
Vascular health determinants in children
Introduction
Atherosclerosis: relevance to youth
The causes of cardiovascular disease (CVD) have been intensively studied, but it is still one of the most causes of death. Therefore, a shift to early risk assessment to describe arterial health determinants is necessary. Since the atherosclerotic progression covers a long phase, it is important to evaluate the arterial health status of children and adolescents and to define arterial health determinants. Early evaluation of the vascular health status in youth has gained increasing scientific interest for individual cardiovascular (CV) risk assessment. Additionally it is important to analyze CV risk factors that are responsible for and in relation with the atherosclerotic progression (1). CV risk factors act early in life and have a major impact on the development of atherosclerosis (2).
One of the early and most significant American multicenter study, organized by fifteen cooperating centers, to determine pathophysiological aspects of atherosclerosis in youth was the PDAY study (Pathobiological Determinants of Atherosclerosis in Youth) (3). Detailed descriptions of methods and results have been presented in a number of publications (4-10). Summarizing the implications of the results from the pathologic studies it can be stated that atherosclerosis begins in childhood—atherosclerotic lesions are present in the aorta of nearly 100% and in the right coronary arteries in about 50% by the late teen (10–15 years old) and increase in extent and severity through the early thirties. Second, risk factors, such as male sex, cholesterol, smoking, hypertension, obesity, hyperglycemia, accelerate the progression in youth; third, there is potential for primary prevention and intervention should begin early—the rapid increase in raised lesions at about the age 25 years suggests that risk factor control should be initiated prior to that age.
These conclusions from the PDAY study are consistent with the observation that serum cholesterol levels in young adults predict coronary heart disease risk at middle age (11). Also Van Horn and Greenland (12) stated that prevention of coronary artery disease is, indeed a pediatric problem.
The Bogalusa Heart Study underlines that early manifestation of atherosclerosis is associated to traditional risk factors (13). The observations from the Bogalusa Heart Study have shown that etiologies of adult CVD, atherosclerosis, coronary artery disease, and essential hypertension begin in childhood, with early documented anatomic pathomorphological changes as early as 5 to 8 years. Further a “tracking” of risk factors into adulthood has been described (14). Furthermore traditional risk factors that are present in childhood predict cardiovascular risk in adulthood (13,15,16).
The role of endothelial function, arterial stiffness and structure
In addition to functioning as a transport system, the large arteries also have a damping function for the pressure fluctuations produced by the pulsatile heart action. Arterial elasticity and compliance lead to reversible extensibility of the artery and thereby converts the cyclic pulsatile blood flow into a continuous-phasic flow which is also referred to as the so called “Windkessel function”.
Arterial vascular stiffness is the generic term for structural and functional properties of the vascular system. Changes in the arterial system such as endothelial dysfunction or stiffening reflect clinically significant pathophysiological changes in the body (17). An impairment of this attenuation function by an increase in the arterial vascular stiffness ultimately leads to an increased afterload of the heart via an increased pulse pressure with the consequences of left ventricular hypertrophy. Furthermore, the myocardial perfusion is impaired and in the long term this can lead to heart failure. This increases the risks for a cardiovascular event (18,19).
Already in young patients a limitation of the elastic properties of the large arteries can be detected in addition to, or before the vascular wall thickening (for example as intima-media measurement of the A. carotis communis) (1).
Methods
Non-invasive diagnostic approaches in pediatric cardiovascular health prevention—imaging of the artery, its structure and function
In the last years, technological advancement made it possible to underline the screening of risk factors non-invasively mostly by ultrasound measurements. Several non-invasive and relatively easy to obtain measures of arterial structure and function have shown to be clinically useful in pediatric screenings and diagnostics, getting an “inside view” of arterial health and endothelial function.
Those measurements are primarily the measurement of carotid intima-media thickness (IMT) of the A. carotis communis, diagnosing anatomic changes, endothelial function (physiologic changes) (conduit-artery endothelial function), by assessing the flow-mediated dilatation (FMD) of the brachial artery (BA) (1,20,21) and arterial compliance (AC), e.g., arterial stiffness (mechanical changes) on the carotid artery, aorta or BA, measuring the change in vessel diameter between systole and diastole or pulse wave velocity (PWV). PWV is a measure of the speed of the arterial pressure wave propagation and is an inverse index of arterial wall stiffness (22,23).
Non-invasive assessment of arterial stiffness: PWV
The PWV directly reflects the vessel elasticity. Their advantage is that their quick and easy measurement is now possible via blood pressure cuffS Furthermore, with this method of measurement, the pulse wave reflection, another parameter for estimating a possible end organ damage of the arterial system, can also be dimensioned via a pulse contour analysis. Preliminary reflected pulse waves increase cardiac afterload and additionally affect coronary perfusion (24). Changes in vascular stiffness mainly occur with increasing age (25). Other risk factors include obesity, hypertension, active smoking, diabetes types I and II (25).
By means of pulse wave analysis, a simple and patient-friendly representation of the cardiovascular risk profile is possible. Also, a quantification of a potential end organ damage for adulthood is now available (26). In addition, the comparison of the measured values with the age-specific norm values enables the explanation in terms of the “biological vascular age”. This plastic presentation of the findings is well understood by the patients (27). Especially in chronic or subclinical diseases of the vascular system, e.g., in the context of arterial hypertension, understanding the patient’s relationships is of fundamental importance for the compliance of preventive measures. One of the most important of these measures is adequate exercise.
It may be possible to verify first subclinical arteriosclerotic changes with PWA in inactive children, and to demonstrate the influence of activity-enhancing programs on PWA.
Measurement of the PWV has been recommended since 2007 by the European professional societies (ESH and ESC) to quantify the cardiovascular risk profile and estimate the potential end organ damage for adulthood (26). Such recommendations do not yet exist for children and adolescents.
Simple and rapid diagnostic methods are needed to initiate appropriate preventive measures as early as infancy, preventing subsequent cardiovascular complications.
Special attention is given on the oscillometric method which is nearly operator independent and easy to perform. PWV is thereby assessed at the A. brachialis with the Mobil-O-Graph (I.E.M, Stolberg, Germany; ARCSolver method), the method consists of a three-level algorithm. First, the single pressure waves are verified for their plausibility. In a second step, a comparison of each single pressure wave is applied to recognize artifacts. Last, a filtered, averaged pulse wave is determined from the measured pulse waves, which is used for calculating the central aortic pulse wave (28) and other hemodynamic parameters like central blood pressure or augmentation index.
Norm values form a large German cohort have been published by Elmenhorst et al. (29).
Non-invasive measure of arterial stiffness and compliance: eTRACKING
Next to PWV vessel function is defined by sonographic stiffness indices (stiffness index, augmentation index, elastic modulus). Here, increased vascular stiffness is positively associated with increased cardiovascular risk (30).
With the eTRACKING system (ProSound Alpha 7®; Aloka/Hitachi Medical Systems GmbH, Wiesbaden, Germany) the movement of the vessel wall during the cardiac cycle is tracked and a diameter change curve in real time can be constructed (Figure 1) (31). The method allows to calculate the local PWVβ from the time delay between two adjacent distension waveforms (Figure 2) (32). An extensive and comprehensive description of underlying methodologies and assumptions to assess carotid local arterial stiffness is provided by Vriz and colleagues (32). The diagnosis is performed after 15 minutes of rest in supine position: the neck is slightly extended and the head is turned 45° opposite the site being scanned, (automatic, oscillometric) blood pressure measurement is followed directly (time delay: max. 5 minutes). Distensibility is assessed using semi-automated B- and M-mode ultrasound, which combines automatic edge-detection with manual correction, with a high frequency linear array probe (5–13 MHz). PWVβ is measured 1 cm proximal to the bulb. Parameters are calculated as average values of four measurements (2 measurements at each side).
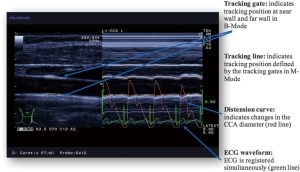
Non-invasive assessment of peripheral vascular function: principles of FMD
Vascular endothelial function of the right BA can be studied with ultrasonography (Aloka/Hitachi, ProSound Alpha 6) with a high-frequency (5–13 MHz) linear-array transducer. Mean arterial pressure need to be simultaneously determined from the contralateral arm. The transducer is placed in the distal third of the upper arm to image the BA. Tracking gates are set on the intima in B-mode, the artery resulting tracking lines, indicating the tracking position, are presented on the monitor in M-mode. The waveform of diameter changes over the cardiac cycle are displayed in real time using the FMD-mode of the eTRACKING system.
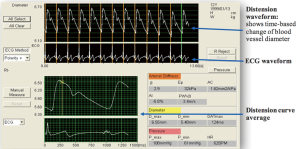
Changes in arterial diameter at baseline (1 minute), ischaemia (5 minutes) and vasodilatation (2 minutes) are measured. Ischemia is developed by a blood pressure cuff placed around the forearm inflated to a pressure of 220 mmHg. After 5 minutes the cuff is deflated, causing increased flow-mediated vasodilatation. Peak artery diameter and the time taken to reach the maximal diameter after the release of the cuff, is recorded. From these data, FMD%, an index indicating the percentage dilated at the maximum vessel diameter in peak vasodilatation after cuff deflation, relative to maximum vessel diameter at baseline, is calculated. BA distensibility is defined by AC, pressure strain elastic modulus (Ep), and PWVβ according to the above-mentioned formula.
AC describes the ability of an artery to change its volume due to a given change in arterial blood pressure. The compliance is calculated from the diameter of the blood vessel (D) and BP.
Pressure strain elastic modulus (Ep) is the ratio of stress and strain on the arterial wall and measures the intrinsic stiffness. (Moo) An increase in stiffness leads to a higher Ep value.
Beta-index (β) is another parameter to depict arterial stiffness. The higher the β-index, the lower is the arterial elasticity.
PWV is the velocity of the pressure wave transmitted between two portions of the arterial tree (33). PWVβ is measured as the local PWV of BA, calculated from β (Figure 3).
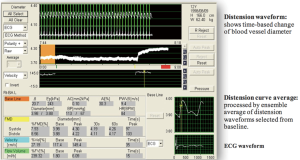
FMD examination is possible in children, however the admission of glyceryl trinitrate to initiate maximal dilatation of the vessel system, assessing individual control values of maximal dilation has not been implemented in the examination of healthy school children in Germany. Before initiating FMD in children the examination needs to be well explained. Children laid down in a supine position and rest for at least 10 minutes to guarantee stable conditions during measurement. The right arm was extended and immobilized with foam supports to guarantee a comfortable position. Since the A. brachialis is located medially, the arm is slightly turned outside (supination) to allow consistent imaging of the BA (34).
Non-invasive assessment of arterial structure—the IMT
In non-invasive diagnostics of atherosclerotic progression, the IMT is measured to quantify the atherosclerotic wall process and serves as a subclinical marker of atherosclerosis and further is a strong marker of cardiovascular disease burden.
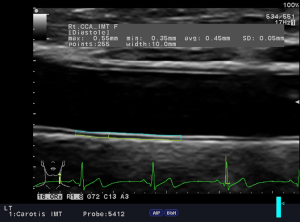
The IMT can be used to predict myocardial infarction and stroke (35). It has been further indicated in adults that an increased IMT is associated with an increased risk of myocardial infarction and stroke, even without a previous history of cardiovascular disease (36). IMT can be viewed as a descriptive index of individual atherosclerosis in adults (37). Figure 4 demonstrates a carotid IMT measurement, assessed with B-mode ultrasound (ProSound Alpha 6, Aloka/Hitachi) using a high frequency linear array probe (5–13 MHz). After 15 minutes rest patients were examined in supine position, the neck slightly extended and their head turned 45° opposite the site being scanned. Carotid IMT was measured according to the Mannheim consensus (35) on the far wall of the CCA, using a semi-automatic edge to edge detection.
Results
Endothelial function in healthy children
Our first results from a longitudinal school-based study, on FMD using the continuous eTRACKING mode, to analyze changes in blood flow velocity and arterial diameter at baseline, ischemia and vasodilatation (ProSound Alpha 6, Hitachi/Aloka) demonstrated sex differences in healthy children. One hundred and nineteen children (53 girls), aged in the median 12.3 years, interquartile range (IQR) 11.9–12.9 years and median body mass index standard deviation score (BMI-SDS) of 0.06 (IQR −0.93 to 1.18) were examined at baseline. At baseline, girls revealed higher (P=0.004) arterial stiffness β=29.25 (IQR 17.67–38.50) than boys: β=22.17 (IQR 16.18–30.16). AC was lower (P=0.004) in girls 0.052 mm2/kPa (IQR 0.039–0.075 mm2/kPa) compared to boys 0.084 mm2/kPa (IQR 0.044–0.104 mm2/kPa). Girls had higher (P=0.024) local PWV 10.89 m/s (IQR 8.61–12.14 m/s) than in boys 9.46 m/s (IQR 8.13–11.28 m/s). The leptin/adiponectin ratio 0.299 (IQR 0.135–0.737) in girls was higher (P<0.001) than in boys 0.134 (IQR 0.075–0.287). Resting heart rate was also higher (P=0.007) in girls. However, no sex difference in systolic FMD (%) and diastolic FMD (%), anthropometric data, systolic and diastolic blood pressure and total cholesterol revealed. This first research in suggestive of the fact that there is a potential for being female negatively influences endothelial properties, since higher arterial stiffness and lower compliance revealed for girls. Associations between FMD and established risk factors in the healthy group of school children could not be demonstrated (34). The investigation is ongoing. A main limitation of the study is that puberty status could not be assessed in this school-based set-up. Further clinical studies should clarify the mechanisms behind.
Arterial structure in children
Our research team determine norm values for IMT in a pediatric population and investigate the validity and feasibility of the sonography of the carotid IMT. Age and sex-specific IMT percentiles for age groups 8/9 to 14/15 years were calculated. The results revealed higher IMT values in boys than in girls at the same age. Systolic blood pressure and IMT were positively related in boys (P<0.001, r=0.31) and girls (P=0.005, r=0.24). Systolic blood pressure was shown to be a predictor (r2=0.10, β=0.31, P<0.001) in boys; weight emerged as a predictor (r2=0.19, β=0.43, P<0.001) in girls for age-adjusted IMT (38).
Later in a larger cohort, using a newer ultrasound device, the age and gender specific percentiles were updated (39) and with earlier methodologies compared (40).
Those non-invasive diagnostic methods that are applicable in children are “attractive” in the pediatric age group to prevent, detect and ameliorate conditions in childhood that lead to atherosclerotic cardiovascular disease later in life (41).
Discussion
Impact of conventional risk factors on vascular function and structure in children
Obesity and its comorbidities
Overweight at any age increases the risk of death from coronary heart disease (42). Increased body weight is often accompanied by certain metabolic disorders such as insulin resistance, hyperinsulinemia, diabetes mellitus and/or dyslipidemia or hyperlipidemia. This symptom complex, known as “metabolic syndrome”, promotes the development of atherosclerotic vascular changes (43,44). Here, too, childhood overweight has been shown to be associated with cardiovascular risk factors (45), and in fact associated with an increased incidence of coronary heart disease in old age (46).
The mechanism behind is multifaceted and mainly explained by the adipose tissue. Adipose tissue is an active endocrine and paracrine tissue that releases mediators such as leptin, adiponectin, interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α), which influence next to body weight, homeostasis, insulin resistance, diabetes, inflammation and atherosclerosis (47,48).
Obesity is also inducing endothelial dysfunction, a surrogate marker of an early accelerated atherogenic process (49,50). Studies with cardiovascular disease risk factors have described impaired FMD (51), greater stiffness (52-55) and increased IMT (56-62).
Even during childhood obesity increases medical problems such as pre-diabetes (59) and cardiovascular diseases (45,46,63). In that context several studies in children and adolescents have detected endothelial dysfunction with risk factors for cardiovascular disease (64,65). Further children with familial hyperlipidemia and family history of type 2 diabetes are known about increased risk for early development of vascular structural and functional pathology (66,67).
The role of physical activity
The development of arteriosclerosis is multifactorial. The lack of physical activity is given special importance (68) and is one of the few risk factors that is considered to be relatively well-established for the development of arteriosclerosis (69). Recent studies in healthy children show that exercise has a positive effect on endothelial function. In the Abbott group, it has been shown that not only in adults is physical activity significantly associated with endothelial vasodilation (70), demonstrating vascular health. Unfortunately, a more inactive lifestyle with a reduction in physical fitness has been observed for years in Western industrialized countries
Work on healthy children is still rare and has provided contradictory findings. Schack-Nielsen and co-workers (71) showed that the amount of time 10-year-old children spent to play or participated in sports is inversely related to arterial stiffness of the aorto-radial and aorto-femoral segments. In contrast, Reed and colleagues (72) found no significant relationships between to total amount of physical activity (7-day questionnaire) and AC in 9–11 years old children.
It has been published that intervention strategies with physical activity could improve arterial function in youth with risk factors (56,73).
In preliminary work of the own working group, regular physical activity in obese children showed an improved metabolic status and thus a reduction of body fat and lowering of blood pressure after 4 weeks of inpatient rehabilitation. However, it should be taken into consideration that the holistic approach of the rehabilitation program also contained a nutritional intervention (74).
The role of fitness and exercise
Next to light or moderate physical activity, different exercise intensities play a decisive role in the adaptation mechanism of vascular function and vascular structure. In young, normally active adults, Boreham and colleagues (75) found an inverse relationship between arterial vascular stiffness and cardiorespiratory fitness and physical activity. Also, Denham and co-workers (76) report a lower vascular stiffness in moderately active men. In contrast, between intensively trained competitive athletes and control persons, negative adaptation reactions to the increased training intensity were found. Thus, Vlachopoulos et al. (77) found significantly higher blood pressure values and a higher PWV (6.89±1.00 vs. 6.33±1.03) in marathon runners. Schmidt-Trucksäss et al. (78) also report increased IMT of the femoral artery (0.33±0.03 vs. 0.27±0.03 mm). In American football players Feairheller and co-workers (79) showed a higher carotid IMT (0.49±0.06 vs. 0.46±0.07 mm) and a larger diameter of the BA (4.3±0.5 vs. 3.7±0.6 mm). Kim and his co-workers (80) observed higher vascular stiffness in American football players (6.2±0.9 vs. 5.6±0.7 mm). All results are based on comparison with a control group.
Green and co-workers (81) suspect increased blood pressure levels as a trigger for changes in vascular structure and function. Depending on their type (chronic or intermittent), these changes are responsible for functional physiological as well as pathological processes. Bertovic and colleagues (82) observe increased vascular stiffness due to blood pressure peaks during strength training. As a mechanism, they suspect a change in the proportion of smooth muscle cells in the intima and a relative increase in the proportion of collagen compared to elastin in the media. Since intensive endurance exercise also causes such pikes in blood pressure, a possible trigger for the pathological adaptation of the vascular structure could be found here. Inflammation markers are also a mechanism that causes changes in the vessel after intensive exercise. For example, increased leukocyte and CRP levels were found in athletes after a marathon and associated with an increase in vascular stiffness (81,83). Sharma and co-workers (84) see a connection between increased arterial shear forces and increased oxidative stress, which may favor the development of atherosclerosis. The consequence of atherosclerotic changes is, inter alia, increased vascular resistance with subsequently enhanced cardiac work. By increasing the level of troponin, intensive training could further lead to fibrotic changes and ventricular arrhythmias.
Positive adaptations due to exercise can be induced by increased shear forces and the associated release of vasoactive substances such as NO (81,85-87). Furthermore, moderate endurance exercise stimulates endothelial progenitor cells that contribute to the maintenance of intimal function (86-88). Moderate aerobic endurance exercise has a positive effect on blood pressure levels and is recommended as a non-pharmacological therapy for the treatment of arterial hypertension (89). Vasodilating substances released during exercise are only slowly degraded after the end of exercise, resulting in low blood pressure values (90). The mechanism of post-exercise hypotension (PEH) can last up to 10 hours with positive modulating effects (increased elasticity or decreased rigidity) on the vascular system (91,92).
Little is currently known regarding exercise and remodeling processes with regard to diameter [wall-to-lumen ratio (W-to-L-ratio)] and arterial stiffness of the carotid artery. Research aims in our ongoing outpatient clinic is to investigate the carotid intima-media thickness (cIMT) in young competitive athletes compared to reference values and further associations between cIMT, W-to-L ratio as well as intraventricular septal thickness (IVSd), left ventricular posterior wall dimensions (LVPWd) and exercise performance are analyzed. First results from 85 boys, all elite youth soccer players, age 14–18 years, training duration 15–20 h/week, revealed a slightly increased cIMT.
cIMT (cIMT right 0.531±0.03 mm; cIMT left 0.519±0.04 mm) were above the 75th percentile compared to age-matched reference values (39). Multivariate regression analysis on cIMT right (adjusted for age and blood pressure) revealed a significant model and could explain 17.4% of the variance [V02%: β=0.000 P=0.312; relative performance (Watt) β=0.037, P=0.024, R2=0.174]. Regarding cardiac structure (IVSd: 10.1±1.8 mm; IVSd z-score: 1.13±0.88; LVPWd: 8.6±1.57, LVPWd z-score: 0.80±0.85) and arterial stiffness no outstanding detecting or significant correlations occurred (93).
The increased cIMT in these young athletes might be considered as a vascular adaptation to exercise. Like adaptation mechanisms in athlete’s heart, the thickening could be interpreted as hypertrophy of smooth muscle cells, in order to economize the arteries work under elevated shear stress. The mechanisms explaining changes in the arterial wall by revealing the arterial elasticity, as a result of exercise training are not fully understood yet. Identifying these stimuli in a larger cohort will help in the design and recommendation of optimal exercise training protocols to attenuate atherosclerosis burden and risk (93).
Cross sectional studies suggest that aerobic fitness may contribute to improve arterial function in healthy children. Treiber and co-workers (94) described that aerobic fitness explained 23% of the variance in femoral FMD among children aged 11–14 years.
Performance on a 20-m-shuttle run appears to contribute to large- and small-artery compliance (15% and 7% respectively), with children who show the highest performance compared to those with the lowest performance scores (72).
However, in large school-based field study that examined 646 healthy boys and girls (316 girls, age 13.9±2.1 years), the relationship between cardiorespiratory fitness and AC in children and adolescents remained controversial. Different surrogates of arterial stiffness demonstrated different relationships with cardiorespiratory fitness in children and adolescents, after correcting for multiple confounders.
The investigators suggested more research in this area to understand the functioning of the juvenile vessels. Furthermore the limitations of many different methodologies, analyzing arterial stiffness should be considered (95).
Conclusions
Traditional cardiovascular risk factors act early in life and have a major impact on the development of atherosclerosis (2). The results of the PDAY study (3) and the Bogalusa Heart Study (13) underline that the prevention strategies and risk factor control should begin in childhood. The emphasis in the present report lied on the determination of vascular health, analyzing arterial structure and function, using non-invasive diagnostic methods. Vascular health and its relation to obesity, hypertension, physical activity and exercise were emphasized. The limitations of many different methodologies, analyzing arterial structure and function should be considered. Harmonization of knowledge and methods would greatly increase the comparability of existing results. The missing knowledge of the puberty status in school-based research set-ups also lead to limitations.
To further elucidate the clinical relevance, the mechanisms linking arterial structure and compliance function with physical activity, fitness and exercise need further clinical investigation.
It is also still unclear, if strategies that reduce risk factors in childhood, lead to an improvement in arterial structure and function in adolescents and adulthood.
However, it is unquestionable that physical activity and exercise improve health- and skill-related physical fitness components that are fundamental for a healthy maturation of a child. Providing healthy, overweight and obese children and adolescents a chance to learn improve their fitness, to be able to learn and improve motor skills, is necessary for a meaningful involvement in physical activity and exercise. By addressing the “first digital generation” (96) innovative approaches and networking of institutions and organizations (97) are essential to reduce inactive behaviors and to work towards the promotion of an active healthy lifestyle for vascular health and the long-term CV disease.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Ethics Committee of the Faculty of Medicine, Technical University of Munich (No. 4027/11). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Celermajer DS, Sorensen KE, Gooch VM, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet 1992;340:1111-5. [Crossref] [PubMed]
- Aggoun Y. Obesity, metabolic syndrome, and cardiovascular disease. Pediatr Res 2007;61:653-9. [Crossref] [PubMed]
- Strong JP, Malcom GT, Oalmann MC, et al. The PDAY Study: natural history, risk factors, and pathobiology. Pathobiological Determinants of Atherosclerosis in Youth. Ann N Y Acad Sci 1997;811:226-35; discussion 235-7. [Crossref] [PubMed]
- Wissler RW. An overview of the quantitative influence of several risk factors on progression of atherosclerosis in young people in the United States. Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Research Group. Am J Med Sci 1995;310 Suppl 1:S29-36. [Crossref] [PubMed]
- McGill HC Jr, McMahan CA, Malcom GT, et al. Relation of glycohemoglobin and adiposity to atherosclerosis in youth. Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Research Group. Arterioscler Thromb Vasc Biol 1995;15:431-40. [Crossref] [PubMed]
- McGill HC Jr, McMahan CA, Malcom GT, et al. Effects of serum lipoproteins and smoking on atherosclerosis in young men and women. The PDAY Research Group. Pathobiological Determinants of Atherosclerosis in Youth. Arterioscler Thromb Vasc Biol 1997;17:95-106. [Crossref] [PubMed]
- McGill HC Jr, McMahan CA, Herderick EE, et al. Effects of coronary heart disease risk factors on atherosclerosis of selected regions of the aorta and right coronary artery. PDAY Research Group. Pathobiological Determinants of Atherosclerosis in Youth. Arterioscler Thromb Vasc Biol 2000;20:836-45. [Crossref] [PubMed]
- McGill HC Jr, McMahan CA, Zieske AW, et al. Effects of nonlipid risk factors on atherosclerosis in youth with a favorable lipoprotein profile. Circulation 2001;103:1546-50. [Crossref] [PubMed]
- McGill HC Jr, McMahan CA, Herderick EE, et al. Obesity accelerates the progression of coronary atherosclerosis in young men. Circulation 2002;105:2712-8. [Crossref] [PubMed]
- Strong JP, Malcom GT, Oalmann MC. Environmental and genetic risk factors in early human atherogenesis: lessons from the PDAY study. Pathobiological Determinants of Atherosclerosis in Youth. Pathol Int 1995;45:403-8. [Crossref] [PubMed]
- Stamler J, Daviglus ML, Garside DB, et al. Relationship of baseline serum cholesterol levels in 3 large cohorts of younger men to long-term coronary, cardiovascular, and all-cause mortality and to longevity. JAMA 2000;284:311-8. [Crossref] [PubMed]
- Van Horn L, Greenland P. Prevention of coronary artery disease is a pediatric problem. JAMA 1997;278:1779-80. [Crossref] [PubMed]
- Berenson GS, Srinivasan SR, Bao W, et al. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N Engl J Med 1998;338:1650-6. [Crossref] [PubMed]
- Li S, Chen W, Srinivasan SR, et al. Childhood cardiovascular risk factors and carotid vascular changes in adulthood: the Bogalusa Heart Study. JAMA 2003;290:2271-6. [Crossref] [PubMed]
- Akerblom HK, Uhari M, Pesonen E, et al. Cardiovascular risk in young Finns. Ann Med 1991;23:35-9. [Crossref] [PubMed]
- Berenson GS. Childhood risk factors predict adult risk associated with subclinical cardiovascular disease. The Bogalusa Heart Study. Am J Cardiol 2002;90:3L-7L. [Crossref] [PubMed]
- Laurent C, Quaglia A, Foroni L, et al. Genomic alteration is not associated with fatty and clear cell change in hepatocellular carcinomas and its precursor nodular lesions in cirrhotic liver. Hepatol Res 2006;36:40-7. [Crossref] [PubMed]
- Weber T, Auer J, O'Rourke MF, et al. Arterial stiffness, wave reflections, and the risk of coronary artery disease. Circulation 2004;109:184-9. [Crossref] [PubMed]
- Baulmann J, Homsi R, Uen S, et al. Pulse wave velocity is increased in patients with transient myocardial ischemia. J Hypertens 2006;24:2085-90. [Crossref] [PubMed]
- Celermajer DS. Testing endothelial function using ultrasound. J Cardiovasc Pharmacol 1998;32 Suppl 3:S29-32. [PubMed]
- Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 2002;39:257-65. [Crossref] [PubMed]
- Davies JI, Struthers AD. Pulse wave analysis and pulse wave velocity: a critical review of their strengths and weaknesses. J Hypertens 2003;21:463-72. [Crossref] [PubMed]
- Aggoun Y, Szezepanski I, Bonnet D. Noninvasive assessment of arterial stiffness and risk of atherosclerotic events in children. Pediatr Res 2005;58:173-8. [Crossref] [PubMed]
- Weber T, Eber B, Zweiker R, et al. Pulse wave velocity, central blood pressure and augmentation index - "Novel" parameters for assessment of end organ damage of the arterial system in hypertension. Pathophysiology, methods, prognostic implications, recommendations. Journal für Hypertonie - Austrian Journal of Hypertension 2008;12:7-13.
- Giannattasio C, Mancia G. Arterial distensibility in humans. Modulating mechanisms, alterations in diseases and effects of treatment. J Hypertens 2002;20:1889-99. [Crossref] [PubMed]
- Mancia G, De Backer G, Dominiczak A, et al. 2007 ESH-ESC Practice Guidelines for the Management of Arterial Hypertension: ESH-ESC Task Force on the Management of Arterial Hypertension. J Hypertens 2007;25:1751-62. [Crossref] [PubMed]
- Baulmann J, Nurnberger J, Slany J, et al. Arterial stiffness and pulse wave analysis. Dtsch Med Wochenschr 2010;135 Suppl 1:S4-14. [Crossref] [PubMed]
- Wassertheurer S, Kropf J, Weber T, et al. A new oscillometric method for pulse wave analysis: comparison with a common tonometric method. J Hum Hypertens 2010;24:498-504. [Crossref] [PubMed]
- Elmenhorst J, Hulpke-Wette M, Barta C, et al. Percentiles for central blood pressure and pulse wave velocity in children and adolescents recorded with an oscillometric device. Atherosclerosis 2015;238:9-16. [Crossref] [PubMed]
- Mackenzie IS, Wilkinson IB, Cockcroft JR. Assessment of arterial stiffness in clinical practice. QJM 2002;95:67-74. [Crossref] [PubMed]
- Pandit D, Kinare A, Chiplonkar S, et al. Carotid arterial stiffness in overweight and obese Indian children. J Pediatr Endocrinol Metab 2011;24:97-102. [Crossref] [PubMed]
- Vriz O, Driussi C, La Carrubba S, et al. Comparison of sequentially measured Aloka echo-tracking one-point pulse wave velocity with SphygmoCor carotid-femoral pulse wave velocity. SAGE Open Med 2013;1:2050312113507563. [Crossref] [PubMed]
- Reusz GS, Cseprekal O, Temmar M, et al. Reference values of pulse wave velocity in healthy children and teenagers. Hypertension 2010;56:217-24. [Crossref] [PubMed]
- Böhm B, Elmenhorst J, Müller J, et al. Flow mediated dilation is not associated with established cardiovascular risk factors in healthy children. Eur J Prev Cardiol 2012;19:S66-97.
- Touboul PJ, Hennerici MG, Meairs S, et al. Mannheim carotid intima-media thickness consensus (2004-2006). An update on behalf of the Advisory Board of the 3rd and 4th Watching the Risk Symposium, 13th and 15th European Stroke Conferences, Mannheim, Germany, 2004, and Brussels, Belgium, 2006. Cerebrovasc Dis 2007;23:75-80. [Crossref] [PubMed]
- O'Leary DH, Polak JF, Kronmal RA, et al. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med 1999;340:14-22. [Crossref] [PubMed]
- Baldassarre D, Werba JP, Tremoli E, et al. Common carotid intima-media thickness measurement. A method to improve accuracy and precision. Stroke 1994;25:1588-92. [Crossref] [PubMed]
- Böhm B, Hartmann K, Buck M, et al. Sex differences of carotid intima-media thickness in healthy children and adolescents. Atherosclerosis 2009;206:458-63. [Crossref] [PubMed]
- Weberruß H, Pirzer R, Bohm B, et al. Increased intima-media thickness is not associated with stiffer arteries in children. Atherosclerosis 2015;242:48-55. [Crossref] [PubMed]
- Weberruß H, Pirzer R, Dalla Pozza R, et al. Intima-Media Thickness Does Not Differ between Two Common Carotid Artery Segments in Children. PLoS One 2016;11:e0149057. [Crossref] [PubMed]
- Slyper AH. Clinical review 168: What vascular ultrasound testing has revealed about pediatric atherogenesis, and a potential clinical role for ultrasound in pediatric risk assessment. J Clin Endocrinol Metab 2004;89:3089-95. [Crossref] [PubMed]
- Calle EE, Thun MJ, Petrelli JM, et al. Body-mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med 1999;341:1097-105. [Crossref] [PubMed]
- Ginsberg HN. Insulin resistance and cardiovascular disease. J Clin Invest 2000;106:453-8. [Crossref] [PubMed]
- Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest 2000;106:473-81. [Crossref] [PubMed]
- Freedman DS, Dietz WH, Srinivasan SR, et al. The relation of overweight to cardiovascular risk factors among children and adolescents: the Bogalusa Heart Study. Pediatrics 1999;103:1175-82. [Crossref] [PubMed]
- Must A, Jacques PF, Dallal GE, et al. Long-term morbidity and mortality of overweight adolescents. A follow-up of the Harvard Growth Study of 1922 to 1935. N Engl J Med 1992;327:1350-5. [Crossref] [PubMed]
- Lau DC, Dhillon B, Yan H, et al. Adipokines: molecular links between obesity and atheroslcerosis. Am J Physiol Heart Circ Physiol 2005;288:H2031-41. [Crossref] [PubMed]
- Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature 2006;444:875-80. [Crossref] [PubMed]
- Celermajer DS, Sorensen KE, Bull C, et al. Endothelium-dependent dilation in the systemic arteries of asymptomatic subjects relates to coronary risk factors and their interaction. J Am Coll Cardiol 1994;24:1468-74. [Crossref] [PubMed]
- Lee S, Gungor N, Bacha F, et al. Insulin resistance: link to the components of the metabolic syndrome and biomarkers of endothelial dysfunction in youth. Diabetes Care 2007;30:2091-7. [Crossref] [PubMed]
- Juonala M, Viikari JS, Laitinen T, et al. Interrelations between brachial endothelial function and carotid intima-media thickness in young adults: the cardiovascular risk in young Finns study. Circulation 2004;110:2918-23. [Crossref] [PubMed]
- Tounian P, Aggoun Y, Dubern B, et al. Presence of increased stiffness of the common carotid artery and endothelial dysfunction in severely obese children: a prospective study. Lancet 2001;358:1400-4. [Crossref] [PubMed]
- Iannuzzi A, Licenziati MR, Acampora C, et al. Carotid artery stiffness in obese children with the metabolic syndrome. Am J Cardiol 2006;97:528-31. [Crossref] [PubMed]
- Zebekakis PE, Nawrot T, Thijs L, et al. Obesity is associated with increased arterial stiffness from adolescence until old age. J Hypertens 2005;23:1839-46. [Crossref] [PubMed]
- Juonala M, Jarvisalo MJ, Maki-Torkko N, et al. Risk factors identified in childhood and decreased carotid artery elasticity in adulthood: the Cardiovascular Risk in Young Finns Study. Circulation 2005;112:1486-93. [Crossref] [PubMed]
- Woo KS, Chook P, Yu CW, et al. Overweight in children is associated with arterial endothelial dysfunction and intima-media thickening. Int J Obes Relat Metab Disord 2004;28:852-7. [Crossref] [PubMed]
- Järvisalo MJ, Raitakari M, Toikka JO, et al. Endothelial dysfunction and increased arterial intima-media thickness in children with type 1 diabetes. Circulation 2004;109:1750-5. [Crossref] [PubMed]
- Reinehr T, Kiess W, de Sousa G, et al. Intima media thickness in childhood obesity: relations to inflammatory marker, glucose metabolism, and blood pressure. Metabolism 2006;55:113-8. [Crossref] [PubMed]
- Singh TP, Groehn H, Kazmers A. Vascular function and carotid intimal-medial thickness in children with insulin-dependent diabetes mellitus. J Am Coll Cardiol 2003;41:661-5. [Crossref] [PubMed]
- Stabouli S, Kotsis V, Papamichael C, et al. Adolescent obesity is associated with high ambulatory blood pressure and increased carotid intimal-medial thickness. J Pediatr 2005;147:651-6. [Crossref] [PubMed]
- Zhu W, Huang X, He J, et al. Arterial intima-media thickening and endothelial dysfunction in obese Chinese children. Eur J Pediatr 2005;164:337-44. [Crossref] [PubMed]
- Mangge H, Schauenstein K, Stroedter L, et al. Low grade inflammation in juvenile obesity and type 1 diabetes associated with early signs of atherosclerosis. Exp Clin Endocrinol Diabetes 2004;112:378-82. [Crossref] [PubMed]
- Clarke WR, Woolson RF, Lauer RM. Changes in ponderosity and blood pressure in childhood: the Muscatine Study. Am J Epidemiol 1986;124:195-206. [Crossref] [PubMed]
- Celermajer DS, Sorensen K, Ryalls M, et al. Impaired endothelial function occurs in the systemic arteries of children with homozygous homocystinuria but not in their heterozygous parents. J Am Coll Cardiol 1993;22:854-8. [Crossref] [PubMed]
- Sorensen KE, Celermajer DS, Georgakopoulos D, et al. Impairment of endothelium-dependent dilation is an early event in children with familial hypercholesterolemia and is related to the lipoprotein(a) level. J Clin Invest 1994;93:50-5. [Crossref] [PubMed]
- Wagenknecht LE, Bowden DW, Carr JJ, et al. Familial aggregation of coronary artery calcium in families with type 2 diabetes. Diabetes 2001;50:861-6. [Crossref] [PubMed]
- McEleavy OD, McCallum RW, Petrie JR, et al. Higher carotid-radial pulse wave velocity in healthy offspring of patients with Type 2 diabetes. Diabet Med 2004;21:262-6. [Crossref] [PubMed]
- Golan M, Crow S. Parents are key players in the prevention and treatment of weight-related problems. Nutr Rev 2004;62:39-50. [Crossref] [PubMed]
- WHO. Global Health Observatory - Deaths from cardiovascular disease and diabetes: WHO; 2014 Available online: http://www.who.int/gho/ncd/mortality_morbidity/cvd/en/
- Abbott RA, Harkness MA, Davies PS. Correlation of habitual physical activity levels with flow-mediated dilation of the brachial artery in 5-10 year old children. Atherosclerosis 2002;160:233-9. [Crossref] [PubMed]
- Schack-Nielsen L, Molgaard C, Larsen D, et al. Arterial stiffness in 10-year-old children: current and early determinants. Br J Nutr 2005;94:1004-11. [Crossref] [PubMed]
- Reed KE, Warburton DE, Lewanczuk RZ, et al. Arterial compliance in young children: the role of aerobic fitness. Eur J Cardiovasc Prev Rehabil 2005;12:492-7. [Crossref] [PubMed]
- Meyer AA, Kundt G, Lenschow U, et al. Improvement of early vascular changes and cardiovascular risk factors in obese children after a six-month exercise program. J Am Coll Cardiol 2006;48:1865-70. [Crossref] [PubMed]
- Kallweit C, Moser J, Böhm B, et al. Kardiovaskuläre Risikofaktoren bei übergewichtigen und adipösen Kindern und ihre Veränderung unter Interventionsprogrammen. Monatsschrift Kinderheilkunde 2007;155:69.
- Boreham C, Robson PJ, Gallagher AM, et al. Tracking of physical activity, fitness, body composition and diet from adolescence to young adulthood: The Young Hearts Project, Northern Ireland. Int J Behav Nutr Phys Act 2004;1:14. [Crossref] [PubMed]
- Denham J, Brown NJ, Tomaszewski M, et al. Aortic augmentation index in endurance athletes: a role for cardiorespiratory fitness. Eur J Appl Physiol 2016;116:1537-44. [Crossref] [PubMed]
- Vlachopoulos C, Kardara D, Anastasakis A, et al. Arterial stiffness and wave reflections in marathon runners. Am J Hypertens 2010;23:974-9. [Crossref] [PubMed]
- Schmidt-Trucksäss A, Schmid A, Dorr B, et al. The relationship of left ventricular to femoral artery structure in male athletes. Med Sci Sports Exerc 2003;35:214-9; discussion 220. [Crossref] [PubMed]
- Feairheller DL, Aichele KR, Oakman JE, et al. Vascular Health in American Football Players: Cardiovascular Risk Increased in Division III Players. Int J Vasc Med 2016;2016:6851256. [Crossref] [PubMed]
- Kim JH, Sher S, Wang F, et al. Impact of American-style football participation on vascular function. Am J Cardiol 2015;115:262-7. [Crossref] [PubMed]
- Green DJ, Spence A, Rowley N, et al. Vascular adaptation in athletes: is there an 'athlete's artery'? Exp Physiol 2012;97:295-304. [Crossref] [PubMed]
- Bertovic DA, Waddell TK, Gatzka CD, et al. Muscular strength training is associated with low arterial compliance and high pulse pressure. Hypertension 1999;33:1385-91. [Crossref] [PubMed]
- Siegel AJ, Stec JJ, Lipinska I, et al. Effect of marathon running on inflammatory and hemostatic markers. Am J Cardiol 2001;88:918-20, A9.
- Sharma S, Merghani A, Mont L. Exercise and the heart: the good, the bad, and the ugly. Eur Heart J 2015;36:1445-53. [Crossref] [PubMed]
- Idris NS, Evelein AM, Geerts CC, et al. Effect of physical activity on vascular characteristics in young children. Eur J Prev Cardiol 2015;22:656-64. [Crossref] [PubMed]
- Schuler G, Adams V, Goto Y. Role of exercise in the prevention of cardiovascular disease: results, mechanisms, and new perspectives. Eur Heart J 2013;34:1790-9. [Crossref] [PubMed]
- Möbius-Winkler S, Hilberg T, Menzel K, et al. Time-dependent mobilization of circulating progenitor cells during strenuous exercise in healthy individuals. J Appl Physiol (1985) 2009;107:1943-50. [PubMed]
- Sandri M, Adams V, Gielen S, et al. Effects of exercise and ischemia on mobilization and functional activation of blood-derived progenitor cells in patients with ischemic syndromes: results of 3 randomized studies. Circulation 2005;111:3391-9. [Crossref] [PubMed]
- Whelton SP, Chin A, Xin X, et al. Effect of aerobic exercise on blood pressure: a meta-analysis of randomized, controlled trials. Ann Intern Med 2002;136:493-503. [Crossref] [PubMed]
- Le VV, Mitiku T, Sungar G, et al. The blood pressure response to dynamic exercise testing: a systematic review. Prog Cardiovasc Dis 2008;51:135-60. [Crossref] [PubMed]
- Chen CY, Bonham AC. Postexercise hypotension: central mechanisms. Exerc Sport Sci Rev 2010;38:122-7. [Crossref] [PubMed]
- Fitzgerald W. Labile hypertension and jogging: new diagnostic tool or spurious discovery? Br Med J (Clin Res Ed) 1981;282:542-4. [Crossref] [PubMed]
- Böhm B, Wimmer T, Müller J, et al. Impact of exercise training on arterial wall thickness and distensibility in young competitive athletes. Rome, Italy, 50th Annual Meeting of the Association for European Paediatric and Congenital Cardiology 2016;26:S1-181.
- Treiber F, Papavassiliou D, Gutin B, et al. Determinants of endothelium-dependent femoral artery vasodilation in youth. Psychosom Med 1997;59:376-81. [Crossref] [PubMed]
- Meyer J, Elmenhorst J, Giegerich T, et al. Controversies in the association of cardiorespiratory fitness and arterial stiffness in children and adolescents. Hypertens Res 2017;40:675-8. [Crossref] [PubMed]
- Harwood PG, Asal V. Educating the first digital generation. Westport, USA: Praeger Frederick, 2007.
- Goran MI, Reynolds KD, Lindquist CH. Role of physical activity in the prevention of obesity in children. Int J Obes Relat Metab Disord 1999;23 Suppl 3:S18-33. [Crossref] [PubMed]


