevent Recurrent Events trial (CURE) Investigators. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet 2001;358:527-33. [Crossref] [PubMed]
- Neumann FJ, Sousa-Uva M, Ahlsson A, et al. ESC Scientific Document Group. ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 2018.2018. [Epub ahead pf print]. [PubMed]
- O’Gara PT, Kushner FG, Ascheim DD, et al. 2013ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;61:e78-140. [Crossref] [PubMed]
- Authors/Task Force members, Windecker S, Kolh P, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 2014;35:2541-619. [Crossref] [PubMed]
- Wiviott SD, Braunwald E, McCabe CH, et al. TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007;357:2001-15. [Crossref] [PubMed]
- Wallentin L, Becker RC, Budaj A, et al. PLATO Investigators. Freij A, Thorsén M. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009;361:1045-57. [Crossref] [PubMed]
- Davì G, Patrono C. Platelet activation and atherothrombosis. N Engl J Med 2007;357:2482-94. [Crossref] [PubMed]
- Furie B, Furie BC. Mechanisms of thrombus formation. N Engl J Med 2008;359:938-49. [Crossref] [PubMed]
- Aradi D, Storey RF, Komócsi A, et al. Working Group on Thrombosis of the European Society of Cardiology. Expert position paper on the role of platelet function testing in patients undergoing percutaneous coronary intervention. Eur Heart J 2014;35:209-15. [Crossref] [PubMed]
- Michelson AD, Frelinger AL 3rd, Furman MI. Current options in platelet function testing. Am J Cardiol 2006;98:4N-10N. [Crossref] [PubMed]
- Pereillo JM, Maftouh M, Andrieu A, et al. Structure and stereochemistry of the active metabolite of clopidogrel. Drug Metab Dispos 2002;30:1288-95. [Crossref] [PubMed]
- Brandt JT, Close SL, Iturria SJ, et al. Common polymorphisms of CYP2C19 and CYP2C9 affect the pharmacokinetic and pharmacodynamics response to clopidogrel but not prasugrel. J Thromb Haemost 2007;5:2429-36. [Crossref] [PubMed]
- Lau WC, Waskell LA, Watkins PB, et al. Atorvastatin reduces the ability of clopidogrel to inhibit platelet aggregation: a new drug-drug interaction. Circulation 2003;107:32-7. [Crossref] [PubMed]
- Aradi D, Komócsi A, Vorobcsuk A, et al. Prognostic significance of high on clopidogrel platelet reactivity after percutaneous coronary intervention: systematic review and meta-analysis. Am Heart J 2010;160:543-51. [Crossref] [PubMed]
- Shuldiner AR, O’Connell JR, Bliden KP, et al. Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA 2009;302:849-57. [Crossref] [PubMed]
- Frere C, Cuisset T, Morange PE, et al. Effect of cytochrome p450 polymorphisms on platelet reactivity after treatment with clopidogrel in acute coronary syndrome. Am J Cardiol 2008;101:1088-93. [Crossref] [PubMed]
- Grosdidier C, Quilici J, Loosveld M, et al. Effect of CYP2C19*2 and *17 genetic variants on platelet response to clopidogrel and prasugrel maintenance dose and relation to bleeding complications. Am J Cardiol 2013;111:985-90. [Crossref] [PubMed]
- Cuisset T, Loosveld M, Morange PE, et al. CYP2C19*2 and *17 alleles have a significant impact on platelet response and bleeding risk in patients treated with prasugrel after acute coronary syndrome. JACC Cardiovasc Interv 2012;5:1280-7. [Crossref] [PubMed]
- Campo G, Parrinello G, Ferraresi P, et al. Prospective evaluation of on-clopidogrel platelet reactivity over time in patients treated with percutaneous coronary intervention relationship with gene polymorphisms and clinical outcome. J Am Coll Cardiol 2011;57:2474-83. [Crossref] [PubMed]
- Mangiacapra F, Patti G, Barbato E, et al. A therapeutic window for platelet reactivity for patients undergoing elective percutaneous coronary intervention: results of the ARMYDA-PROVE (Antiplatelet therapy for Reduction of MYocardial Damage during Angioplasty-Platelet Reactivity for Outcome Validation Effort) study. JACC Cardiovasc Interv 2012;5:281-9. [Crossref] [PubMed]
- Sibbing D, Schulz S, Braun S, et al. Antiplatelet effects of clopidogrel and bleeding in patients undergoing coronary stent placement. J Thromb Haemost 2010;8:250-6. [Crossref] [PubMed]
- Nguyen TA, Diodati JG, Pharand C. Resistance to clopidogrel: A review of the evidence. J Am Coll Cardiol 2005;45:1157-64. [Crossref] [PubMed]
- Pankert M, Quilici J, Loundou AD, et al. Impact of obesity and the metabolic syndrome on response to clopidogrel or prasugrel and bleeding risk in patients treated after coronary stenting. Am J Cardiol 2014;113:54-9. [Crossref] [PubMed]
- Tantry US, Bonello L, Aradi D, et al. Working Group on On-Treatment Platelet Reactivity. Consensus and update on the definition of on-treatment platelet reactivity to adenosine diphosphate associated with ischemia and bleeding. J Am Coll Cardiol 2013;62:2261-73. [Crossref] [PubMed]
- Cuisset T, Grosdidier C, Loundou AD, et al. Clinical implications of very low on-treatment platelet reactivity in patients treated with thienopyridine: the POBA study (predictor of bleedings with antiplatelet drugs). JACC Cardiovasc Interv 2013;6:854-63. [Crossref] [PubMed]
- Brar SS, ten Berg J, Marcucci R, et al. Impact of platelet reactivity on clinical outcomes after percutaneous coronary intervention. A collaborative meta-analysis of individual participant data. J Am Coll Cardiol 2011;58:1945-54. [Crossref] [PubMed]
- Stuckey TD, Kirtane AJ, Brodie BR, et al. ADAPT-DES Investigators. Impact of Aspirin and Clopidogrel Hyporesponsiveness in Patients Treated With Drug-Eluting Stents: 2-Year Results of a Prospective, Multicenter Registry Study. JACC Cardiovasc Interv 2017;10:1607-17. [Crossref] [PubMed]
- Parodi G, Marcucci R, Valenti R, et al. High residual platelet reactivity after clopidogrel loading and long-term cardiovascular events among patients with acute coronary syndromes undergoing PCI. JAMA 2011;306:1215-23. [Crossref] [PubMed]
- Stone GW, Witzenbichler B, Weisz G, et al. ADAPT-DES Investigators. Platelet reactivity and clinical outcomes after coronary artery implantation of drug-eluting stents (ADAPT-DES): a prospective multicentre registry study. Lancet 2013;382:614-23. [Crossref] [PubMed]
- Aradi D, Kirtane A, Bonello L, et al. Bleeding and stent thrombosis on P2Y12-inhibitors: collaborative analysis on the role of platelet reactivity for risk stratification after percutaneous coronary intervention. Eur Heart J 2015;36:1762-71. [Crossref] [PubMed]
- Michelson AD, Frelinger AL 3rd, Braunwald E, et al. TRITON-TIMI 38 Investigators. Pharmacodynamic assessment of platelet inhibition by prasugrel vs. clopidogrel in the TRITON-TIMI 38 trial. Eur Heart J 2009;30:1753-63. [Crossref] [PubMed]
- Ferri N, Corsini A, Bellosta S. Pharmacology of the new P2Y12 receptor inhibitors: insights on pharmacokinetic and pharmacodynamic properties. Drugs 2013;73:1681-709. [Crossref] [PubMed]
- Storey RF, Angiolillo DJ, Patil SB, et al. Inhibitory effects of ticagrelor compared with clopidogrel on platelet function in patients with acute coronary syndromes: the PLATO (PLATelet inhibition and patient Outcomes) PLATELET substudy. J Am Coll Cardiol 2010;56:1456-62. [Crossref] [PubMed]
- Bassez C, Deharo P, Pankert M, et al. Effectiveness of switching 'low responders' to prasugrel to ticagrelor after acute coronary syndrome. Int J Cardiol 2014;176:1184-5. [Crossref] [PubMed]
- Kerneis M, Silvain J, Abtan J, et al. Switching acute coronary syndrome patients from prasugrel to clopidogrel. JACC Cardiovasc Interv 2013;6:158-65. [Crossref] [PubMed]
- Deharo P, Bassez C, Bonnet G, et al. Prasugrel versus ticagrelor in acute coronary syndrome: a randomized comparison. Int J Cardiol 2013;170:e21-2. [Crossref] [PubMed]
- Kirtane AJ, Parikh PB, Stuckey TD, et al. Is There an Ideal Level of Platelet P2Y12-Receptor Inhibition in Patients Undergoing Percutaneous Coronary Intervention?: "Window" Analysis From the ADAPT-DES Study (Assessment of Dual AntiPlatelet Therapy With Drug-Eluting Stents). JACC Cardiovasc Interv 2015;8:1978-87. [Crossref] [PubMed]
- Sibbing D, Steinhubl SR, Schulz S, et al. Platelet aggregation and its association with stent thrombosis and bleeding in clopidogrel-treated patients: Initial evidence of a therapeutic window. J Am Coll Cardiol 2010;56:317-8. [Crossref] [PubMed]
- Price MJ, Berger PB, Teirstein PS, et al. GRAVITAS Investigators. Standard- vs high-dose clopidogrel based on platelet function testing after percutaneous coronary intervention: the GRAVITAS randomized trial. JAMA 2011;305:1097-105. [Crossref] [PubMed]
- Trenk D, Stone GW, Gawaz M, et al. A randomized trial of prasugrel versus clopidogrel in patients with high platelet reactivity on clopidogrel after elective percutaneous coronary intervention with implantation of drug-eluting stents. J Am Coll Cardiol 2012;59:2159-64. [Crossref] [PubMed]
- Collet JP, Cuisset T, Rangé G, et al. ARCTIC Investigators. Bedside monitoring to adjust antiplatelet therapy for coronary stenting. N Engl J Med 2012;367:2100-9. [Crossref] [PubMed]
- Cayla G, Cuisset T, Silvain J, et al. ANTARCTIC investigators. Platelet function monitoring to adjust antiplatelet therapy in elderly patients stented for an acute coronary syndrome (ANTARCTIC): An open-label, blinded-endpoint, randomized controlled superiority trial. Lancet 2016;388:2015-22. [Crossref] [PubMed]
- Antman EM, Wiviott SD, Murphy SA, et al. Early and late benefits of prasugrel in patients with acute coronary syndromes undergoing percutaneous coronary intervention: a TRITON-TIMI 38 (TRial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet InhibitioN with Prasugrel-Thrombolysis In Myocardial Infarction) analysis. J Am Coll Cardiol 2008;51:2028-33. [Crossref] [PubMed]
- Becker RC, Bassand JP, Budaj A, et al. Bleeding complications with the P2Y12 receptor antagonists clopidogrel and ticagrelor in the PLATelet inhibition and patient Outcomes (PLATO) trial. Eur Heart J 2011;32:2933-44. [Crossref] [PubMed]
- Sibbing D, Aradi D, Jacobshagen C, et al. TROPICAL-ACS Investigators. Guided de-escalation of antiplatelet treatment in patients with acute coronary syndrome undergoing percutaneous coronary intervention (TROPICAL-ACS): a randomised, open-label, multicentre trial. Lancet 2017;390:1747-57. [Crossref] [PubMed]
- Cuisset T, Deharo P, Quilici J, et al. Benefit of switching dual antiplatelet therapy after acute coronary syndrome: the TOPIC (timing of platelet inhibition after acute coronary syndrome) randomized study. Eur Heart J 2017;38:3070-8. [Crossref] [PubMed]
- Deharo P, Quilici J, Camoin-Jau L, et al. Benefit of Switching Dual Antiplatelet Therapy After Acute Coronary Syndrome According to On-Treatment Platelet Reactivity: The TOPIC-VASP Pre-Specified Analysis of the TOPIC Randomized Study. JACC Cardiovasc Interv 2017;10:2560-70. [Crossref] [PubMed]
- Angiolillo DJ, Rollini F, Storey RF, et al. International Expert Consensus on Switching Platelet P2Y12 Receptor-Inhibiting Therapies. Circulation 2017;136:1955-75. [Crossref] [PubMed]
- Cuisset T, Frere C, Quilici J, et al. Aspirin noncompliance is the major cause of "aspirin resistance" in patients undergoing coronary stenting. Am Heart J. 2009;157:889-93. [Crossref] [PubMed]
- Mehran R, Baber U, Steg PG, et al. Cessation of dual antiplatelet treatment and cardiac events after percutaneous coronary intervention (PARIS): 2 year results from a prospective observational study. Lancet 2013;382:1714-22. [Crossref] [PubMed]
- Deharo P, Quilici J, Bonnet G, et al. Fixed-dose aspirin-clopidogrel combination enhances compliance to aspirin after acute coronary syndrome. Int J Cardiol 2014;172:e1-2. [Crossref] [PubMed]


