Ultrasound imaging of the arterial system
Introduction and basic physics
Ultrasound (US) imaging (also known as conventional B-mode) uses equipment that generates acoustic energy that transmit through tissues resulting in interference patterns with tissues within the body. These interactions result in formation of an US image (1). It poses no radiation risk, is readily available in most clinical settings and is relatively inexpensive. It is however operator dependent. Hence a combination of operator and interpretative skills are required in answering a particular clinical question, this is what makes US imaging unique.
The use of Doppler imaging is an extension of B-mode US, using the same basic physics of acoustic energy transmission. It enables visualizing and quantifying blood flow by analyzing interactions of acoustic pulses with flowing blood within the vessel lumen. Because rapidly moving targets such as red blood cells, produce a unique set of echoes, in effect one can visualize blood flow patterns, this is commonly displayed as a color flow map using red and blue (hence the term color Doppler), although this can be altered within a given system. When portrayed in form of a wave pattern, it is known as spectral Doppler (2).
The review that follows aims to highlight the various applications of US in imaging of the arterial system.
Applications
There remains a common pre-requisite among the various applications of US in imaging the arteries, to get the best image the target vessel needs to be within the depth range for the probe being used, the focal point of the probe is ideally placed at the level of the blood vessel. Further, appropriate steering of the Doppler angle allows for accurate measurement of blood flow while eliminating artefacts. High frequency probes are usually used to achieve better resolution, crucial in vessel wall imaging and measurement of intima-media thickness (IMT). Understandably, operator experience plays a pivotal role in Doppler interpretation. This review also focuses on advanced techniques such as contrast enhanced ultrasound (CEUS), this will be dealt with in the appropriate sections.
Upper limb
Vasculitis imaging
US plays a crucial role in the imaging and follow up of patients with large vessel vasculitis (LVV) (3). It retains the advantages of US used elsewhere—it is readily available, uses no ionizing radiation, universal application by radiologists, angiologists and rheumatologists alike. This enables multidisciplinary management of the patient.
According to the Chapel Hill Consensus Conference (CHCC) nomenclature in 2012 (4), LVV has two primary variants i.e., giant cell arteritis (GCA) and Takayasu arteritis (TA). US diagnosis and follow up of LVV primarily hinges on detection of intimal and/or adventitial wall thickening as a result of inflammation (5).
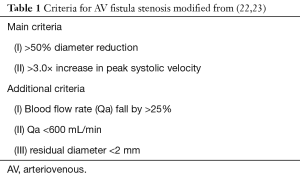
B-mode US (of shallow vessels with high frequency probe) clearly depicts three layers of the arterial wall (3) two are hyperechoic with a hypoechoic layer in between (Figure 1). The two innermost layers are referred to as the IMT (IMT stands for intima-medial thickness and is the cardiovascular risk factor test measured in the distal 1 cm of the posterior carotid wall-cIMT).
GCA is the most common of the LVV, affecting persons over the age of 50 years, it has cranial and extracranial manifestation. The temporal, ophthalmic and axillary arteries are particularly affected. The primary sign in an abnormal vessel is an increased IMT. There are readily available published normal values for IMT in the facial, temporal and axillary arteries (6).
In 1997 a landmark study by Schmidt et al. (7) proposed the ‘halo sign’ of vasculitis, it described a hypoechoic halo around the lumen of affected arteries. The sign had a good sensitivity (73%) and excellent specificity (100%) in the diagnosis of GCA. Another reliable sign in evaluating GCA is compression US (8) that showed similar diagnostic performance to the halo sign. Strict definitions of normal and abnormal US findings (compression US and CDUS), as recently proposed by the OMERACT US LVV task force have also been published (9). The role of US in follow up is less apparent. It has been noted that the halo sign persists for months after steroid therapy (10), hence temporal artery biopsy (TAB) still has a role.
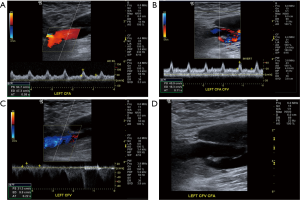
Subclavian steal syndrome
The subclavian and vertebral arteries are readily imaged in the entire course (11), as a result there are identifiable changes in the vertebral artery waveform that take place in relation to severity of subclavian artery stenosis, pre-steal is the earliest change which manifests as a mid-systolic notch also known as a “bunny waveform” (12) (Figures 2,3), flow remains antegrade throughout the cardiac cycle. If clinically indicated the waveform changes may be elicited by provocative maneuvers such as ipsilateral arm exercise or blood pressure cuff induced arm hyperemia. It should be noted though that subclavian steal phenomenon is almost always harmless.
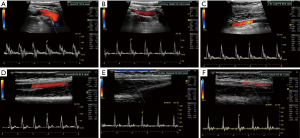
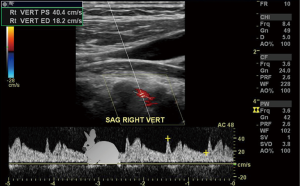
On the other hand, partial steal occurs with intermittent flow reversal in the ipsilateral vertebral artery.
Complete steal occurs with high grade stenosis or occlusion in the subclavian artery prior to origin of the vertebral artery. The flow is reversed throughout the cardiac cycle.
Usually caused by atherosclerotic occlusion, it has been reported to occur commonly in an iatrogenic setting such as during thoracic aortic endovascular repair (13), the role of left subclavian artery to carotid artery transposition is not established.
Arterial access
With rapid advancement in interventional radiology safe access to the arterial system is essential. The common femoral artery has been and still is the vessel of choice for most intravascular procedures. However, with miniaturization of catheters and endovascular devices the radial artery approach is rapidly gaining popularity.
Focal hematoma and bleeding at the site of vascular access (VA) is rare and particularly prevalent in octogenarians (14) vascular puncture site complications are usually minor, major bleeding is rare only about 6% of patients need a blood transfusion (15). Bleeding into the retroperitoneum can occur when VA is in the groin. In this case female gender, low body surface area and high femoral puncture are primary risk factors (16). Sonographic evaluation in these cases is limited and further imaging with computed tomography (CT) and/or angiography is needed depending on the clinical scenario.
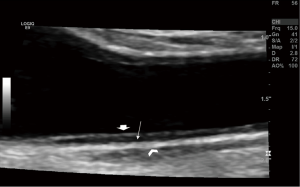
A Pseudoaneurysm is defined as a localized extravascular sac without all the vascular layers connected to the feeding artery by a narrow neck (16). Clinically presenting as a pulsatile and often tender mass following VA, Color Doppler US is the method of choice for evaluation of pseudoaneurysms. A typical “to-fro” spectral Doppler pattern is noted within the lesion with a yin and yang appearance on color Doppler (17) (Figures 4,5). US guided compression and thrombin injections have been used successfully in treating this entity (18,19).
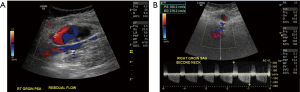
Arteriovenous fistula (AVF) is a less common VA complication than pseudoaneurysm. Data on its exact incidence is scant. Although clinically obvious due to a palpable bruit, CDUS is crucial in confirming clinical suspicion. On Doppler, there is arterialization of flow within the vein as a result of direct fistulous connection with the adjacent artery (16).
Dialysis fistula
Adequate VA is crucial for the dialysis patient (20). At the time of writing CDUS has three primary applications in this clinical setting:
- evaluating forearm vessels for surgical planning;
- assessment of maturation of the AVF;
- detecting complications at VA site.
Current international guidelines support the routine use of CDUS in pre-surgical planning of VA (21). Early surgical referral is essential in end stage renal failure, the goal is to achieve good arterial inflow and venous outflow compliance with optimal positioning of the arteriovenous (AV) anastomosis. Thus, CDUS is crucial in vascular mapping and detecting vessel characteristics such as calcifications which may make it unsuitable for fistula placement, anatomical variants can also be highlighted. It is unclear however if the role of CDUS in surgical planning translates into increased post op patency rates of the AVF.
Maturation of the VA is a dynamic process. Multiple factors that alter arterial inflow (stenosis, atherosclerosis etc.) or venous outflow (surgical traction, fibrotic valves etc.) may predispose the AVF to failure, CDUS therefore should be the first step in assessment of AVF maturation. The “rule of sixes “are often used as a guideline for adequacy—a blood flow rate in excess of 600 mL/min, diameter greater than 0.6 cm and depth of about 0.6 cm (21).
A prospective study conducted in 2,792 patients showed that reduced blood flow rate measurements were the primary predictor of AVF failure (22). Further criteria based on diameter assessment have also been proposed, as detailed in Table 1.
Lower limb
Peripheral arterial disease (PAD)
Catheter angiography remains the gold standard in diagnosis of PAD (24), however although it is almost always performed prior to endovascular intervention, its role in screening a symptomatic patient is unclear. Noninvasive imaging in PAD is useful for inpatient screening and stratification. It also facilitates appropriate patient selection and optimal PAD intervention outcomes (25).
The position statement on noninvasive imaging of PAD by the Society of Interventional Radiology (SIR) and the Canadian Interventional Radiology Association (26) describes two broad categories of noninvasive PAD testing, functional and anatomical.
Functional tests include ankle brachial index (ABI), segmental limb pressures, pulse volume recordings and segmental Doppler US. Anatomical tests include computed tomography angiography (CTA) and magnetic resonance angiography (MRA) and US. Hence US and physiologic testing together provide a comprehensive evaluation of PAD with anatomical and functional aspects. The reported sensitivity and specificity of CDUS is between 70–90% (27).
Color Doppler US should be performed in the relevant vascular segments of the limb being evaluated. It reliably assesses blood flow patterns in normal and diseased vessels. The normal peripheral arterial waveform is triphasic (1) the first positive deflection is forward systolic flow, the negative deflection or dip is the diastolic reversed flow and the third small positive deflection is late diastolic forward flow due to wall recoil, it correlates with wall elasticity. The ‘dip’ below baseline denotes the high resistance characteristics of all lower limb arteries, primarily contributed to by the muscular bed. Mild disease results in a biphasic waveform with loss of late diastolic forward flow (Figure 6). With increasing severity of narrowing the waveform then becomes monophasic with loss of flow reversal, slow systolic upstroke results with a classic ‘parvus tardus’ appearance (26) (Figure 7).
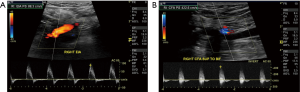

The limitations of Doppler US deserves mention here, the Doppler angle of insonation should be optimum at ≤60 degrees, an incorrect angle can alter the waveform and potentially change interpretation. The operators experience and awareness of this artefact is important. Another limitation of CDUS is in the evaluation of multilevel or ‘tandem’ stenosis. A stenotic lesion, once present alters hemodynamics distally resulting in reduced sensitivity of picking up further downstream stenotic lesions. Also, several studies have reported poor performance of CDUS in differentiating high grade stenosis from occlusion (28,29).
The normal peak systolic velocity (PSV) in peripheral lower limb arteries varies from 45–180 cm/s (30). Severe arterial disease manifests as a PSV in excess of 200 cm/s, monophasic waveform and spectral broadening of the Doppler waveform.
CDUS evaluation in the pre-surgical/endovascular intervention patient provides crucial information as to the number, extent of stenotic lesions and their precise location. It can also be used to mark skin incision sites to reduced contrast CT or MRI use (31).
Graft Evaluation is readily performed by CDUS. Arterial bypass graft flow velocities depend on conduit diameter, cardiac output and status of inflow and outflow arteries. At least 3–4 normal graft segments are evaluated (for a synthetic graft a minimum of 5 and many more for an in situ/reverse in situ). The normal velocities should range from 50–80 cm/s (30,32). Further, there are specific criteria for graft stenosis based on PSV as described below.
Abdomen and viscera
Abdominal aorta
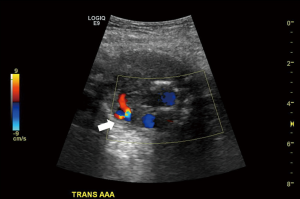
Although CT Angiography is the preferred modality of imaging the aorta, US is the screening test of choice, especially in smokers between the ages of 65–75 years (33). It is used to detect, document size of the aneurysm and monitor its progress, if any (11). Often, during the same scan it is valuable to exclude coexistent iliac artery aneurysms.
Dissection is characterized by a characteristic flow pattern on color Doppler (Figure 8). The use of CDUS is not limited to pre-operative imaging but is crucial in the post endovascular repair graft surveillance as well (34). An endoleak is the most frequent complication following an endovascular aortic repair (EVAR) procedure (35). The graft is close examined for integrity of its landing zones, very sensitive Doppler settings are needed to detect extra-stent flow. Operator experience plays a role as ‘bleed over’ of the color flow can simulate an endoleak. However, there is a subtle difference, the flow from a true endoleak is uniform, whereas apparent flow due to artefact occurs in flashes. A type I endoleak is characterized by flow at the landing zones of the graft, type II on the other hand reveals retrograde flow within the inferior mesenteric and lumbar arteries with ‘to-fro’ flow in the excluded sac (Figure 9). Additionally, the limb of the grafts is evaluated to look for stenosis.

The limitations of aortic US are common to most US examinations in the abdomen, Poor sonographic windows due to bowel gas, obesity and deep location of the area of interest often compromises the study. CT and MRA fare better in this respect.
Recent work has focused on CEUS, this uses an echogenic microbubble intravascular contrast agent injected IV while imaging the target vessel/organ. Normally only between 0.1 to 5 mL of contrast needs to be injected to visualize the vascular lumen. It has been known for some time that CEUS offers sensitivity and specificity comparable to CTA in aortic imaging (36). In the imaging of the post-operative aorta, a CTA provides only a snapshot of blood flow in the vessel, CEUS on the other hand has the potential to provide continuous imaging of flow for up to 3 minutes (37). Recent work has also highlighted the disadvantages of longstanding gadolinium administration such as deposition in the globus pallidus which can be detected even years after administration (38-40). CEUS thus may be an alternative to CTA and MRA, especially if contrast allergy, toxicity and radiation dose are of concern. CEUS adds significant value to a CDUS study especially by real time visualization of blood leaks for example in aortic rupture without delaying surgical treatment (41), further it was shown to be as effective as CTA and less invasive. Obviously, this requires a multidisciplinary approach and coordination between multiple specialties is crucial. CEUS has shown to be superior to CDUS in the imaging of endoleaks, some studies have shown it to be superior to even CTA (42,43). US contrast agents can be used safely in patients with renal dysfunction, further if the results of CEUS scanning are equivocal, the patient can always be referred for follow up CTA/MRA.
The use of CEUS is not without risk, the most common of which is an anaphylactic reaction, thought to occur in one of 100,000 cases (35). Further risks documented in medical literature deal with complications of venipuncture, which are exceedingly rare.
Renal arteries
The visualization of native renal arteries is heavily operator dependent and on the sonographic window, bowel gas in particular is responsible for the low rate of visualization of the proximal main renal arteries, quoted as being between 25–40% (44). This limitation has been circumvented by some investigators by measuring intra-renal waveforms, these are often readily visualized with reported diagnostic accuracy of >90% (45). The rationale being that the presence of a main renal artery stenosis (RAS) results in a parvus tardus waveform of the intrarenal vessels, with low PSV and extended acceleration times, a cutoff of >70 ms acceleration time and an acceleration of <3 m/s/s has been shown to be 96% accurate in detecting significant stenosis (46) (Figure 10).
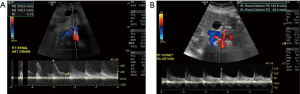
The imaging of renal transplant arteries is a natural extension of native renal artery Doppler. Vascular complications occur in fewer than 10% of renal transplants (47). RAS is the most common, usually occurring within the first year, most commonly at the anastomotic site. Findings include turbulent flow at the stenosis with parvus tardus waveform downstream, the PSV usually exceeds 250 cm/s and ratio of Renal artery to iliac PSV is in excess of 1.8 (Figure 11) (48). Rarely this finding may be caused by iliac artery stenosis.

Renal artery thrombosis is rare and occurs in less than 1% of cases (47), it occurs in the early post-operative period and is usually due to surgical technique causing kinking of the vessel. Absent arterial and venous flow with intraluminal arterial thrombus is the hallmark on CDUS, supported by clinical finding of a poor ‘blush’ in the graft on the operating table after vascular clamp release.
A Renal AVF or pseudoaneurysm can occur after a renal biopsy, biopsy is often performed for diagnosis of parenchymal renal disease in a native kidney or rejection in a transplant graft. An AVF is more common and is known to occur in 7.3% of cases after biopsy of a transplant graft (49). A ‘yin-yang’ color flow pattern is typical of a pseudoaneurysm. An AVF appears as a localized area of turbulent flow in the renal cortex, corresponding to the biopsy site. This represents the damaged segmental artery and the paired vein, large AVF may ‘steal’ blood flow from the rest of the graft resulting in ischemia. Angiography and embolization are usually required to manage these conditions.
The role of CEUS in evaluation of RAS in the transplanted and native kidneys is unclear (50).
Liver transplant
In the past few decades liver transplant has evolved into the definitive treatment for end stage liver failure. The indications have expanded as well with few contraindications (51). There is no upper age limit. Due to long waiting lists, one way to increase the pool of available organs is to split the liver from a living donor into the recipient, called a living donor liver transplant (LDLT). Usually the donor’s right lobe is transplanted into the recipient.
Although pre-operative evaluation is performed nowadays using CTA, US and CDUS evaluation still plays a role in the post op examination of these patients. It can be readily performed in the operating theatre or by the bedside in the immediate post op period.
Fulminant hepatic failure in the immediate post op period is usually caused by vascular complications, most commonly hepatic artery (HA) thrombosis (52). The HA anastomosis can be challenging due to small diameter of the vessel and difference in diameters of the host and recipient vessels, different anastomotic techniques have been used to circumvent this challenge (53). In the healthy graft the HA displays a biphasic waveform, the resistive index (RI) is the most common measurement acquired. The normal HA has an RI of between 0.55–0.8 (53) (Figure 12). A high RI in the HA during the early post op course is not unusual, this usually returns to normal in a few days. Close monitoring is essential though.
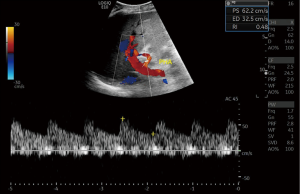
HA vasospasm can be seen in the post op period, this occurs due to excessive manipulation of the vessel on the operating table. It is difficult to differentiate other causes of high RI from vasospasm but reduction of RI values after vasodilator therapy aid in diagnosis.
HA thrombosis is a graft threating condition and needs emergent management, it occurs in between 1–12% of cases (54). This is readily diagnosed by US and CDUS by visualizing the echogenic thrombus in an artery without flow. Dampening of the PSV is a cardinal feature of impending thrombosis, as opposed to vasospasm where the PSV is high and shows rapid upstroke (53). Management is usually by revision surgery, or if unavailable, endovascular thrombolysis.
HA dissection is not uncommon, it is a risk factor for HA stenosis or occlusion and can occur on the recipient or donor side of the anastomosis. It is seen as an echogenic flap within the lumen of the artery with a ‘double lumen’ appearance. HA stenosis is a distinct entity, it is insidious in onset and can occur at the anastomotic site or due to a diseased donor artery. It can result in graft failure, biliary necrosis and sepsis. Prompt recognition is therefore, essential. The waveform is a typical parvus tardus with slow upstroke (acceleration time >0.08 s), the RI is reduced to <0.55. a turbulent jet at the anastomosis showing PSV in excess of 3 times that in the pre-anastomotic segment is usually diagnostic. Surgical revision or Endovascular dilation is the recommended treatment.
Conventional CDUS does not prove to be useful in 13% of cases (55), in these cases CEUS has demonstrated sensitivity and specificity close to 100%. It provides better visualization of the vascular flow. In fact, the use of CEUS can obviate the need for vascular angiography in more than 60% of cases (56,57).
Pancreas transplant
Whole organ pancreas transplant is an established treatment for diabetes and end stage renal failure. The most common transplant is a combined pancreas and kidney transplant (also known as simultaneous pancreas-kidney or SPK) which accounts for 78% of cases (58). This allows the recipient to be free of regular insulin use and from dialysis for end stage renal failure. The second most common form, accounting for about 16% of cases, is a pancreas transplant after kidney transplant (PAK), the recipient may receive a kidney from a living donor and can be free of dialysis while on waiting list for a cadaveric pancreas (59). The pancreatic graft is harvested with the donor duodenum and vascular supply. It is placed in the right lower quadrant of abdomen in the peritoneal cavity.
Surgical techniques vary but some basic principles are common. The donor superior mesenteric artery (SMA) and splenic artery supply the head and body/tail respectively. The donor common, internal and external iliac arteries are then attached to the SMA and splenic arteries forming a Y graft. This common iliac artery is then anastomosed to the recipient iliac artery.
Venous outflow contains the exocrine secretions, the donor portal vein serves as the main vein for the graft, this is usually anastomosed to the recipient superior mesenteric vein. The duodenum attached to the graft and containing the ampulla is usually anastomosed to an adjacent small bowel loop or bladder, to allow secretions to drain (58).
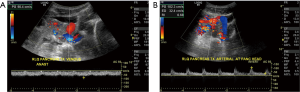
In the post op period the Y graft, artery and vein are readily visualized by CDUS. The artery shows rapid upstroke, the vein is anechoic and shows monophasic waveforms. The major role of US and CDUS in pancreatic transplant is to exclude vascular thrombosis and to guide biopsy (60). Appearances of the normal postoperative Doppler must be kept in mind (Figure 13). With arterial thrombosis the echogenic thrombus is seen within the lumen with absent flow. A venous thrombus is also readily visualized, the arterial waveforms in this case display high RI values. CDUS is also useful for detection of anastomotic vascular strictures and pseudoaneurysms (60), the findings are similar to stenosis or pseudoaneurysms in other arteries.
Conclusions
The applications of US and CDUS in arterial imaging are widespread and ever evolving. Noninvasive nature, portability and availability are the key strengths in clinical practice and in the hospital setting. In particular, the introduction of CEUS has the potential to revolutionize vascular imaging. With recent research highlighting the disadvantages of MRI contrast agents, this area of research has been renewed and will likely flourish.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Rumack CM. Diagnostic ultrasound. St. Louis: Elsevier Mosby, 2011.
- Merritt CR. Doppler US: the basics. RadioGraphics 1991;11:109-19. [Crossref] [PubMed]
- Czihal M, Lottspeich C, Hoffmann U. Ultrasound imaging in the diagnosis of large vessel vasculitis. Vasa 2017;46:241-53. [Crossref] [PubMed]
- Jennette JC. Overview of the 2012 revised International Chapel Hill Consensus Conference nomenclature of vasculitides. Clin Exp Nephrol 2013;17:603-6. [Crossref] [PubMed]
- Muratore F, Pipitone N, Salvarani C, et al. Imaging of vasculitis. State of the art. Best Pract Res Clin Rheumatol 2016;30:688-706. [Crossref] [PubMed]
- Schäfer VS, Juche A, Ramiro S, et al. Ultrasound cut-off values for intima-media thickness of temporal, facial and axillary arteries in giant cell arteritis. Rheumatology 2017;56:1479-83. [Crossref] [PubMed]
- Schmidt WA, Kraft HE, Vorpahl K, et al. Color Duplex Ultrasonography in the Diagnosis of Temporal Arteritis. N Engl J Med 1997;337:1336-42. [Crossref] [PubMed]
- Aschwanden M, Daikeler T, Kesten F, et al. Temporal artery compression sign--a novel ultrasound finding for the diagnosis of giant cell arteritis. Ultraschall Med 2013;34:47-50. [PubMed]
- Duftner C, Dejaco C, Moller Dohn U, et al. FRI0518 Ultrasound Definitions for Vasculitis in Cranial and Large Vessel Giant Cell Arteritis. Results of A Delphi Survey of The Omeract Ultrasound Large Vessel Vasculitis Group. Ann Rheum Dis 2016;75:626. [Crossref]
- Diamantopoulos AP, Myklebust G. Long-term inflammation in the temporal artery of a giant cell arteritis patient as detected by ultrasound. Ther Adv Musculoskelet Dis 2014;6:102-3. [Crossref] [PubMed]
- Phillips GW. Review of Arterial Vascular Ultrasound. World J Surg 2000;24:232-40. [Crossref] [PubMed]
- Ginat DT, Bhatt S, Sidhu R, et al. Carotid and Vertebral Artery Doppler Ultrasound Waveforms. Ultrasound Q 2011;27:81-5. [Crossref] [PubMed]
- Gulati M, Khadem N, Lekht I, et al. Subclavian steal following left subclavian artery occlusion during thoracic endovascular aortic repair. Doppler findings and literature review. J Ultrasound Med 2015;34:926-9. [Crossref] [PubMed]
- Dick P, Barth B, Mlekusch W, et al. Complications After Peripheral Vascular Interventions in Octogenarians. J Endovasc Ther 2008;15:383-9. [Crossref] [PubMed]
- Lauer MA, Karweit JA, Cascade EF, et al. Practice patterns and outcomes of percutaneous coronary interventions in the United States. 1995 to 1997. Am J Cardiol 2002;89:924-9. [Crossref] [PubMed]
- Mlekusch W, Mlekusch I, Sabeti-Sandor S. Vascular puncture site complications - diagnosis, therapy, and prognosis. Vasa 2016;45:461-9. [Crossref] [PubMed]
- Eisenberg L, Paulson EK, Kliewer MA, et al. Sonographically guided compression repair of pseudoaneurysms. further experience from a single institution. AJR Am J Roentgenol 1999;173:1567-73. [Crossref] [PubMed]
- Pezzullo JA, Dupuy DE, Cronan JJ. Percutaneous Injection of Thrombin for the Treatment of Pseudoaneurysms After Catheterization. AJR Am J Roentgenol 2000;175:1035-40. [Crossref] [PubMed]
- Hendricks NJ, Saad WE. Ultrasound-Guided Management of Vascular Access Pseudoaneurysms. Ultrasound Clin 2012;7:299-307. [Crossref]
- Lomonte C, Meola M, Petrucci I, et al. The Key Role of Color Doppler Ultrasound in the Work-up of Hemodialysis Vascular Access. Semin Dial 2015;28:211-5. [Crossref] [PubMed]
- Access Vascular. 2006 Work Group. Clinical Practice Guidelines for Vascular Access. Am J Kidney Dis 2006;48:S176-247. [PubMed]
- Malik J, Kudlicka J, Novakova L, et al. Surveillance of arteriovenous accesses with the use of duplex Doppler ultrasonography. J Vasc Access 2014;15 Suppl 7:S28-32.
- Lockhart ME. Doppler of hemodialysis fistula. In: Mohler ER, Gerhard-Herman M, Jaff MR, editors. Essentials Vasc Lab Diagnosis. Chichester: John Wiley & Sons, 2008:160-72.
- Rooke TW, Hirsch AT, Misra S, et al. 2011 ACCF/AHA Focused Update of the Guideline for the Management of Patients With Peripheral Artery Disease (updating the 2005 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2011;58:2020-45. [Crossref] [PubMed]
- Rooke TW, Hirsch AT, Misra S, et al. 2011 ACCF/AHA focused update of the guideline for the management of patients with peripheral artery disease (updating the 2005 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society for Vascular Medicine, and Society for Vascular Surgery. Catheter Cardiovasc Interv 2012;79:501-31. [Crossref] [PubMed]
- Dhanoa D, Baerlocher MO, Benko AJ, et al. Position Statement on Noninvasive Imaging of Peripheral Arterial Disease by the Society of Interventional Radiology and the Canadian Interventional Radiology Association. J Vasc Interv Radiol 2016;27:947-51. [Crossref] [PubMed]
- Tang GL, Chin J, Kibbe MR. Advances in diagnostic imaging for peripheral arterial disease. Expert Rev Cardiovasc Ther 2010;8:1447-55. [Crossref] [PubMed]
- Sensier Y, Hartshorne T, Thrush A, et al. A prospective comparison of lower limb colour-coded Duplex scanning with arteriography. Eur J Vasc Endovasc Surg 1996;11:170-5. [Crossref] [PubMed]
- Aly S, Sommerville K, Adiseshiah M, et al. Comparison of duplex imaging and arteriography in the evaluation of lower limb arteries. Br J Surg 1998;85:1099-102. [Crossref] [PubMed]
- AbuRahma AF. Overview of Noninvasive Vascular Techniques in Peripheral Arterial Disease. Noninvasive Peripher Arter Diagnosis. London: Springer London, 2010;15-24.
- Hingorani A, Ascher E, Marks N. Preprocedural imaging. new options to reduce need for contrast angiography. Semin Vasc Surg 2007;20:15-28. [Crossref] [PubMed]
- AbuRahma AF. Overview of Peripheral Arterial Disease of the Lower Extremity. Noninvasive Peripher Arter Diagnosis. London: Springer London, 2010:1-14.
- Anderson JL, Halperin JL, Albert NM, et al. Management of Patients With Peripheral Artery Disease (Compilation of 2005 and 2011 ACCF/AHA Guideline Recommendations). A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013;127:1425-43. [Crossref] [PubMed]
- Arko FR, Filis KA, Heikkinen MA, et al. Duplex scanning after endovascular aneurysm repair. an alternative to computed tomography. Semin Vasc Surg 2004;17:161-5. [Crossref] [PubMed]
- Mehta KS, Lee JJ, Taha AA, et al. Vascular applications of contrast-enhanced ultrasound imaging. J Vasc Surg 2017;66:266-74. [Crossref] [PubMed]
- Clevert DA, Schick K, Chen MH, et al. Role of contrast enhanced ultrasound in detection of abdominal aortic abnormalities in comparison with multislice computed tomography. Chin Med J (Engl) 2009;122:858-64. [PubMed]
- Rafailidis V, Fang C, Yusuf GT, et al. Contrast-enhanced ultrasound (CEUS) of the abdominal vasculature. Abdom Radiol (NY) 2018;43:934-47. [Crossref] [PubMed]
- Malikova H, Holesta M. Gadolinium contrast agents – are they really safe? J Vasc Access 2017;18:1-7.
- Ramalho J, Ramalho M. Gadolinium Deposition and Chronic Toxicity. Magn Reson Imaging Clin N Am 2017;25:765-78. [Crossref] [PubMed]
- Ramalho M, Ramalho J, Burke LM, et al. Gadolinium Retention and Toxicity—An Update. Adv Chronic Kidney Dis 2017;24:138-46. [Crossref] [PubMed]
- Catalano O, Lobianco R, Cusati B, et al. Contrast-Enhanced Sonography for Diagnosis of Ruptured Abdominal Aortic Aneurysm. AJR Am J Roentgenol 2005;184:423-7. [Crossref] [PubMed]
- Bendick PJ, Bove PG, Long GW, et al. Efficacy of ultrasound scan contrast agents in the noninvasive follow-up of aortic stent grafts. J Vasc Surg 2003;37:381-5. [Crossref] [PubMed]
- Napoli V, Bargellini I, Sardella SG, et al. Abdominal aortic aneurysm. contrast-enhanced US for missed endoleaks after endoluminal repair. Radiology 2004;233:217-25. [Crossref] [PubMed]
- Middleton WD. Doppler US evaluation of renal artery stenosis. past, present, and future. Radiology 1992;184:307-8. [Crossref] [PubMed]
- Taylor DC, Kettler MD, Moneta GL, et al. Duplex ultrasound scanning in the diagnosis of renal artery stenosis. a prospective evaluation. J Vasc Surg 1988;7:363-9. [Crossref] [PubMed]
- Stavros AT, Parker SH, Yakes WF, et al. Segmental stenosis of the renal artery. pattern recognition of tardus and parvus abnormalities with duplex sonography. Radiology 1992;184:487-92. [Crossref] [PubMed]
- Dodd GD, Tublin ME, Shah A, et al. Imaging of vascular complications associated with renal transplants. AJR Am J Roentgenol 1991;157:449-59. [Crossref] [PubMed]
- Rodgers SK, Sereni CP, Horrow MM. Ultrasonographic evaluation of the renal transplant. Radiol Clin North Am 2014;52:1307-24. [Crossref] [PubMed]
- Schwarz A, Gwinner W, Hiss M, et al. Safety and adequacy of renal transplant protocol biopsies. Am J Transplant 2005;5:1992-6. [Crossref] [PubMed]
- Granata A, Clementi S, Londrino F, et al. Renal transplant vascular complications. the role of Doppler ultrasound. J Ultrasound 2014;18:101-7. [Crossref] [PubMed]
- Chong WK. Ultrasound evaluation of liver transplants. Abdom Imaging 2004;29:180-8. [Crossref] [PubMed]
- Duffy JP, Hong JC, Farmer DG, et al. Vascular complications of orthotopic liver transplantation. experience in more than 4,200 patients. J Am Coll Surg 2009;208:896-903; discussion 903-5. [Crossref] [PubMed]
- Abdelaziz O, Attia H. Doppler ultrasonography in living donor liver transplantation recipients. Intra- and post-operative vascular complications. World J Gastroenterol 2016;22:6145. [Crossref] [PubMed]
- Abdelaziz O, Hosny K, Amin A, et al. Endovascular management of early hepatic artery thrombosis after living donor liver transplantation. Transpl Int 2012;25:847-56. [Crossref] [PubMed]
- Berstad AE, Brabrand K, Foss A. Clinical utility of microbubble contrast-enhanced ultrasound in the diagnosis of hepatic artery occlusion after liver transplantation. Transpl Int 2009;22:954-60. [Crossref] [PubMed]
- Claudon M, Dietrich CF, Choi BI, et al. Guidelines and Good Clinical Practice Recommendations for Contrast Enhanced Ultrasound (CEUS) in the Liver – Update 2012. Ultrasound Med Biol 2013;39:187-210. [Crossref] [PubMed]
- Sidhu PS, Shaw AS, Ellis SM, et al. Microbubble ultrasound contrast in the assessment of hepatic artery patency following liver transplantation. role in reducing frequency of hepatic artery arteriography. Eur Radiol 2004;14:21-30. [Crossref] [PubMed]
- Vandermeer FQ, Manning MA, Frazier AA, et al. Imaging of Whole-Organ Pancreas Transplants. RadioGraphics 2012;32:411-35. [Crossref] [PubMed]
- Sollinger HW, Odorico JS, Becker YT, et al. One thousand simultaneous pancreas-kidney transplants at a single center with 22-year follow-up. Ann Surg 2009;250:618-30. [PubMed]
- Nikolaidis P, Amin RS, Hwang CM, et al. Role of Sonography in Pancreatic Transplantation. RadioGraphics 2003;23:939-49. [Crossref] [PubMed]