Pre-hepatic and pre-pancreatic transplant donor evaluation
Introduction
The first successful solid organ transplant in humans was performed by Harrison and Murray in 1954, between two identical twins (1). Subsequent work on immunosuppression in the three decades after 1950 revolutionized the area of transplantation, facilitating the work of Starzl when he completed the first successful liver transplant (LT) in 1967 (2-5). In association with improvements in organ preservation and extended donor eligibility, organ transplantation has become an increasingly important and successful component of modern medicine with more than 34,000 transplants performed in the United States alone in 2017 (6). Imaging plays a critical role in organ transplantation as it allows assessment of donors and recipients both before and after transplantation. The growing use of living candidates to meet the demand for organ donors has resulted in frequent imaging of donors during pre-transplantation assessment. These requests are not restricted solely to tertiary transplant centers given the increasing development of regional and national transplant networks. Identification of donor anatomy, variants and any associated pathology is essential for the selection of appropriate surgical candidates and suitable surgical technique. This process has been facilitated by the improved multimodality protocols and techniques available to radiologists. Detailed knowledge and understanding of these options and protocols is critical to ensuring optimal patient outcomes, and ultimately survival. This article will focus on imaging evaluation of donor candidates for liver and pancreas transplantation.
Liver transplantation
First described in 1968, liver transplantation has since become the definitive treatment for end-stage liver disease in suitable recipient candidates (3). As a result, the number of LTs has increased substantially, with 8082 transplants performed in 2017 in the United States (US) compared to 1713 in 1988 according to the United Network for Organ Sharing (UNOS) (7). While living donor liver transplantation (LDLT) has become an increasingly accepted alternative to deceased donors over this period, it still only accounted for 11.5% of LTs in the US in 2014–2016, compared to 0% in 1988 (7-11). However, almost 14,000 candidates remain on the LT waiting list in the US as of May 2018 (12).
Types of liver transplantation
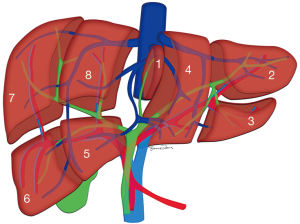
Depending on the nature of the organ used, liver transplantation broadly includes deceased donor transplantation and LDLT (13). Evaluation of the donor liver requires a knowledge of the segmental anatomy of the liver (Figure 1).
Deceased donor transplant
The most commonly performed LT surgical technique involves the replacement of the native liver with a donor liver from a deceased donor. LT can involve the transplant of the whole donor liver into one recipient, or a split liver transplant (SLT), whereby one donor liver is split into independent anatomical segments to allow for two recipients to be transplanted (11). SLT evolved from techniques developed to resize adult donor livers for pediatric recipients (14) and has been considered a means of expanding the donor pool in the context of a limited donor organ supply (15-17). SLT accounted for 1.2% of LT in adults in the US in 2016 (11).
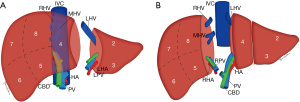
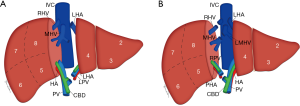
The splitting of the donor liver into separate grafts can be performed in the donor prior to procurement or as a back-table procedure after retrieval, known as in situ and ex vivo techniques, respectively (18). The original technique allowed for the transplant of a child and adult, with the liver split along the falciform ligament into a left lateral segment incorporating segments II and III for the child and a larger tri-segment which includes segments I, IV, V, VI, VII and VIII for the adult (Figure 2A) (19). Subsequent advances have led to the development of a technique that allows for the creation of two comparably sized grafts, one including segments I–IV and the other segments V–VIII. The conventional dissection plane is made to the right of, or along, the middle hepatic vein (MHV) given the smaller volume of the left liver lobe, but alternative approaches and reconstructive techniques have been described in the literature (Figure 2B) (20-23).
Living donor liver transplant
LDLT involves removal of a portion of healthy liver from a matched donor and placement into a recipient. Current techniques and expertise result in an overall donor complication rate of 40%, a major donor complication rate of 1.3% and mortality rate of 0.2% (24-26). The critical factor to be considered when contemplating a LDLT is the health of the donor and that sufficient residual liver volume remains in the donor to avoid donor liver failure after hepatectomy. Sufficient residual liver volume should be at least 30–35% of the donor’s total liver volume depending on the quality of the underlying liver parenchyma (24,27,28). Ensuring that the graft weight/recipient body weight ratio (GWBWR) remains greater than 0.8% is also critical to minimize the risk of the recipient developing small for size syndrome (SFSS) (29). Both considerations influence the approach and technique to the donor hepatectomy.
If a graft is required for a pediatric recipient, a left lateral segmentectomy along the falciform ligament to include segments II and III can be performed as described for the deceased donor graft procurement. An alternate approach for an adult recipient includes a full left hepatectomy with the transection margin passing to the left of, or including, the MHV and sometimes segment I (Figure 3A). However, to avoid SFSS in the recipient, a full right hepatectomy is also a consideration, which involves segment V, VI, VII and VIII, and sometimes segment I (Figure 3B). This transection occurs along the MHV which is sometimes included in the donor graft depending on the risk to the donor (8,27,30).
Liver donor: imaging evaluation
Organ Procurement and Transplantation Network (OPTN) donor evaluation includes anatomical assessment of certain living liver donor parameters; projected graft volume, the donor’s remnant volume, vascular anatomy and determination of hepatic steatosis (31). This is predicated on the fact that up to 11% of potential LDLT donor candidates have been shown to be excluded from consideration for anatomic reasons (32), while another study found that 38% of donor candidates were excluded in total (33). Tailored multidetector computed tomography (MDCT) (Table 1) and magnetic resonance imaging (MRI) (Table 2) studies can be performed to achieve a comprehensive assessment of the donor anatomy (Table 3) (31).

Full table

Full table

Full table
Multiphasic contrast-enhanced CT provides improved spatial resolution relative to MRI, permitting evaluation of the liver parenchyma itself. A sample multiphasic protocol includes a limited non-contrast phase incorporating four 10 mm slices of the mid liver to allow for hepatic steatosis evaluation, an arterial phase of the whole liver with image acquisition triggered by bolus tracking from a region of interest over the aorta to evaluate the arterial anatomy and a portal venous phase of the whole liver acquired 60 seconds after contrast injection to assess the portal and hepatic venous anatomy (Table 1). Both post-contrast phases play a role in assessing for focal liver lesions. Isotropic MDCT data can be post-processed as necessary to allow for the creation of three-dimensional (3-D) image construction, maximum intensity projection and volume rendering. In addition, it allows for more accurate characterization of the hepatic vasculature [hepatic arteries (HAs), portal veins (PVs) and hepatic veins (HVs)] (37).
Dual energy CT (DECT) is now increasingly established in mainstream radiology practice since its commercial release in 2006 (38,39). It has been shown to improve the conspicuity of hypovascular liver metastases, as well as improved diagnostic accuracy and characterization of hypervascular liver lesions (39). In addition, the development of improved virtual enhanced images, as well as a reduced intravenous iodinated contrast requirement, may in future help to reduce donor radiation exposure (40,41).
Newer MRI techniques acquired over shorter periods allow for increasing spatial resolution in studies without any associated radiation exposure to the donor. The availability of hepatobiliary contrast agents also permits the delineation of biliary anatomy, which is particularly important given the discontinuation of the CT cholangiographic contrast agent, iodipamide meglumine (42). These agents, including gadoxetate disodium and gadobenate dimeglumine, allow for sensitivity and specificity of up to 88% and 93% respectively when combined with heavily T2 weighted 2D and reconstructed 3D maximum intensity projection magnetic resonance cholangiograms (43,44). Given the equivalent biliary image quality associated with both agents, gadoxetate disodium (Eovist; Bayer AG, Leverkusen, Germany) is increasingly favored due to its shorter hepatobiliary phase image acquisition timing of 10–20 minutes versus up to 60 minutes for gadobenate dimeglumine (34,45). The use of post-processing MRI techniques, such as multiplanar reconstruction (MPR), allows for the creation of clear images which can be of assistance in defining the anatomy and surgical planning (Figure 4).
In the context of MRI utilization, radiologists must remain cognizant of the consequences of gadolinium administration. While the incidence of adverse reactions is reported to be 0.01–2.4%, lower than that associated with iodinated CT contrast agents, and with an exceedingly low mortality rate of 0.00008% to 0.0019% (46), the use of gadolinium in those patients with renal impairment remains controversial. Nephrogenic system fibrosis (NSF) is a systemic fibrosing process with high morbidity and mortality first described in 1997 and subsequently linked to gadolinium-based contrast agents (GBCAs) in 2006 (47). It is most closely linked with the use of certain linear non-ionic GBCA in the setting of renal dysfunction, either chronic kidney disease (CKD) or acute kidney injury (AKI) (48). However since its identification and characterization, NSF has been almost eliminated after changes in the Food and Drug Administration (FDA) and European Medicines Agency (EMA) regulatory framework (49,50), and subsequently in the European Society of Urogenital Radiology (ESUR) and the American College of Radiology (ACR) guidelines (51,52), resulting in the withdrawal or restriction of the GBCAs of concern and more rigorous evaluation of contrast use in those patients with renal impairment. An ongoing discussion centers on the recently established entity of gadolinium deposition within bone and the central nervous system (CNS), even in the absence of renal impairment or increased blood-brain barrier permeability (53-58). Recent studies have also demonstrated a relationship between the degree of deposition and the numbers of doses and types of GBCAs, particularly linear agents, administered (59). Despite an updated FDA Drug Safety Communication being issued in May 2018 (60), no adverse consequences to tissue deposition of gadolinium have been identified but clinical discretion regarding the judicious use of GBCAs remains important (51).
Hepatic parenchymal evaluation
Imaging of the donor hepatic parenchyma should allow for evaluation for both focal and diffuse hepatic processes. The incidence of hepatic steatosis (HS) amongst donor candidates has been estimated at 25% (61). Moderate-severe HS (30–60% hepatic steatosis) in deceased donor livers is associated with decreased graft survival, while severe HS (>60% hepatic steatosis) is associated with an increased risk of poor graft function and primary graft non-function requiring re-transplantation (62,63). Recipient outcomes using grafts with varying fatty infiltration beneath 30% do not vary significantly and therefore, deceased donor grafts with greater than 30% fatty infiltration are not commonly considered (64-66), while this threshold falls to 10–15% for LDLT grafts at most centers (67). A further influencing factor limiting use of moderate-severe HS LDLT grafts is the increased perioperative mortality and morbidity associated with major hepatectomies (68), and the additional risk to the donor. While liver biopsy remains the gold standard for assessment of HS, it still carries complication and mortality rates of 0.6% and 0.03% respectively (69), and therefore non-invasive assessment is preferred in living donors. CT can be used to quantify moderate-severe HS by a number of means; an absolute liver parenchymal attenuation less than 40 Hounsfield Units (HU), a liver/spleen attenuation ratio of less than 0.8, both on a non-contrast CT, an attenuation of 10 HU or greater less the spleen (35,70,71). Overall, CT demonstrates high accuracy in detecting moderate-severe HS in liver donors (72) but is less effective in quantitative assessment of HS (73). MRI Dixon-based protocols which demonstrate 30% signal drop out on the out of phase sequences relative to in phase sequences normalized to spleen have been shown to effectively differentiate mild-moderate HS from moderate-severe HS (34,74,75). In addition to hepatic steatosis, it is important not to overlook any other parenchymal abnormality such as hemochromatosis, or a focal liver lesion (FLL), such as a cyst, hemangioma, hepatic adenoma or focal nodular hyperplasia. In a cohort of LDLT donors assessed with CT, 25% were shown to have a FLL (76), while a similar study with MR showed 29% with FLL (77), however all were benign. Depending on location and size, diffuse or focal processes can exclude a potential donor from consideration.
Liver volumetry
In LDLT scenarios, liver volumes are calculated to ensure that sufficient liver will be available to both the donor and graft to the recipient to prevent donor liver failure and SFSS in the recipient respectively. Both MRI and MDCT can both be used to calculate volumes as long as there is sufficient tissue contrast and minimal movement artifact (34), with both producing safe estimates (37,78,79). Precise delineation of the transection planes is particularly important in the setting of right liver lobe donation given the absence of clear anatomical landmarks (80).
Vascular evaluation
Assessment of hepatic vascular anatomy in the donor is very important for optimal donor performance after hepatectomy and successful liver transplantation in the recipient. Only 35% of the population has been shown to possess conventional HA, HV and PV anatomy, with variant HA and HV each found in 40% of the population, and variant PV anatomy found in 20% (81). Therefore, knowledge and awareness of variant hepatic vasculature is critical in liver donor evaluation and surgical planning.
Hepatic arterial anatomy
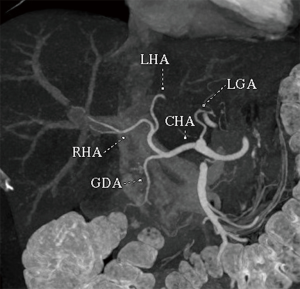
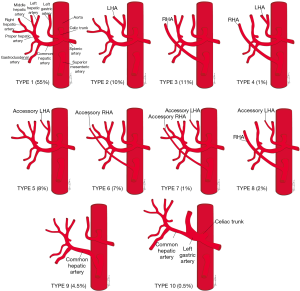
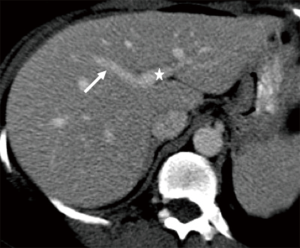
Conventional hepatic arterial anatomy (HAA) is defined as a common hepatic artery arising from the celiac axis supplying the whole liver through right and left hepatic arteries, resulting from normal hepatic embryological development (Figure 4) (82). This normal anatomical configuration has been reported in as low as 55% of the population (83,84). The Michels Classification describes ten anatomic variant categories (Figure 5), but only a subset of these are surgically relevant for donor LT evaluation and are outlined in Table 4 (81,85,86). Accessory HAs are variant arteries present in addition to the conventional HAA while replaced HAs are present in place of the conventional HAA (Figures 6,7). An important variation not covered by the Michels Classification is the origin of the segment IV HA or middle hepatic artery (MHA), a relevant finding for both right and left donor hepatectomy donor candidates (87). These anatomical variants are most relevant in living donors where arterial supply to both the graft and the remnant liver has to be fully maintained. They also are important in deceased donors, to determine the possibility of splitting the liver into two grafts if that was intended, and, in the case of a full liver graft, to determine the arterial reconstruction required to maintain arterial supply. This reconstruction is often performed ex-vivo prior to implanting the graft.

Full table
Portal venous anatomy
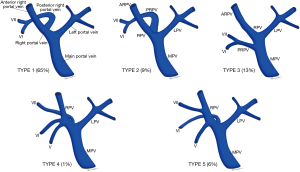
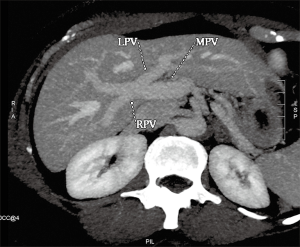
Conventional PV anatomy (PVA) has been reported in 65–80% of the population, but up to a fifth of donor candidates have been excluded from consideration due to PVA variants (84,88,89) (Figure 8). Conventional PVA describes the main portal vein (MPV) formed by the confluence of the splenic vein (SV) and superior mesenteric vein (SMV) before it branches into the right portal vein (RPV) and left portal vein (LPV) at the hilum. The RPV then divides into anterior RPV (ARPV) which supplies segments V and VIII, and the posterior RPV (PRPV) which supplies segments VI and VII. The LPV supplies segments II, III and IV, and branches from both the RPV and LPV supply segment I (90). PV anatomical variants of surgical relevance are detailed in Table 5 (Figures 9,10) (85). Additional surgical considerations are the angle of the MPV bifurcation, with too small an angle allowing for possible compromised portal vein supply in the recipient of whole livers as the donor liver hypertrophies and encases both the RPV and LPV, as well as the length of the MPV and its diameter at the anticipated site of anastomosis, both of which also influence surgical technique (91).

Full table
Hepatic venous anatomy
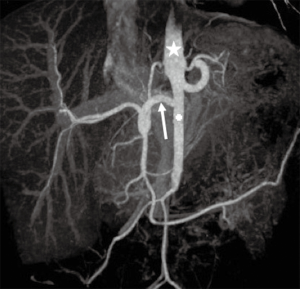
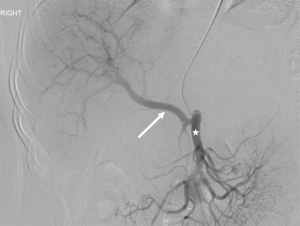
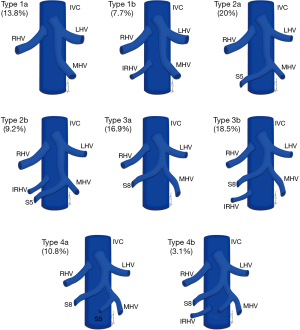
Normal HV anatomy is seen in up to 70% of the population and describes three hepatic veins; the right hepatic vein (RHV) draining segments V, VI, VII and VIII, the MHV draining segments IV, V and VIII, and the left hepatic vein (LHV) draining segments II and III, with segment I draining directly into the inferior vena cava (IVC) (Figure 11) (84,93). Approximately 60% of patients demonstrate common drainage of the LHV and MHV into the IVC. Surgically relevant HV anatomical variants are detailed in Table 6 (92). Pre-operative identification of accessory HVs in the donor is important to minimize excessive bleeding during the transplant hepatectomy, while accurate estimation of their distance from the HV/IVC confluence permits accurate surgical planning as a distance greater than 4 cm often precludes a single anastomosis with the conventional donor HV to the recipient IVC (Figures 12,13) (94). Accessory HVs smaller than 0.3 cm in diameter can be ligated with minimal risk of graft congestion (85). The most common accessory HV, an accessory inferior RHV draining predominantly segments VI and VII directly into the IVC, can be present in up to 47% of donors, while variant segment VIII drainage via a separate accessory vein is seen in 9% (81,87).
Full table
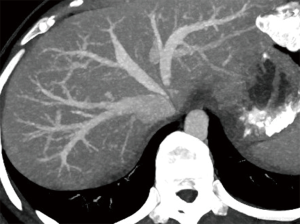
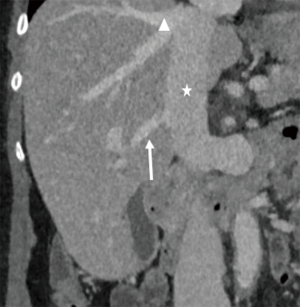
Location and orientation of the HV anatomy is critically important, as the transection plane for a right hepatectomy in LDLT is usually positioned 1.0 cm to the right of the MHV. Pre-operative knowledge of accessory hepatic veins within the donor graft are essential as failure to identify and anastomose these vessels to the recipient structures can potentially lead to hepatic parenchymal congestion, graft failure and/or parenchymal atrophy (81,85).
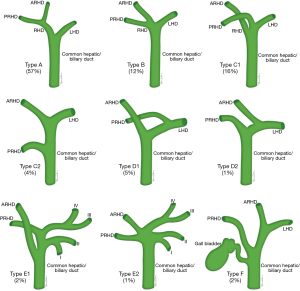
Biliary anatomy
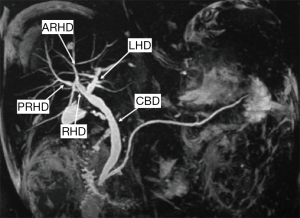
Conventional biliary anatomy is seen in approximately 60–70% of the population (Figures 14,15) (95-99). This describes a right hepatic duct (RHD), comprised of an anterior right hepatic duct (ARHD) draining segments V and VIII and a posterior right hepatic duct (PRHD) draining segments VI and VII, joining with a left hepatic duct (LHD) which drains segments II, III and IV to form a common hepatic duct (CHD). Segment I drains into the more central segments of the LHD or RHD. The cystic duct then joins with the CHD to form the common bile duct (CBD). Biliary duct complications occur in 10–25% of recipients and up to 1.8% of donors (85,100). The risk of biliary complications is increased with the number of anastomoses, HA thrombosis and small hepatic duct caliber (101,102). Bile duct strictures are the most common recipient complication in LT, causing around 40% of all biliary complications, and are seen in up to 5% of deceased donor LT and between 7.4–60% of LDLT depending on the type of donor hepatectomy performed (103). Normal anatomy allows for an uncomplicated single duct-to-duct anastomosis but appropriate pre-operative donor evaluation and tailored surgical techniques allow for the risk of biliary complications to be minimized (104). The relevant biliary anatomy variants are described in Table 7.

Full table
Often overlooked in the overall liver donor evaluation process is the importance of effective, accurate, reproducible and succinct communication of the radiologist’s findings to the transplant surgeon and hepatologist. To facilitate this process, the use of structured reporting can be of benefit, providing for effective communication and referring physician satisfaction (105,106). This also helps to ensure that all relevant information is included in reports, reduces the risk for oversights or errors and ensures that critical findings are conveyed to the referrer (107-109).
Pancreatic transplantation
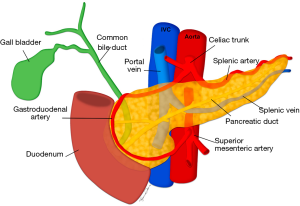
Achieving normoglycemia in insulin-dependent diabetic patients is the primary indication for pancreas transplant (PT), first performed in 1966, with the secondary benefit of limiting the sequelae of uncontrolled diabetes, such as diabetic nephropathy and retinopathy (110-112). While not performed as frequently as LTs, PTs are increasingly performed with 213 performed in the United States in 2017 compared to 78 in 1988, all of which were from deceased donors (7). 75% of PTs are performed simultaneously with kidney transplants, known as simultaneous pancreas-kidney (SPK) transplants, with pancreas after kidney (PAK) transplants and pancreas transplants alone (PTA) making up the remainder with 18% and 7% respectively (113). Given the lack of living donors, imaging plays no formal role in pre-transplant donor evaluation, with intra-operative appraisal by the transplant surgeon the most important assessment (114). However in limited situations, prior imaging of a deceased donor may be available for review and knowledge of the surgical techniques used and salient vascular anatomy may be beneficial if the radiologist’s opinion is sought (Figure 16). The graft can also be assessed for fibrosis, fatty infiltration and calcification.
The donor pancreas is procured with the adjacent duodenal stump including the ampulla of Vater and local vasculature, as well as the donor common, internal and external iliac arteries in continuity, to allow the creation of an arterial Y graft (115). The Y graft is used to anastomose the donor superior mesenteric artery (SMA) to the donor external iliac artery to supply the pancreatic head, and the internal iliac artery to the splenic artery to supply the pancreatic body and tail. The donor common iliac artery is then anastomosed to a recipient common or external iliac artery to facilitate arterial blood supply to the graft. The donor portal vein is also resected intact to facilitate superior mesenteric and splenic vein drainage, and this is anastomosed to the recipient superior mesenteric vein (SMV) or an iliac vein in portal or systemic venous techniques respectively (116-118).
The two most prevalent surgical techniques in use currently include systemic venous-enteric exocrine drainage (Figure 17A), where the donor portal vein and recipient common or external iliac vein are anastomosed for systemic venous drainage while the donor duodenum is anastomosed to a recipient jejunal loop to allow for exocrine drainage and portal venous-enteric drainage (Figure 17B) where the donor portal vein is anastomosed to the recipient SMV. Rarely used is the bladder drainage of the donor duodenum (Figure 17C), given the complications associated with fluid and bicarbonate loss in the urine and cystitis secondary to the pancreatic enzymes effect in the bladder (116-119).
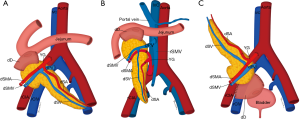
As a result, it may be of some benefit to the radiologist’s surgical colleagues if it is possible to establish the patency of the relevant arterial and venous structures on prior imaging, as well as the infrequent presence of relevant SMA variant anatomy, such as a common origin of the celiac trunk and SMA (120).
Conclusions
Imaging plays a critical role in the evaluation of liver donors prior to transplantation allowing for assessment of hepatic parenchymal, biliary and vascular anatomy. Adequate knowledge and understanding of these anatomic considerations is required for radiologists to provide precise information to the surgeons for preoperative planning to enable successful transplantation.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Harrison JH, Merrill JP, Murray JE. Renal homotransplantation in identical twins. Surg Forum 1956;6:432-6. [PubMed]
- Dangoor JY, Hakim DN, Singh RP, et al. Transplantation: a brief history. Exp Clin Transplant 2015;13:1-5. [PubMed]
- Starzl TE, Groth CG, Brettschneider L, et al. Orthotopic homotransplantation of the human liver. Ann Surg 1968;168:392-415. [Crossref] [PubMed]
- Starzl TE, Porter KA, Putnam CW, et al. Orthotopic liver transplantation in ninety-three patients. Surg Gynecol Obstet 1976;142:487-505. [PubMed]
- Porrett PM, Hashmi SK, Shaked A. Immunosuppression: trends and tolerance? Clin Liver Dis 2014;18:687-716. [Crossref] [PubMed]
- Sharing UNfO. Transplant trends. In: United Network for Organ Sharing, 2018.
- Sharing UNfO. Transplant trends. In: United Network for Organ Sharing: United Network for Organ Sharing, 2017.
- Tulla KA, Jeon H. Living Donor Liver Transplantation: Technical Innovations. Gastroenterol Clin North Am 2018;47:253-65. [Crossref] [PubMed]
- Yamaoka Y, Washida M, Honda K, et al. Liver transplantation using a right lobe graft from a living related donor. Transplantation 1994;57:1127-30. [Crossref] [PubMed]
- Vagefi PA, Ascher NL, Freise CE, et al. Use of living donor liver transplantation varies with the availability of deceased donor liver transplantation. Liver Transpl 2012;18:160-5. [Crossref] [PubMed]
- Kim WR, Lake JR, Smith JM, et al. OPTN/SRTR 2016 Annual Data Report: Liver. Am J Transplant 2018;18 Suppl 1:172-253. [Crossref] [PubMed]
- Network OPaT. National Data. In: U.S. Department of Health & Human Services, 2018.
- Eghtesad B, Kadry Z, Fung J. Technical considerations in liver transplantation: what a hepatologist needs to know (and every surgeon should practice). Liver Transpl 2005;11:861-71. [Crossref] [PubMed]
- Bismuth H, Houssin D. Reduced-sized orthotopic liver graft in hepatic transplantation in children. Surgery 1984;95:367-70. [PubMed]
- Busuttil RW, Goss JA. Split liver transplantation. Ann Surg 1999;229:313-21. [Crossref] [PubMed]
- Halac E, Dip M, Quinonez E, et al. Split liver transplantation: Report of right and left graft outcomes from a multicenter Argentinean group. Liver Transpl 2016;22:63-70. [Crossref] [PubMed]
- Vagefi PA, Parekh J, Ascher NL, et al. Outcomes with split liver transplantation in 106 recipients: the University of California, San Francisco, experience from 1993 to 2010. Arch Surg 2011;146:1052-9. [Crossref] [PubMed]
- Liu H, Li R, Fu J, et al. Technical Skills Required in Split Liver Transplantation. Ann Transplant 2016;21:408-15. [Crossref] [PubMed]
- Hashimoto K, Fujiki M, Quintini C, et al. Split liver transplantation in adults. World J Gastroenterol 2016;22:7500-6. [Crossref] [PubMed]
- Vagefi PA, Parekh J, Ascher NL, et al. Ex vivo split-liver transplantation: the true right/left split. HPB (Oxford) 2014;16:267-74. [Crossref] [PubMed]
- Emre S, Umman V. Split liver transplantation: an overview. Transplant Proc 2011;43:884-7. [Crossref] [PubMed]
- Ferla F, Lauterio A, Di Sandro S, et al. Split-liver full-left full-right: proposal for an operative protocol. Transplant Proc 2014;46:2279-82. [Crossref] [PubMed]
- Sguinzi R, Ferla F, De Carlis R, et al. Split liver technique with middle hepatic vein reconstruction on livers from transplant hepatectomies: a useful tool for surgical improvement. Updates Surg 2018;70:491-4. [Crossref] [PubMed]
- Hwang S, Lee SG, Lee YJ, et al. Lessons learned from 1,000 living donor liver transplantations in a single center: how to make living donations safe. Liver Transpl 2006;12:920-7. [Crossref] [PubMed]
- Fisher RA. Living donor liver transplantation: eliminating the wait for death in end-stage liver disease? Nat Rev Gastroenterol Hepatol 2017;14:373-82. [Crossref] [PubMed]
- Abecassis MM, Fisher RA, Olthoff KM, et al. Complications of living donor hepatic lobectomy--a comprehensive report. Am J Transplant 2012;12:1208-17. [Crossref] [PubMed]
- Lee SG. A complete treatment of adult living donor liver transplantation: a review of surgical technique and current challenges to expand indication of patients. Am J Transplant 2015;15:17-38. [Crossref] [PubMed]
- Marcos A, Ham JM, Fisher RA, et al. Single-center analysis of the first 40 adult-to-adult living donor liver transplants using the right lobe. Liver Transpl 2000;6:296-301. [Crossref] [PubMed]
- Hill MJ, Hughes M, Jie T, et al. Graft weight/recipient weight ratio: how well does it predict outcome after partial liver transplants? Liver Transpl 2009;15:1056-62. [Crossref] [PubMed]
- Jung DH, Hwang S, Song GW, et al. In Situ Split Liver Transplantation for 2 Adult Recipients: A Single-Center Experience. Ann Transplant 2017;22:230-40. [Crossref] [PubMed]
- Network OPaT. Organ Procurement and Transplantation Network Policies. In: Organ Procurement and Transplantation Network, 2018.
- Tsang LL, Chen CL, Huang TL, et al. Preoperative imaging evaluation of potential living liver donors: reasons for exclusion from donation in adult living donor liver transplantation. Transplant Proc 2008;40:2460-2. [Crossref] [PubMed]
- Hahn LD, Emre SH, Israel GM. Radiographic features of potential donor livers that precluded donation. AJR Am J Roentgenol 2014;202:W343-8. [Crossref] [PubMed]
- Jhaveri KS, Guo L, Guimaraes L. Current State-of-the-Art MRI for Comprehensive Evaluation of Potential Living Liver Donors. AJR Am J Roentgenol 2017;209:55-66. [Crossref] [PubMed]
- Zhang YN, Fowler KJ, Hamilton G, et al. Liver fat imaging - a clinical overview of ultrasound, CT, and MR imaging. Br J Radiol 2018;91:20170959. [Crossref] [PubMed]
- Lee MW, Lee JM, Lee JY, et al. Preoperative evaluation of the hepatic vascular anatomy in living liver donors: comparison of CT angiography and MR angiography. J Magn Reson Imaging 2006;24:1081-7. [Crossref] [PubMed]
- Schroeder T, Malago M, Debatin JF, et al. "All-in-one" imaging protocols for the evaluation of potential living liver donors: comparison of magnetic resonance imaging and multidetector computed tomography. Liver Transpl 2005;11:776-87. [Crossref] [PubMed]
- McCollough CH, Leng S, Yu L, et al. Dual- and Multi-Energy CT: Principles, Technical Approaches, and Clinical Applications. Radiology 2015;276:637-53. [Crossref] [PubMed]
- Lestra T, Mule S, Millet I, et al. Applications of dual energy computed tomography in abdominal imaging. Diagn Interv Imaging 2016;97:593-603. [Crossref] [PubMed]
- Chung YE, You JS, Lee HJ, et al. Possible Contrast Media Reduction with Low keV Monoenergetic Images in the Detection of Focal Liver Lesions: A Dual-Energy CT Animal Study. PloS One 2015;10:e0133170. [Crossref] [PubMed]
- De Cecco CN, Muscogiuri G, Schoepf UJ, et al. Virtual unenhanced imaging of the liver with third-generation dual-source dual-energy CT and advanced modeled iterative reconstruction. Eur J Radiol 2016;85:1257-64. [Crossref] [PubMed]
- Administration USFaD. FDA Drug Shortages. In: U.S. Department of Health and Human Services, 2018.
- Artioli D, Tagliabue M, Aseni P, et al. Detection of biliary and vascular anatomy in living liver donors: value of gadobenate dimeglumine enhanced MR and MDCT angiography. Eur J Radiol 2010;76:e1-5. [Crossref] [PubMed]
- Lewis S, Vasudevan P, Chatterji M, et al. Comparison of gadoxetic acid to gadobenate dimeglumine for assessment of biliary anatomy of potential liver donors. Abdom Radiol (NY) 2016;41:1300-9. [Crossref] [PubMed]
- Lee MS, Lee JY, Kim SH, et al. Gadoxetic acid disodium-enhanced magnetic resonance imaging for biliary and vascular evaluations in preoperative living liver donors: comparison with gadobenate dimeglumine-enhanced MRI. J Magn Reson Imaging 2011;33:149-59. [Crossref] [PubMed]
- Ramalho M, Ramalho J. Gadolinium-Based Contrast Agents: Associated Adverse Reactions. Magn Reson Imaging Clin N Am 2017;25:755-64. [Crossref] [PubMed]
- Kaewlai R, Abujudeh H. Nephrogenic systemic fibrosis. AJR Am J Roentgenol 2012;199:W17-23. [Crossref] [PubMed]
- Daftari Besheli L, Aran S, Shaqdan K, et al. Current status of nephrogenic systemic fibrosis. Clinical radiology 2014;69:661-8. [Crossref] [PubMed]
- Administration FaD. FDA Drug Safety Communication: New warnings for using gadolinium-based contrast agents in patients with kidney dysfunction. In: Silver Spring M, ed.: Federal Drug Administration, 2010.
- European Medicines Agency. Questions and answers on the review of gadolinium-containing contrast agents. 2010:3. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/gadolinium_31/WC500015635.pdf
- Radiology ACo. ACR Manual on Contrast Media Version 10.3, 2018.
- Radiology ESoU. 10.0 Contrast Media Safety Guidelines, 2018.
- McDonald RJ, McDonald JS, Kallmes DF, et al. Intracranial Gadolinium Deposition after Contrast-enhanced MR Imaging. Radiology 2015;275:772-82. [Crossref] [PubMed]
- Kanda T, Fukusato T, Matsuda M, et al. Gadolinium-based Contrast Agent Accumulates in the Brain Even in Subjects without Severe Renal Dysfunction: Evaluation of Autopsy Brain Specimens with Inductively Coupled Plasma Mass Spectroscopy. Radiology 2015;276:228-32. [Crossref] [PubMed]
- Kanda T, Osawa M, Oba H, et al. High Signal Intensity in Dentate Nucleus on Unenhanced T1-weighted MR Images: Association with Linear versus Macrocyclic Gadolinium Chelate Administration. Radiology 2015;275:803-9. [Crossref] [PubMed]
- Gibby WA, Gibby KA, Gibby WA. Comparison of Gd DTPA-BMA (Omniscan) versus Gd HP-DO3A (ProHance) retention in human bone tissue by inductively coupled plasma atomic emission spectroscopy. Invest Radiol 2004;39:138-42. [Crossref] [PubMed]
- Kanda T, Ishii K, Kawaguchi H, et al. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 2014;270:834-41. [Crossref] [PubMed]
- McDonald RJ, McDonald JS, Kallmes DF, et al. Gadolinium Deposition in Human Brain Tissues after Contrast-enhanced MR Imaging in Adult Patients without Intracranial Abnormalities. Radiology 2017;285:546-54. [Crossref] [PubMed]
- Kang H, Hii M, Le M, et al. Gadolinium Deposition in Deep Brain Structures: Relationship with Dose and Ionization of Linear Gadolinium-Based Contrast Agents. AJNR Am J Neuroradiol 2018;39:1597-603. [Crossref] [PubMed]
- Administration FaD. FDA Drug Safety Communication: FDA warns that gadolinium-based contrast agents (GBCAs) are retained in the body; requires new class warnings. In, 2018.
- Selzner M, Clavien PA. Fatty liver in liver transplantation and surgery. Semin Liver Dis 2001;21:105-13. [Crossref] [PubMed]
- Chu MJ, Dare AJ, Phillips AR, et al. Donor Hepatic Steatosis and Outcome After Liver Transplantation: a Systematic Review. J Gastrointest Surg 2015;19:1713-24. [Crossref] [PubMed]
- Spitzer AL, Lao OB, Dick AA, et al. The biopsied donor liver: incorporating macrosteatosis into high-risk donor assessment. Liver Transpl 2010;16:874-84. [Crossref] [PubMed]
- McCormack L, Dutkowski P, El-Badry AM, et al. Liver transplantation using fatty livers: always feasible? J Hepatol 2011;54:1055-62. [Crossref] [PubMed]
- Kwon CH, Joh JW, Lee KW, et al. Safety of donors with fatty liver in liver transplantation. Transplant Proc 2006;38:2106-7. [Crossref] [PubMed]
- Cho JY, Suh KS, Kwon CH, et al. Mild hepatic steatosis is not a major risk factor for hepatectomy and regenerative power is not impaired. Surgery 2006;139:508-15. [Crossref] [PubMed]
- Zezos P, Renner EL. Liver transplantation and non-alcoholic fatty liver disease. World J Gastroenterol 2014;20:15532-8. [Crossref] [PubMed]
- Behrns KE, Tsiotos GG, DeSouza NF, et al. Hepatic steatosis as a potential risk factor for major hepatic resection. J Gastrointest Surg 1998;2:292-8. [Crossref] [PubMed]
- Garcia-Tsao G, Boyer JL. Outpatient liver biopsy: how safe is it? Ann Intern Med 1993;118:150-3. [Crossref] [PubMed]
- Limanond P, Raman SS, Lassman C, et al. Macrovesicular hepatic steatosis in living related liver donors: correlation between CT and histologic findings. Radiology 2004;230:276-80. [Crossref] [PubMed]
- Cheng YF, Yu CY, Ou HY, et al. Section 1. Image evaluation of fatty liver in living donor liver transplantation. Transplantation 2014;97 Suppl 8:S3-6. [Crossref] [PubMed]
- Zheng D, Tian W, Zheng Z, et al. Accuracy of computed tomography for detecting hepatic steatosis in donors for liver transplantation: A meta-analysis. Clin Transplant 2017.31. [PubMed]
- Park SH, Kim PN, Kim KW, et al. Macrovesicular hepatic steatosis in living liver donors: use of CT for quantitative and qualitative assessment. Radiology 2006;239:105-12. [Crossref] [PubMed]
- Kreft BP, Tanimoto A, Baba Y, et al. Diagnosis of fatty liver with MR imaging. J Magn Reson Imaging 1992;2:463-71. [Crossref] [PubMed]
- Idilman IS, Aniktar H, Idilman R, et al. Hepatic steatosis: quantification by proton density fat fraction with MR imaging versus liver biopsy. Radiology 2013;267:767-75. [Crossref] [PubMed]
- Schroeder T, Radtke A, Kuehl H, et al. Evaluation of living liver donors with an all-inclusive 3D multi-detector row CT protocol. Radiology 2006;238:900-10. [Crossref] [PubMed]
- Fulcher AS, Szucs RA, Bassignani MJ, et al. Right lobe living donor liver transplantation: preoperative evaluation of the donor with MR imaging. AJR Am J Roentgenol 2001;176:1483-91. [Crossref] [PubMed]
- Emiroglu R, Coskun M, Yilmaz U, et al. Safety of multidetector computed tomography in calculating liver volume for living-donor liver transplantation. Transplant Proc 2006;38:3576-8. [Crossref] [PubMed]
- D'Onofrio M, De Robertis R, Demozzi E, et al. Liver volumetry: Is imaging reliable? Personal experience and review of the literature. World J Radiol 2014;6:62-71. [Crossref] [PubMed]
- Ishifuro M, Horiguchi J, Nakashige A, et al. Use of multidetector row CT with volume renderings in right lobe living liver transplantation. Eur Radiol 2002;12:2477-83. [Crossref] [PubMed]
- Erbay N, Raptopoulos V, Pomfret EA, et al. Living donor liver transplantation in adults: vascular variants important in surgical planning for donors and recipients. AJR Am J Roentgenol 2003;181:109-14. [Crossref] [PubMed]
- Couinaud C. Surgical anatomy of the liver revisited. Embryology, 1989.
- Michels NA. Newer anatomy of the liver and its variant blood supply and collateral circulation. Am J Surg 1966;112:337-47. [Crossref] [PubMed]
- Kamel IR, Kruskal JB, Pomfret EA, et al. Impact of multidetector CT on donor selection and surgical planning before living adult right lobe liver transplantation. AJR Am J Roentgenol 2001;176:193-200. [Crossref] [PubMed]
- Hennedige T, Anil G, Madhavan K. Expectations from imaging for pre-transplant evaluation of living donor liver transplantation. World J Radiol 2014;6:693-707. [Crossref] [PubMed]
- Catalano OA, Singh AH, Uppot RN, et al. Vascular and biliary variants in the liver: implications for liver surgery. Radiographics 2008;28:359-78. [Crossref] [PubMed]
- Kamel IR, Kruskal JB, Raptopoulos V. Imaging for right lobe living donor liver transplantation. Semin Liver Dis 2001;21:271-82. [Crossref] [PubMed]
- Sureka B, Patidar Y, Bansal K, et al. Portal vein variations in 1000 patients: surgical and radiological importance. Br J Radiol 2015;88:20150326. [Crossref] [PubMed]
- Covey AM, Brody LA, Getrajdman GI, et al. Incidence, patterns, and clinical relevance of variant portal vein anatomy. AJR Am J Roentgenol 2004;183:1055-64. [Crossref] [PubMed]
- Mathew RP, Venkatesh SK. Liver vascular anatomy: a refresher. Abdom Radiol (NY) 2018;43:1886-95. [Crossref] [PubMed]
- Sahani D, D'Souza R, Kadavigere R, et al. Evaluation of living liver transplant donors: method for precise anatomic definition by using a dedicated contrast-enhanced MR imaging protocol. Radiographics 2004;24:957-67. [Crossref] [PubMed]
- Varotti G, Gondolesi GE, Goldman J, et al. Anatomic variations in right liver living donors. J Am Coll Surg 2004;198:577-82. [Crossref] [PubMed]
- Soyer P, Heath D, Bluemke DA, et al. Three-dimensional helical CT of intrahepatic venous structures: comparison of three rendering techniques. J Comput Assist Tomogr 1996;20:122-7. [Crossref] [PubMed]
- Sahani D, Mehta A, Blake M, et al. Preoperative hepatic vascular evaluation with CT and MR angiography: implications for surgery. Radiographics 2004;24:1367-80. [Crossref] [PubMed]
- Brunicardi FAD, Billiar TR, Dunn DL, et al. Liver. In: Principles of Surgery. McGraw-Hill Professional Publishing, 2014.
- Mortelé KJ, Ros PR. Anatomic variants of the biliary tree: MR cholangiographic findings and clinical applications. AJR Am J Roentgenol 2001;177:389-94. [Crossref] [PubMed]
- Xu X, Wei X, Ling Q, et al. Inaccurate preoperative imaging assessment on biliary anatomy not increases biliary complications after living donor liver transplantation. Eur J Radiol 2012;81:e457-60. [Crossref] [PubMed]
- Choi JW, Kim TK, Kim KW, et al. Anatomic variation in intrahepatic bile ducts: an analysis of intraoperative cholangiograms in 300 consecutive donors for living donor liver transplantation. Korean J Radiol 2003;4:85-90. [Crossref] [PubMed]
- Macdonald DB, Haider MA, Khalili K, et al. Relationship between vascular and biliary anatomy in living liver donors. AJR Am J Roentgenol 2005;185:247-52. [Crossref] [PubMed]
- Lee JG, Lee KW, Kwon CHD, et al. Donor safety in living donor liver transplantation: The Korean organ transplantation registry study. Liver Transpl 2017;23:999-1006. [Crossref] [PubMed]
- Jeong S, Wang X, Wan P, et al. Risk factors and survival outcomes of biliary complications after adult-to-adult living donor liver transplantation. United European Gastroenterol J 2017;5:997-1006. [Crossref] [PubMed]
- Alawi K, Khalaf H, Medhat Y, et al. Risk factors for biliary complications after living-donor liver transplant: a single-center experience. Exp Clin Transplant 2008;6:101-4. [PubMed]
- Rao HB, Prakash A, Sudhindran S, et al. Biliary strictures complicating living donor liver transplantation: Problems, novel insights and solutions. World J Gastroenterol 2018;24:2061-72. [Crossref] [PubMed]
- Nakamura T, Tanaka K, Kiuchi T, et al. Anatomical variations and surgical strategies in right lobe living donor liver transplantation: lessons from 120 cases. Transplantation 2002;73:1896-903. [Crossref] [PubMed]
- Powell DK, Silberzweig JE. State of structured reporting in radiology, a survey. Acad Radiol 2015;22:226-33. [Crossref] [PubMed]
- Larson DB, Towbin AJ, Pryor RM, et al. Improving consistency in radiology reporting through the use of department-wide standardized structured reporting. Radiology 2013;267:240-50. [Crossref] [PubMed]
- Marcovici PA, Taylor GA. Journal Club: Structured radiology reports are more complete and more effective than unstructured reports. AJR Am J Roentgenol 2014;203:1265-71. [Crossref] [PubMed]
- Hawkins CM, Hall S, Zhang B, et al. Creation and implementation of department-wide structured reports: an analysis of the impact on error rate in radiology reports. J Digit Imaging 2014;27:581-7. [Crossref] [PubMed]
- Buckley BW, Daly L, Allen GN, et al. Recall of structured radiology reports is significantly superior to that of unstructured reports. Br J Radiol 2018;91:20170670. [Crossref] [PubMed]
- Kelly WD, Lillehei RC, Merkel FK, et al. Allotransplantation of the pancreas and duodenum along with the kidney in diabetic nephropathy. Surgery 1967;61:827-37. [PubMed]
- Gruessner RW, Gruessner AC. What defines success in pancreas and islet transplantation-insulin independence or prevention of hypoglycemia? A review. Transplant Proc 2014;46:1898-9. [Crossref] [PubMed]
- Heller MT, Bhargava P. Imaging in pancreatic transplants. Indian J Radiol Imaging 2014;24:339-49. [Crossref] [PubMed]
- Gruessner AC. 2011 update on pancreas transplantation: comprehensive trend analysis of 25,000 cases followed up over the course of twenty-four years at the International Pancreas Transplant Registry (IPTR). Rev Diabet Stud 2011;8:6-16. [Crossref] [PubMed]
- Tolat PP, Foley WD, Johnson C, et al. Pancreas transplant imaging: how I do it. Radiology 2015;275:14-27. [Crossref] [PubMed]
- Gallego Ferrero P, Crespo Del Pozo J. Imaging in pancreas transplantation complications: Temporal classification. J Med Imaging Radiat Oncol 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Freund MC, Steurer W, Gassner EM, et al. Spectrum of imaging findings after pancreas transplantation with enteric exocrine drainage: Part 1, posttransplantation anatomy. AJR Am J Roentgenol 2004;182:911-7. [Crossref] [PubMed]
- Fridell JA, Powelson JA, Kubal CA, et al. Retrieval of the pancreas allograft for whole-organ transplantation. Clin Transplant 2014;28:1313-30. [Crossref] [PubMed]
- Vandermeer FQ, Manning MA, Frazier AA, et al. Imaging of whole-organ pancreas transplants. Radiographics 2012;32:411-35. [Crossref] [PubMed]
- Rogers J, Farney AC, Orlando G, et al. Pancreas transplantation with portal venous drainage with an emphasis on technical aspects. Clin Transplant 2014;28:16-26. [Crossref] [PubMed]
- Farghadani M, Momeni M, Hekmatnia A, et al. Anatomical variation of celiac axis, superior mesenteric artery, and hepatic artery: Evaluation with multidetector computed tomography angiography. J Res Med Sci 2016;21:129. [Crossref] [PubMed]