Protected percutaneous coronary intervention with Impella CP in a patient with left main disease, severe left ventricular systolic dysfunction and established hemolysis
Introduction
The use of the Impella device in patients with reduced left ventricular (LV) systolic function undergoing unprotected left main (LM) percutaneous coronary intervention (PCI) has been growing exponentially. Data from observational studies and registries demonstrate that Impella-assisted high-risk PCI is safe and effective with a low rate of peri-procedural complications (1-3). Hemolysis is a potential limitation of all percutaneous mechanical circulatory support devices and a small incidence of hemolysis has also been associated with Impella use (4). The safety and feasibility of Impella use in patients with established pre-existing hemolysis has not been evaluated. We report the case of Impella-assisted left main stem (LMS) PCI in a patient with severe LV systolic dysfunction and autoimmune hemolytic anemia (AIHA).
Case presentation
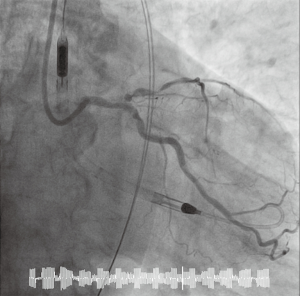
An 82-year-old male was admitted to our institution with a non-ST elevation myocardial infarction (NSTEMI). His cardiovascular risk factors were systemic arterial hypertension and a history of smoking. His medical background included chronic kidney disease, with a baseline eGFR of 40 mL/min, and myelodysplastic syndrome with recurrent anemia and red cell transfusion dependence. Blood count analysis on admission showed two-lineage cytopenia (hemoglobin 69 g/L, platelet count 114×109). Lactate dehydrogenase (LDH), bilirubin and reticulocyte count were all elevated while haptoglobin was reduced. Blood film microscopy showed features of AIHA with spherocytosis and polychromasia. After initial treatment with oral antiplatelet therapy (aspirin and clopidogrel), and intravenous methylprednisolone to suppress the hemolysis, the patient underwent coronary angiography which revealed severe, heavily calcified coronary artery disease involving the distal LM bifurcation and proximal left anterior descending (LAD) artery (Figure 1). An echocardiogram demonstrated a dilated left ventricle with global severe LV systolic dysfunction (ejection fraction of 32%) and a cardiac MRI showed viability in 16 out of 17 LV segments. In view of the patient’s severe comorbidities, the heart team concluded that the risks of surgical revascularization were unacceptably high, and it was decided to offer Impella-assisted LM-PCI instead.
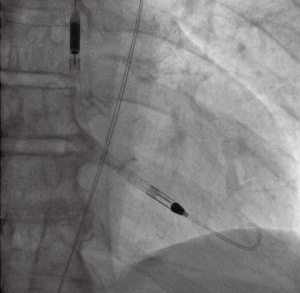
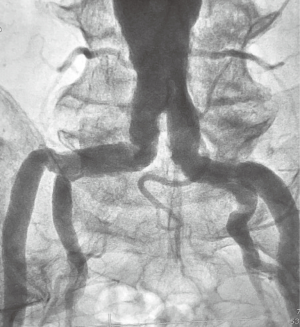
Arterial access was gained via the right radial artery with a 7 Fr Terumo Glidesheath Slender sheath (Terumo Medical Corporation, Japan). A 125 cm 6 Fr pigtail catheter (Cordis, Fremont, California, USA) was passed to the descending aorta via the right radial artery and an aortogram of the iliac and femoral systems was obtained in order to assess the suitability for transfemoral Impella delivery. There was severe tortuosity of the right iliac artery and severe bilateral iliac calcification (Figure 2). The left femoral artery was chosen as the site of access due to less tortuosity and visually better dimensions for device delivery. The left femoral artery was punctured under fluoroscopic guidance using a Micropuncture Kit (Cook Medical, Bloomington, IN, USA) and a 14 Fr sheath (Abiomed Europe, Aachen, Germany) was inserted. An Impella CP (Abiomed, Danvers, Massachusetts, USA) was positioned in the left ventricle (Figure 3) and the flow was set and maintained at 3 L per minute. A total of 11,000 international units of unfractionated heparin were given and the activated clotting time (ACT) was monitored closely, aiming for an ACT of >250 s for the duration of the procedure.
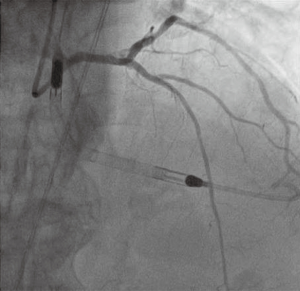
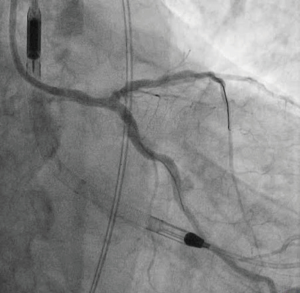
PCI was performed via the right radial artery without complication. The LM coronary artery was engaged with a 7 Fr EBU 3.5 guiding catheter (Medtronic Limited, MN, USA). A Whisper MS J-Tip guidewire (Abbott Vascular, Abbott, Illinois, USA) was advanced to the distal left circumflex artery (LCx) and an Asahi Sion black guidewire (Asahi Intecc Co., Ltd., Aichi, Japan) was advanced to the distal LAD. The LM and LAD were assessed with intravascular ultrasound using a Volcano IVUS catheter (Volcano Corporation, San Diego, California, USA). Following pre-dilatation of the LCx and LAD ostia and proximal LAD with non-compliant balloons (NC Emerge, Boston Scientific, Massachusetts, USA), the distal LM bifurcation was stented using the Mini-Crush technique. A 2.75 mm × 36 mm drug eluting stent (Biofreedom, Biosensors International Ltd, Singapore) was deployed in the LM/LAD and a 3.0 mm × 24 mm Biofreedom stent was deployed in the LCx. Post-dilatation was performed with a 3.0 mm × 15 mm NC Emerge balloon in the LAD and LCx and a 4.0 mm × 8 mm NC Emerge balloon in the LM. Final kissing at the LAD/LCx bifurcation was performed with 3.0 mm NC Emerge balloons (Figures 4,5). The Impella CP was removed at the end of the procedure. Femoral artery hemostasis was achieved using the double Angio-Seal technique as previously described (5). Two standard 0.035” wires were introduced through the 14 Fr femoral artery sheath. Following removal of the 14 Fr sheath, a 6 Fr sheath was introduced over one wire and an 8 Fr Angio-Seal vascular closure device (ANGIO-SEAL VIP Vascular Closure Device, Terumo International Systems, Somerset, New Jersey, USA) was deployed over the other wire. Following this, the 6 Fr sheath was replaced by a second 8 Fr Angio-Seal. Radial artery hemostasis was achieved with a radial artery compression device (TR band, Terumo International Systems, Somerset, New Jersey, USA).
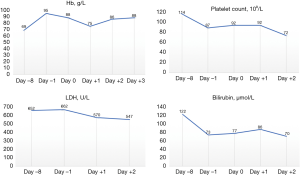
The Impella CP remained in situ for a total of 1 hour and 39 minutes. At the end of the procedure the patient was transferred to the coronary care unit for monitoring. Hemoglobin, LDH, haptoglobin and bilirubin levels were closely monitored with no evidence of deterioration of the patient’s hemolysis (Figure 6). The patient had an episode of epistaxis a few hours after the procedure which resolved with nasal packing. There was a transient hemoglobin drop on day 1 after the procedure, which we believe was related to blood loss during the procedure, as well as the episode of epistaxis. The patient did not require any specific treatment and hemoglobin returned to its pre-procedure level on the following day. The patient was discharged home 4 days after the procedure.
Discussion
The Impella device is one of the most commonly used percutaneous LV assist devices and consists of a catheter-mounted axial flow pump which is inserted in the left ventricle in a retrograde fashion via the femoral artery. The Impella CP provides a maximum flow of 4 L/min, thereby unloading the left ventricle throughout the cardiac cycle and increasing end-organ perfusion (6). The Impella 2.5 and Impella 5.0 provide flows of 2.5 and 5.0 L/min, respectively.
Use of the Impella device has been shown to be safe and effective during high-risk PCI in several publications. The large, multicenter, retrospective Europella registry demonstrated the safety and feasibility of Impella 2.5 hemodynamic support for high-risk PCI (2). Fifty-three percent of the patients in the Europella registry underwent LM-PCI and 35% had LV systolic dysfunction. The 30-day mortality of patients undergoing Impella assisted high-risk PCI was 5.5%. Similarly, results from a single centre retrospective evaluation of the USpella registry strongly supported the feasibility, safety and hemodynamic usefulness of the Impella device for unprotected LM intervention in patients with LV systolic dysfunction without cardiogenic shock (1). The 30-day mortality in this study was 2.36%. Finally, a meta-analysis of 12 studies with 1,346 patients who underwent Impella 2.5 placement for support of high-risk PCI reinforced the feasibility and safety of Impella use in this setting with a 30-day mortality of 3.5% and an acceptable risk of peri-procedural complications (3).
While these data establish the safety and feasibility of Impella use in high-risk PCI, a small incidence of hemolysis has been observed in patients undergoing Impella placement (2,7,8). The reported incidence of hemolysis in published registries is 5–10% (2,7). The degree of hemolysis that has been observed is typically mild and resolves with removal of the device. Although Impella-associated hemolysis might contribute to the development of anemia and increased need for red cell transfusion, it does not appear to affect short-term patient outcomes or survival (9). Even in the rare reported cases of massive hemolysis, there appears to be rapid and complete resolution of hemolysis after device removal with no reported short-term adverse events apart from the need of blood transfusion (10,11).
We report the case of an Impella CP-assisted LM-PCI in a patient with severe LV systolic dysfunction and established AIHA. The patient’s bleeding risk was further increased by the use of high-dose steroids administered for the treatment of hemolysis, the presence of thrombocytopenia and the patient’s impaired renal function. Despite these risks, the procedure was carried out safely without immediate or short-term complications. Monitoring of markers of hemolysis after the procedure did not demonstrate any evidence of an exacerbation of the patient’s previous hemolysis.
Hemolysis is a potential limitation of virtually all mechanical circulatory support systems (12,13). The small size of the Impella device, which enables percutaneous delivery, appears to contribute to an increased risk of hemolysis. Computational flow models have suggested that Impella-associated hemolysis is caused by the high rotatory shear forces of the impeller and the small clearing space between the impeller and its housing (14). Factors that appear to exacerbate hemolysis include the higher impeller speeds required to achieve high flow rates (15), prolonged duration of support (15,16) and improper positioning of the device (11). However, in experienced, high-volume centers the incidence of hemolysis is very low and clinically relevant hemolysis virtually never occurs. In our case, meticulous attention was paid to minimize modifiable factors potentially exacerbating the pre-existing hemolysis. More specifically, care was taken to position the device correctly in the left ventricle therefore avoiding inlet or outlet obstruction and the device was removed early as soon as hemodynamic stability was confirmed to avoid unnecessarily prolonged use.
Our case demonstrates that the Impella device can be safely used in the context of pre-existing or ongoing hemolysis. Precautions should be taken to reduce all possible modifiable factors that can contribute to hemolysis and patients should be closely monitored for any clinical or biochemical evidence of hemolysis.
Acknowledgements
None.
Footnote
Conflicts of Interest: Dr. Napp has received modest, personal fees for lectures, proctorship, consulting and travel support from Abiomed, for lectures from Maquet, for lectures, consulting and travel support from Cytosorbents, consulting and travel support from Bayer, and travel support from Zoll, Amgen, Biotronik, Merit Medical, Servier, Volcano and Terumo. The other authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Schreiber T, Wah Htun W, Blank N, et al. Real-world supported unprotected left main percutaneous coronary intervention with impella device; data from the USpella registry. Catheter Cardiovasc Interv 2017;90:576-81. [Crossref] [PubMed]
- Sjauw KD, Konorza T, Erbel R, et al. Supported high-risk percutaneous coronary intervention with the Impella 2.5 device the Europella registry. J Am Coll Cardiol 2009;54:2430-4. [Crossref] [PubMed]
- Briasoulis A, Telila T, Palla M, et al. Meta-Analysis of Usefulness of Percutaneous Left Ventricular Assist Devices for High-Risk Percutaneous Coronary Interventions. Am J Cardiol 2016;118:369-75. [Crossref] [PubMed]
- Lauten A, Engström AE, Jung C, et al. Percutaneous left-ventricular support with the Impella-2.5-assist device in acute cardiogenic shock: results of the Impella-EUROSHOCK-registry. Circ Heart Fail 2013;6:23-30. [Crossref] [PubMed]
- Abi Rafeh N, Quevedo HC, DeAndrade KB, et al. The double angio-seal technique for arterial closure following large-bore access. J Invasive Cardiol 2013;25:412-4. [PubMed]
- Kar B, Basra SS, Shah NR, et al. Percutaneous circulatory support in cardiogenic shock: interventional bridge to recovery. Circulation 2012;125:1809-17. [Crossref] [PubMed]
- Dixon SR, Henriques JP, Mauri L, et al. A prospective feasibility trial investigating the use of the Impella 2.5 system in patients undergoing high-risk percutaneous coronary intervention (The PROTECT I Trial): initial U.S. experience. JACC Cardiovasc Interv 2009;2:91-6. [Crossref] [PubMed]
- Henriques JP, Remmelink M, Baan J Jr, et al. Safety and feasibility of elective high-risk percutaneous coronary intervention procedures with left ventricular support of the Impella Recover LP 2.5. Am J Cardiol 2006;97:990-2. [Crossref] [PubMed]
- Badiye AP, Hernandez GA, Novoa I, et al. Incidence of Hemolysis in Patients with Cardiogenic Shock Treated with Impella Percutaneous Left Ventricular Assist Device. ASAIO J 2016;62:11-4. [Crossref] [PubMed]
- Tanawuttiwat T, Chaparro SV. An unexpected cause of massive hemolysis in percutaneous left ventricular assist device. Cardiovasc Revasc Med 2013;14:66-7. [Crossref] [PubMed]
- Cardozo S, Ahmed T, Belgrave K. Impella induced massive hemolysis: reemphasizing echocardiographic guidance for correct placement. Case Rep Cardiol 2015;2015:464135. [Crossref] [PubMed]
- Slaughter MS. Hematologic effects of continuous flow left ventricular assist devices. J Cardiovasc Transl Res 2010;3:618-24. [Crossref] [PubMed]
- Goldstein DJ. Worldwide experience with the MicroMed DeBakey Ventricular Assist Device as a bridge to transplantation. Circulation 2003;108 Suppl 1:II272-7. [Crossref] [PubMed]
- Apel J, Paul R, Klaus S, et al. Assessment of hemolysis related quantities in a microaxial blood pump by computational fluid dynamics. Artif Organs 2001;25:341-7. [Crossref] [PubMed]
- Jurmann MJ, Siniawski H, Erb M, et al. Initial experience with miniature axial flow ventricular assist devices for postcardiotomy heart failure. Ann Thorac Surg 2004;77:1642-7. [Crossref] [PubMed]
- Meyns B, Dens J, Sergeant P, et al. Initial experiences with the Impella device in patients with cardiogenic shock - Impella support for cardiogenic shock. Thorac Cardiovasc Surg 2003;51:312-7. [Crossref] [PubMed]