
Folate supplementation for prevention of congenital heart defects and low birth weight: an update
Introduction
Maternal folate insufficiency during early pregnancy is associated with increased risk of anaemia, pregnancy complications and birth defects. The biological functions of folate “Lucy factor” have been discovered in the late 1920th. In particular, the role of folate during pregnancy has been discovered by the British scientist Lucy Wills [1888–1964] through her pioneering survey studies in Hindu Indian women. Women of a particularly poor population subgroup manifested severe anemia during pregnancy, early abortions, and malformed births. The vitamin folate has been discovered and purified in 1945, only few years before the discovery of another important B-vitamin, vitamin B12 (cobalamin). Due to the interaction between folate and B12 metabolisms, also vitamin B12 deficiency causes megaloblastic anaemia that is not treatable with folate. During the first 2 decades after folate discovery, large doses of folic acid have been used to treat vitamin B12-deficiency with variable success in correcting anaemia and/or neurological manifestations. Later, specific folate and vitamin B12 assays in blood have become available. Anaemia is no longer considered a diagnostic test for folate or B12 deficiencies.
Dietary folate (mainly as 5-methyltetrahydrofolate) serves as methyl donor in the cells. It provides a methyl group to homocysteine that is converted to methionine simultaneously releasing tetrahydrofolate (Figure 1). Tetrahydrofolate is further converted to be used for nucleotides synthesis (mainly from formyltetrahydrofolate derivatives). The folate cycle (exists in the cytosol, mitochondria, and cell nucleus) ensures the transfer of C1-units (glycine, serine, and format) between these cell compartments. Folate metabolism is particularly active during cell division such as in pregnancy and early childhood.
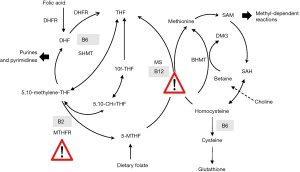
Vitamin B12 is necessary for the transfer of methyl groups form methylfolate to homocysteine. Thus, in individuals with vitamin B12 deficiency, folate becomes trapped and not further utilized to produce nucleotides thus leading to cell growth arrest (1,2). Avoidance of vitamin B12 deficiency and a balance between folate and vitamin B12 are necessary in women of reproductive age.
The importance of sufficient folate status during pregnancy has gained attention since the discovery of the vitamin (3). Experimental folate deficiency in pregnant rats (dietary or genetic models) causes foetal death or congenital malformation. These defects are known to be sensitive to low folate at early pregnancy. Epidemiological studies in populations with prevalent folate deficiency have shown that a clinically-manifested folate deficiency was often diagnosed (or detected) at late pregnancy possibly reflecting that folate depletion occurred over the previous months (at early pregnancy). However, the literature is not conclusive regarding the prevalence of undetected folate-deficiency anaemia at early or mid-pregnancy. Folate insufficiency is believed to influence embryogenesis even before becoming clinically manifested (i.e., anaemia) in the mother.
Since experimental folate deficiency in animals can cause abortions or congenital malformations (4), and since there is an association between low folate and the incidence of congenital defects in human (5), it has been assumed that low folate could be an aetiological factor in congenital heart defects (4).
Folate prevents many neural tube defects (NTDs) (primary outcome)
Folate deficiency during early pregnancy is associated with a particularly severe form of congenital malformations called NTDs (6-10). In the folate-sensitive NTDs, the development of the central nervous system is arrested during early pregnancy and the neural tube remains open. Spina bifida is a less severe form of the disease, it occurs when the neural tube opening in the lower part of the spinal cord fails to close at around the 25–28 days after conception. Live births with spina bifida have high morbidities and mortalities compared with healthy children (11). The risk of NTDs is particularly high in women who already experienced this outcome in a previous pregnancy (recurrent NTDs) (12).
Two randomized controlled trials (RCTs) using folic acid (dose 0.8 and 4 mg/d) as part of multivitamin mix were conducted in the early 1990s. In 1991, the MRC study in women with a previous pregnancy affected with NTD (i.e., high-risk group) found that a high-dose folic acid (4 mg/d) can reduce the risk of recurrent NTD by 72% [relative risk (RR) =0.28, 95% confidence interval (95% CI): 0.12–0.71] (12). In a primary prevention RCT (1992), supplemental 800 µg/d folic acid or multivitamins without folic acid was administered to 4,753 Hungarian pregnant women (13). Czeizel et al. have shown that all NTD cases could be prevented in the women who received 800 g/d folic acid vs. 6 cases occurred in the multivitamin arm without folic acid (P=0.029) (13). After this Hungarian primary prevention trial (13), the lowest dose of supplemental folate that can prevent all folate-responsive NTDs (cases that can be prevented by folate) has never been established. Observational studies over many years supported the notion that sufficient folate status at early pregnancy reduces neonatal mortality from NTDs (14). The definition of sufficiency has not been agreed on until recently. Women of reproductive age are the main target group of all public health interventions aiming at preventing NTDs. Nevertheless, there are still NTD cases that do not respond to folate supplements.
Today, women of reproductive age are recommended to achieve sufficiently high red blood cell (RBC)-folate levels mainly by taking supplemental folate (from multivitamin pills or fortified foods) for a sufficiently long period prior to closure of the neural tube. Optimal RBC-folate concentrations for prevention of NTDs have been recently defined [>906 nmol/L using a microbiological assay, World Health Organization (WHO, 2015)] (15). This threshold of RBC-folate concentrations is markedly higher than the population mean or the mean of levels that can be achieved by consuming a regular non-fortified diet. In a Bayesian regression model on population RBC-folate and NTD risk, Crider et al. have shown that the risk of NTD declines from 48/10,000 in women with RBC-folate concentrations <340 nmol/L to 5/10,000 in women with RBC-folate of approximately 1,200 nmol/L (16). In general, the cutoff for RBC-folate is not meant to predict the individual NTD risk in women, since NTD may still occur even when folate status is high (folate-non-responsive NTD). However, the average RBC-folate of a population provides a good estimate of the risk of NTDs in that population (17).
Supplementation of folic acid before and during early pregnancy is strongly recommended but not widely practiced (especially preconceptionally) (18). Many countries have applied mandatory fortification of staple foods with folic acid in order to raise folate status in young women in the time of conception. The primary aim of this public health strategy is to reduce the prevalence of NTDs among live births. All available reports have shown a reduction of NTDs following the fortification with folic acid (19). The molecular mechanisms of folate deficiency during pregnancy in the pathophysiology of birth defects are not clear yet. NTDs account for a relatively small number of congenital defects. For example, the prevalence of NTDs among live births in Germany is approximately 12/10,000. At an annual birth rate of approximately 800,000 births, it is estimated that 960 cases per year would be affected and at least 50% of these cases can be prevented by folate supplementation.
Influence of folic acid supplementation on secondary outcomes
Recent studies have shown that high folate intake is associated with a reduced risk of birth defects other than NTDs. Higher maternal folate or periconceptional use of folic acid is associated with a lower risk of congenital heart defects (20-23) and oral clefts (24). A recent meta-analysis of 1 randomized controlled trial, 1 cohort study, and 16 case-control studies has shown that maternal folate supplementation is associated with a lowered CHD risk (RR =0.72, 95% CI: 0.63–0.82) (25). However, the results showed considerable heterogeneity, but after excluding the outliers the risk estimate was almost unchanged: the corresponding pooled RRs were not materially altered (RR =0.78, 95% CI: 0.69–0.89) (25).
The prevalence of congenital heart defects in Germany is approximately 1% of live births (EUROCAT Website Database: http://www.eurocat-network.eu, 1980–2016). This is in agreement with previous studies showing a prevalence of 1.1% based on a multicentre study including 7,245 infants with CHD (26). The most common lesions were: ventricular septal defect (all types) (48.9%), atrial septal defect (17.0%), valvular pulmonary stenosis (6.1%), persistent arterial duct (4.3%) and aortic coarctation (3.6%) (26). Compared to healthy infants, prematurity (18.7% vs. 9.1%), a low birth weight <2,500 g (17.5% vs. 6.8%) and multiple births (6.2% vs. 3.3%) were more common outcomes in infants with CHD. Diagnosis of CHD in the infants was made after birth in over 80% of the cases (26). A recent European study reported an unexplained increase (up to 4.6%) in the annual proportional change in prevalence of several forms of CHDs between 2003 and 2012 (27).
In general studies on birth defects are subject to selection bias due to birth defects associated with low survival of the foetus. Approximately 10% of all registered CHD cases in Germany between 1988 and 2016 were associated with chromosomal defects (source EUROCAT-register: EUROCAT Website Database: http://www.eurocat-network.eu, 1980–2016). Complex birth defects may have a higher likelihood of stillbirth and/or prenatal diagnosis and elective termination before detecting CHD (e.g., chromosomal anomaly), suggesting that the prevalence of CHD among live births is underestimated.
Available evidence on prevention of CHD by prenatal folate supplementation is not as strong as that on prevention of NTD (28). However, if folic acid is proven to prevent even some CHD, a significant public health impact can be expected because CHD have approximately 10-fold higher incidence than NTD and it is associated with high health care costs.
Maternal obesity, diabetes (29), and smoking are risk factors for CHD, but in general maternal risk factors are shared with other pregnancy complications (30) and birth defects. Primary prevention trials for CHD are not available. Maternal socioeconomic status has shown an association with CHD (31). However, this association could be also due to poor nutrition, obesity, less use of vitamin supplements or other unhealthy behaviours. The prevalence of birth anomalies was approximately 47% higher and for various cardiac and central nervous system anomalies up to a 3- to 5-fold higher in births from Canadian women with diabetes than those born to nondiabetic mothers (32). Although there was a decline in the prevalence of birth anomalies in women with and those without diabetes after the fortification in Canada, the prevalence remained higher in women with diabetes (32). Agha et al. proposed using high doses of folic acid for women at high risk for birth defects (32). A recent study hypothesized that high-dose folic acid may prevent preeclampsia in women at risk (pre-existing hypertension, prepregnancy diabetes (type 1 or 2), pre-eclampsia in a previous pregnancy, or obesity) (33). However, pregnant women who received 4 mg/d folic acid from 8–16 weeks until delivery did not show lower risk of preeclampsia as compared to those who received placebo (33). Maternal obesity is associated with lower micronutrient status including folate and vitamin B12 (34). Except for using high doses folic acid (i.e., 4–5 mg/d) to prevent recurrent NTDs (has been shown in RCT), there is currently no evidence that such high doses of folic acid are necessary to prevent NTDs, CHD or pregnancy complications in pregnant women with obesity or diabetes.
Periconceptional use of folic acid has been shown to be associated with approximately 20% reduced CHD risk in a Dutch EUROCAT-register based study (22). Approximately one in four major cardiac defects is predicted to be prevented by timely multivitamin use (20). A retrospective Hungarian study covering 17 years between 1980 and 1996 has shown significantly less children with ventricular septal defect [odds ratio (OR) 0.57, 95% CI: 0.45–0.73], tetralogy of Fallot (OR 0.53, 95% CI: 0.17–0.94), d-transposition of great arteries (OR 0.47, 95% CI: 0.26–0.86) and atrial septal defect secundum (OR 0.63, 95% CI: 0.40–0.98) when the mothers had taken high doses of folic acid (mean 5.6 mg/d; range, 3–9 mg/d) during pregnancy compared to children whose mothers did not take folic acid (35).
A Chinese study has shown that the use of folic acid-containing multivitamins for ≥3 months before pregnancy is associated with 69% reduction in CHD risk (OR 0.31, 95% CI: 0.18–0.54) (23). Ionescu-Ittu et al. analysed CHD trends in Quebec, Canada between 1990 and 2005 and found that the risk of severe CHD (tetralogy of Fallot, endocardial cushion defects, univentricular hearts, truncus arteriosus, or transposition complexes) has declined by approximately 6% in the years after starting the fortification with folic acid in Canada in 1998 (36). Whereas in Alberta, only the prevalence of left ventricular outflow tract obstruction appears to have declined when comparing the pre-fortification [1995–1997] and post-fortification [1999–2002] periods (37). Liu et al. investigated the temporal trends in CHD subtypes following fortification with folic acid in Canada (38). The authors observed a decline in most of CHD subtypes between 1990 and 2011, while the rate of atrial septal defects increased significantly (38). Ecologic analysis of the association between CHD prevalence and the fortification is not a strong design to show causality. However, the possibility of CHD prevention by using folic acid prior and during pregnancy should be further evaluated.
Folic acid fortification is associated with a decline in the overall birth prevalence of CHDs, while this association differs according to the disease subtype (38,39). A case-control study in a Californian birth cohort of 1987–1988 (i.e., the fortification in the USA started in 1998) confirmed the inverse association between taking folate supplements and some CHD forms [OR 0.53 (0.34–0.85)] (40). In contrast, data obtained after the mandatory fortification does not support an association between folate biomarkers in maternal blood collected in mid-pregnancy and the risk of CHD in Californian women [2002–2007] (41). The fortification with folic acid significantly increased folate biomarkers in the U.S population, including the target group of women of reproductive age. The lack of association between folate markers and CHD risk in the post fortification period suggests a theoretically folic acid-responsive proportion of CHD cases that has levelled off after enhancing folate status.
Taken together, the overall evidence suggests that folic acid could prevent some CHD cases. The reduction of CHD risk when supplementing folic acid appears to be specific to severe CHD lesions. The risk reduction differed between populations which could be related to the presence of additional risk factors such as obesity (i.e., risk modification) (41).
Multivitamin supplements containing (iron and folic acid) are associated with fewer cases presenting with low birthweight and small for gestational age (42-47). The higher birth weight could be explained by extension of the gestational age or prevention of preterm birth. In a study including Hungarian pregnant women, mean gestational age was slightly longer (by 0.3 week) and mean birth weight was higher (by 37 g) in babies born to women taking supplemental folic acid (5.6 mg/d), compared to babies born to women taking no supplements (43). The rate of preterm births was significantly lower in the folic acid group compared with the group without vitamin supplement [7.6% vs. 11.8%: OR (95% CI) =0.68 (0.63–0.73)], although the OR for low birth weight was not significantly different according to maternal supplement use [OR (95% CI) =0.88 (0.62–1.14)] (43). Likewise, Chinese women who received 400 µg/d folic acid starting periconceptionally have shown lower risk for spontaneous preterm birth (by ≈50%) compared to women not taking the supplements (48).
Further support came from studies linking folate biomarkers in blood with adverse birth outcomes. In a study in Dutch women, newborns were on average 167 g heavier when maternal serum folate was >25.8 nmol/L compared to those from mothers with serum folate <9.3 nmol/L (46). An elevated plasma homocysteine, a marker of low folate status, is associated with a higher risk for having a small for gestational age birth (49). A 1.9-µmol/L increase in maternal plasma homocysteine is associated with 31 g (95% CI: −13, –51 g) lower weight (49). Overall, the association between low maternal folate or hyperhomocysteinemia and the risk of low birth weight and the association of supplemental folate with a lower risk suggest that supplemental folate could reduce the number of births with small for gestational age. There are open questions regarding the associations between folate intake or markers and secondary birth outcomes (outcomes beyond NTDs) (Table 1).

Full table
Folate biomarkers in children affected with congenital heart defects
Several studies investigated plasma folate, vitamin B12 and homocysteine in children with congenital heart defects and/or in mothers of those children. Low folate status, hyperhomocysteinemia and polymorphisms in the folate metabolizing enzyme, methylenetetrahydrofolate reductase (MTHFR C677T or MTHFR A1298C) were common in the group of patients or their mothers compared to the controls or their mothers, respectively (50,51). In a large case-control study designed to evaluate genetic, environmental, and behavior factors associated with the occurrence of major non-syndromic birth defects, Tang et al. investigated the association between CHD (sub-form of obstructive heart defects) and folate genetic polymorphisms in 569 case families and 1,644 control families enrolled between 1997 and 2008 (overlapping with the fortification period in the USA) (52). A SNP in MTHFR gene has been shown to be associated with CHD. Furthermore, multiple SNPs in betaine-homocysteine methyltransferase (BHMT and BHMT2) showed an association with CHD and an interaction with maternal use of folic acid supplements (52).
Determinants of folate status in young women
The average daily intake of folate from the diet in European women is less than half of the proposed optimal intake in pregnancy (approximately 600 µg/d). Folate insufficiency is common in young women especially in those with poor nutrition, low education or socioeconomic status, multiple previous pregnancies and short between-pregnancy intervals. Those women are also less likely to take multivitamin supplements before or during pregnancy. Smoking is associated with low maternal folate (53) and also with many side effects in the newborns (54,55). The negative effects of smoking on birth outcomes could be at least partly mediated by lowering maternal folate. Additionally, common polymorphisms in folate metabolizing genes are associated with low folate and pregnancy complications or poor outcomes (52). The lack of association in some studies (56) could be related to sufficient folate status suggesting a gene-nutrient interaction in determining the risk of birth defects (57).
Dietary folate intake was not sufficient to explain variations in serum or RBC-folate levels in German young women (58). Whereas, the intakes of fiber (positive effect) and carbohydrate (negative effect) have shown associations with plasma or blood folate biomarkers suggesting that dietary patterns (sources of folate in the diet) determine folate status (58). Studies from different European countries have shown that the average RBC-folate in young women is approximately 50% lower than the WHO suggested lowest cutoff for optimal blood folate range in this age group (>906 nmol/L) (59). Moreover, the short time window available for preventing NTDs is a limiting factor because an optimal folate status should be achieved before the closure of the neural tube in the first 28 days post conception. Achieving optimal RBC-folate concentrations by the time of neural tube closure while taking supplements containing 400 µg/d folate is time- and dose-dependent (17). Supplementation trials have shown that German women did not achieve the lowest protective RBC-folate concentration even when receiving 400 µg folic acid/d for 4 or 8 weeks (59,60). Approximately 50% of women with baseline folate <906 nmol/L cannot achieve the protective levels within 8 weeks of supplementation and may thus remain at a residual risk for NTD.
The dose-response relationship between folic acid intake and RBC-folate concentrations in women has been intensively studied (61). A meta-analysis by Berti et al. has shown a 23% higher RBC-folate concentration for doubling the intake (61). Another meta-analysis investigated the dose-response relationship between folate intake from food (natural plus fortified) and serum and RBC-folate concentrations among healthy women (12–49 years) (62). It has been shown that every 10% increase in folate intake is associated with a 6% increase (95% CI: 4–9%) in RBC-folate level and a 7% (95% CI: 1–12%) increase in serum/plasma folate (62). An intake ≥450 µg DFE/d (on long term) is associated with protective RBC-folate concentrations (~1,050 nmol/L) necessary for reducing NTDs risk (62).
It has been estimated that more than half of the German women of reproductive age may need 800 µg/d to achieve optimal RBC-folate concentrations within 2 months (Table 2). Whereas, a folate dose of 400 µg/d taken for 2 months would be sufficient only in women with RBC-folate levels above the population mean or >550 nmol/L (63). In countries applying fortification, approximately 150 µg/d folic acid intake is achieved from the fortified foods. When this amount is consumed over a long time (several months) it appears to be sufficient in eliminating folate deficiency and improving folate status in women. Still, in countries applying fortification, pregnant women are recommended to take additional supplemental sources of folate.

Full table
Several interesting alternative approaches to improve women folate status before closure of the neural tube have been applied or are under discussion. For instance, an oral contraceptive that contains 5-methyltetrahydrofolate (i.e., metafolin) has been developed with the aim of counteracting folate depletion when women stope oral contraceptive with the aim of planning pregnancy (64). Another concept was to use rescue oral pills containing a large dose of methylfolate (7.5 mg) administered several times over 4 days in order to rapidly increase maternal folate supply after a positive pregnancy test (65).
Folate supplementation during pregnancy and possible side effects for the child
Some studies have shown positive associations between using folic acid-containing supplements before and/or during pregnancy and the risk of asthma (66,67) or autism (68) in the child. However, the results regarding these outcomes are controversial. Other studies have shown no association with asthma (69) or even a negative association with autism (70,71). A recent meta-analysis has shown a negative association between taking folic acid-containing supplement during pregnancy and autism in the child (72), while a meta-analysis on maternal folic acid use and asthma was not possible because of a strong heterogeneity in the measurements of the exposures and the outcomes (73). In general, there is still no clear hypothesis on when and how may folic acid intake in the mother influence the risk of these outcomes in the child. The overall evidence does not support a role for folic acid in the aetiology of asthma or autism in the child if the mother had taken folic acid supplement before and during pregnancy.
Conclusions
- Low folate status in the mother is associated with congenital birth defects including NTDs and congenital heart defects in addition to low birth weight and preterm births;
- The experimental evidence available from RCTs in human has proven that folate supplementation can prevent many, although not all, NTDs;
- The association between folate supplementation and CHD has been confirmed by several studies, though experimental evidence is still lacking. However, folate supplementation before and during first pregnancy trimester is highly recommended for prevention of NTDs. Therefore, placebo-controlled studies with the aim of showing a reduction in CHDs are not ethical;
- Overall, there is a good evidence that folate supplementation may have a protective effect against severe types of CHD. However, the optimal folate dose and time of supplementation before or during pregnancy to prevent CHD are not well defined;
- If folic acid/folate is proven to prevent CHD (or some sub-types), the public health impact is expected to exceed that of preventing NTDs;
- The search for additional nutrients that may prevent CHD and show complementary or synergetic effects with folate is continuing. However, more research is needed to clarify the mechanisms.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Hoffbrand AV, Jackson BF. Correction of the DNA synthesis defect in vitamin B12 deficiency by tetrahydrofolate: evidence in favour of the methyl-folate trap hypothesis as the cause of megaloblastic anaemia in vitamin B12 deficiency. Br J Haematol 1993;83:643-7. [Crossref] [PubMed]
- Shane B, Stokstad EL. Vitamin B12-folate interrelationships. Annu Rev Nutr 1985;5:115-41. [Crossref] [PubMed]
- Hibbard BM. The role of folic acid in pregnancy; with particular reference to anaemia, abruption and abortion. J Obstet Gynaecol Br Commonw 1964;71:529-42. [Crossref] [PubMed]
- Miller PN, Pratten MK, Beck F. Growth of 9.5-day rat embryos in folic-acid-deficient serum. Teratology 1989;39:375-85. [Crossref] [PubMed]
- Smithells RW, Sheppard S, Schorah CJ. Vitamin deficiencies and neural tube defects. Arch Dis Child 1976;51:944-50. [Crossref] [PubMed]
- Recommendations for the use of folic acid to reduce the number of cases of spina bifida and other neural tube defects. MMWR Recomm Rep 1992;41:1-7. [PubMed]
- Bower C, Stanley FJ. Periconceptional vitamin supplementation and neural tube defects; evidence from a case-control study in Western Australia and a review of recent publications. J Epidemiol Community Health 1992;46:157-61. [Crossref] [PubMed]
- Miller RK, Faber W, Asai M, et al. The role of the human placenta in embryonic nutrition. Impact of environmental and social factors. Ann N Y Acad Sci 1993;678:92-107. [Crossref] [PubMed]
- Kirke PN, Molloy AM, Daly LE, et al. Maternal plasma folate and vitamin B12 are independent risk factors for neural tube defects. Q J Med 1993;86:703-8. [PubMed]
- Werler MM, Shapiro S, Mitchell AA. Periconceptional folic acid exposure and risk of occurrent neural tube defects. JAMA 1993;269:1257-61. [Crossref] [PubMed]
- Kancherla V, Druschel CM, Oakley GP Jr. Population-based study to determine mortality in spina bifida: New York State Congenital Malformations Registry, 1983 to 2006. Birth Defects Res A Clin Mol Teratol 2014;100:563-75. [Crossref] [PubMed]
- Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. MRC Vitamin Study Research Group. Lancet 1991;338:131-7. [Crossref] [PubMed]
- Czeizel AE, Dudas I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N Engl J Med 1992;327:1832-5. [Crossref] [PubMed]
- Blencowe H, Cousens S, Modell B, et al. Folic acid to reduce neonatal mortality from neural tube disorders. Int J Epidemiol 2010;39 Suppl 1:i110-21. [Crossref] [PubMed]
- Cordero AM, Crider KS, Rogers LM, et al. Optimal serum and red blood cell folate concentrations in women of reproductive age for prevention of neural tube defects: World Health Organization guidelines. MMWR Morb Mortal Wkly Rep 2015;64:421-3. [PubMed]
- Crider KS, Devine O, Hao L, et al. Population red blood cell folate concentrations for prevention of neural tube defects: Bayesian model. BMJ 2014;349:g4554. [Crossref] [PubMed]
- Bailey LB, Hausman DB. Folate status in women of reproductive age as basis of neural tube defect risk assessment. Ann N Y Acad Sci 2018;1414:82-95. [Crossref] [PubMed]
- de Walle HE, de Jong-van den Berg LT. Ten years after the Dutch public health campaign on folic acid: the continuing challenge. Eur J Clin Pharmacol 2008;64:539-43. [Crossref] [PubMed]
- Botto LD, Mastroiacovo P. Triple surveillance: a proposal for an integrated strategy to support and accelerate birth defect prevention. Ann N Y Acad Sci 2018;1414:126-36. [Crossref] [PubMed]
- Botto LD, Mulinare J, Erickson JD. Occurrence of congenital heart defects in relation to maternal mulitivitamin use. Am J Epidemiol 2000;151:878-84. [Crossref] [PubMed]
- Czeizel AE. Reduction of urinary tract and cardiovascular defects by periconceptional multivitamin supplementation. Am J Med Genet 1996;62:179-83. [Crossref] [PubMed]
- van Beynum IM, Kapusta L, Bakker MK, et al. Protective effect of periconceptional folic acid supplements on the risk of congenital heart defects: a registry-based case-control study in the northern Netherlands. Eur Heart J 2010;31:464-71. [Crossref] [PubMed]
- Li X, Li S, Mu D, et al. The association between periconceptional folic acid supplementation and congenital heart defects: a case-control study in China. Prev Med 2013;56:385-9. [Crossref] [PubMed]
- Jahanbin A, Shadkam E, Miri HH, et al. Maternal Folic Acid Supplementation and the Risk of Oral Clefts in Offspring. J Craniofac Surg 2018;29:e534-41. [Crossref] [PubMed]
- Feng Y, Wang S, Chen R, et al. Maternal folic acid supplementation and the risk of congenital heart defects in offspring: a meta-analysis of epidemiological observational studies. Sci Rep 2015;5:8506. [Crossref] [PubMed]
- Lindinger A, Schwedler G, Hense HW. Prevalence of congenital heart defects in newborns in Germany: Results of the first registration year of the PAN Study (July 2006 to June 2007). Klin Padiatr 2010;222:321-6. [Crossref] [PubMed]
- Morris JK, Springett AL, Greenlees R, et al. Trends in congenital anomalies in Europe from 1980 to 2012. PLoS One 2018;13:e0194986. [Crossref] [PubMed]
- De-Regil LM, Fernández-Gaxiola AC, Dowswell T, et al. Effects and safety of periconceptional folate supplementation for preventing birth defects. Cochrane Database Syst Rev 2010;(10):CD007950. Update in Cochrane Database Syst Rev 2015;12:CD007950. [PubMed]
- Leirgul E, Brodwall K, Greve G, et al. Maternal Diabetes, Birth Weight, and Neonatal Risk of Congenital Heart Defects in Norway, 1994-2009. Obstet Gynecol 2016;128:1116-25. [Crossref] [PubMed]
- Maslen CL. Recent Advances in Placenta-Heart Interactions. Front Physiol 2018;9:735. [Crossref] [PubMed]
- Yu D, Feng Y, Yang L, et al. Maternal socioeconomic status and the risk of congenital heart defects in offspring: a meta-analysis of 33 studies. PLoS One 2014;9:e111056. [Crossref] [PubMed]
- Agha MM, Glazier RH, Moineddin R, et al. Congenital abnormalities in newborns of women with pregestational diabetes: A time-trend analysis, 1994 to 2009. Birth Defects Res A Clin Mol Teratol 2016;106:831-9. [Crossref] [PubMed]
- Wen SW, White RR, Rybak N, et al. Effect of high dose folic acid supplementation in pregnancy on pre-eclampsia (FACT): double blind, phase III, randomised controlled, international, multicentre trial. BMJ 2018;362:k3478. [Crossref] [PubMed]
- Scholing JM, Olthof MR, Jonker FA, et al. Association between pre-pregnancy weight status and maternal micronutrient status in early pregnancy. Public Health Nutr 2018;21:2046-55. [Crossref] [PubMed]
- Czeizel AE, Vereczkey A, Szabo I. Folic acid in pregnant women associated with reduced prevalence of severe congenital heart defects in their children: a national population-based case-control study. Eur J Obstet Gynecol Reprod Biol 2015;193:34-9. [Crossref] [PubMed]
- Ionescu-Ittu R, Marelli AJ, Mackie AS, et al. Prevalence of severe congenital heart disease after folic acid fortification of grain products: time trend analysis in Quebec, Canada. BMJ 2009;338:b1673. [Crossref] [PubMed]
- Bedard T, Lowry RB, Sibbald B, et al. Folic acid fortification and the birth prevalence of congenital heart defect cases in Alberta, Canada. Birth Defects Res A Clin Mol Teratol 2013;97:564-70. [Crossref] [PubMed]
- Liu S, Joseph KS, Luo W, et al. Effect of Folic Acid Food Fortification in Canada on Congenital Heart Disease Subtypes. Circulation 2016;134:647-55. [Crossref] [PubMed]
- Parnell AS, Correa A. Analyses of trends in prevalence of congenital heart defects and folic acid supplementation. J Thorac Dis 2017;9:495-500. [Crossref] [PubMed]
- Shaw GM, O'Malley CD, Wasserman CR, et al. Maternal periconceptional use of multivitamins and reduced risk for conotruncal heart defects and limb deficiencies among offspring. Am J Med Genet 1995;59:536-45. [Crossref] [PubMed]
- Shaw GM, Yang W, Carmichael SL, et al. One-carbon metabolite levels in mid-pregnancy and risks of conotruncal heart defects. Birth Defects Res A Clin Mol Teratol 2014;100:107-15. [Crossref] [PubMed]
- Fekete K, Berti C, Trovato M, et al. Effect of folate intake on health outcomes in pregnancy: a systematic review and meta-analysis on birth weight, placental weight and length of gestation. Nutr J 2012;11:75. [Crossref] [PubMed]
- Czeizel AE, Puho EH, Langmar Z, et al. Possible association of folic acid supplementation during pregnancy with reduction of preterm birth: a population-based study. Eur J Obstet Gynecol Reprod Biol 2010;148:135-40. [Crossref] [PubMed]
- Timmermans S, Jaddoe VW, Hofman A, et al. Periconception folic acid supplementation, fetal growth and the risks of low birth weight and preterm birth: the Generation R Study. Br J Nutr 2009;102:777-85. [Crossref] [PubMed]
- Papadopoulou E, Stratakis N, Roumeliotaki T, et al. The effect of high doses of folic acid and iron supplementation in early-to-mid pregnancy on prematurity and fetal growth retardation: the mother-child cohort study in Crete, Greece (Rhea study). Eur J Nutr 2013;52:327-36. [Crossref] [PubMed]
- Bergen NE, Jaddoe VW, Timmermans S, et al. Homocysteine and folate concentrations in early pregnancy and the risk of adverse pregnancy outcomes: the Generation R Study. BJOG 2012;119:739-51. [Crossref] [PubMed]
- Haider BA, Bhutta ZA. Multiple-micronutrient supplementation for women during pregnancy. Cochrane Database Syst Rev 2017;4:CD004905. [PubMed]
- Li Z, Ye R, Zhang L, et al. Periconceptional folic acid supplementation and the risk of preterm births in China: a large prospective cohort study. Int J Epidemiol 2014;43:1132-9. [Crossref] [PubMed]
- Hogeveen M, Blom HJ. den HM. Maternal homocysteine and small-for-gestational-age offspring: systematic review and meta-analysis. Am J Clin Nutr 2012;95:130-6. [Crossref] [PubMed]
- Elizabeth KE, Praveen SL, Preethi NR, et al. Folate, vitamin B12, homocysteine and polymorphisms in folate metabolizing genes in children with congenital heart disease and their mothers. Eur J Clin Nutr 2017;71:1437-41. [Crossref] [PubMed]
- Surmiak P, Baumert M, Paprotny M. Abnormal Biomarkers of Homocysteine Metabolism in Neonates with Conotruncal Heart Defects. Biomed Res Int 2017. Biomed Res Int 2017;2017:7404397. [PubMed]
- Tang X, Cleves MA, Nick TG, et al. Obstructive heart defects associated with candidate genes, maternal obesity, and folic acid supplementation. Am J Med Genet A 2015;167:1231-42. [Crossref] [PubMed]
- Obeid R, Munz W, Jager M, et al. Biochemical indexes of the B vitamins in cord serum are predicted by maternal B vitamin status. Am J Clin Nutr 2005;82:133-9. [Crossref] [PubMed]
- Witt SH, Frank J, Gilles M, et al. Impact on birth weight of maternal smoking throughout pregnancy mediated by DNA methylation. BMC Genomics 2018;19:290. [Crossref] [PubMed]
- Stark KD, Pawlosky RJ, Sokol RJ, et al. Maternal smoking is associated with decreased 5-methyltetrahydrofolate in cord plasma. Am J Clin Nutr 2007;85:796-802. [Crossref] [PubMed]
- Shaw GM, Lu W, Zhu H, et al. 118 SNPs of folate-related genes and risks of spina bifida and conotruncal heart defects. BMC Med Genet 2009;10:49. [Crossref] [PubMed]
- Molloy AM, Mills JL, Kirke PN, et al. Low blood folates in NTD pregnancies are only partly explained by thermolabile 5,10-methylenetetrahydrofolate reductase: low folate status alone may be the critical factor. Am J Med Genet 1998;78:155-9. [Crossref] [PubMed]
- Obeid R, Schön C, Wilhelm M, et al. Dietary and lifestyle predictors of folate insufficiency in non-supplemented German women. Int J Food Sci Nutr 2019;70:367-76. [PubMed]
- Obeid R, Schön C, Wilhelm M, et al. The effectiveness of daily supplementation with 400 or 800 µg/day folate in reaching protective red blood folate concentrations in non-pregnant women: a randomized trial. Eur J Nutr 2018;57:1771-80. [Crossref] [PubMed]
- Brämswig S, Prinz-Langenohl R, Lamers Y, et al. Supplementation with a multivitamin containing 800 microg of folic acid shortens the time to reach the preventive red blood cell folate concentration in healthy women. Int J Vitam Nutr Res 2009;79:61-70. [Crossref] [PubMed]
- Berti C, Fekete K, Dullemeijer C, et al. Folate intake and markers of folate status in women of reproductive age, pregnant and lactating women: a meta-analysis. J Nutr Metab 2012;2012:470656. [Crossref] [PubMed]
- Marchetta CM, Devine OJ, Crider KS, et al. Assessing the association between natural food folate intake and blood folate concentrations: a systematic review and Bayesian meta-analysis of trials and observational studies. Nutrients 2015;7:2663-86. [Crossref] [PubMed]
- Obeid R, Schön C, Wilhelm M, et al. Response of Red Blood Cell Folate to Supplementation in Nonpregnant Women is Predictable: A Proposal for Personalized Supplementation. Mol Nutr Food Res 2018;62. [Crossref] [PubMed]
- Holzgreve W, Pietrzik K, Koletzko B, et al. Adding folate to the contraceptive pill: a new concept for the prevention of neural tube defects. J Matern Fetal Neonatal Med 2012;25:1529-36. [Crossref] [PubMed]
- Bailey SW, Ayling JE. The pharmacokinetic advantage of 5-methyltetrahydrofolate for minimization of the risk for birth defects. Sci Rep 2018;8:4096. [Crossref] [PubMed]
- Yang L, Jiang L, Bi M, et al. High dose of maternal folic acid supplementation is associated to infant asthma. Food Chem Toxicol 2015;75:88-93. [Crossref] [PubMed]
- Whitrow MJ, Moore VM, Rumbold AR, et al. Effect of supplemental folic acid in pregnancy on childhood asthma: a prospective birth cohort study. Am J Epidemiol 2009;170:1486-93. [Crossref] [PubMed]
- Raghavan R, Riley AW, Volk H, et al. Maternal Multivitamin Intake, Plasma Folate and Vitamin B12 Levels and Autism Spectrum Disorder Risk in Offspring. Paediatr Perinat Epidemiol 2018;32:100-11. [Crossref] [PubMed]
- Martinussen MP, Risnes KR, Jacobsen GW, et al. Folic acid supplementation in early pregnancy and asthma in children aged 6 years. Am J Obstet Gynecol 2012;206:72.e1-7. [Crossref] [PubMed]
- Surén P, Roth C, Bresnahan M, et al. Association between maternal use of folic acid supplements and risk of autism spectrum disorders in children. JAMA 2013;309:570-7. [Crossref] [PubMed]
- Schmidt RJ, Tancredi DJ, Ozonoff S, et al. Maternal periconceptional folic acid intake and risk of autism spectrum disorders and developmental delay in the CHARGE (CHildhood Autism Risks from Genetics and Environment) case-control study. Am J Clin Nutr 2012;96:80-9. [Crossref] [PubMed]
- Wang M, Li K, Zhao D, et al. The association between maternal use of folic acid supplements during pregnancy and risk of autism spectrum disorders in children: a meta-analysis. Mol Autism 2017;8:51. [Crossref] [PubMed]
- Brown SB, Reeves KW, Bertone-Johnson ER. Maternal folate exposure in pregnancy and childhood asthma and allergy: a systematic review. Nutr Rev 2014;72:55-64. [Crossref] [PubMed]


