Discrepancies between two lipid-lowering guidelines for CVD prevention in seemingly healthy individuals—case study Lebanon
Introduction
Estimating cardiovascular disease (CVD) risk is at the center of CVD prevention guidelines (1,2). Several evidence-based risk assessment models exist, including the Framingham Risk Scoring (FRS) (3), Heart Score (4), and Reynold’s (5) among others. The Canadian Cardiovascular Society (CCS) guidelines rely on the FRS system, whereas the European Society of Cardiology (ESC) relies on Heart Score. In many countries where no locally developed/adapted scoring systems exist, adopted models and guidelines are utilized instead. The questionable applicability of guidelines in communities different from those where they were developed has been suggested previously (6). To address this challenge, the World Health Organization (WHO) proposed in its Global Program on Evidence for Health Policy. Guidelines for WHO Guidelines, four criteria to judge applicability: (I) efficacy and safety; (II) cost-effectiveness; (III) affordability; and (IV) population benefits.
Recognizing the above gap, practitioners in the Middle East region, where no applicability studies have been conducted, endure several shortcomings of extrapolation of these guidelines imported from Western countries. For example, the ESC states in the 2011 Prevention of CVD Guidelines that scoring systems underestimate risk in (I) persons with family history of CVD and (II) in countries were the mortality from CVD has risen. Both of these factors are present in Middle Eastern countries, thus potentially leading to underestimation of risk and consequently denying therapy to persons in need. On another note, because of concerns of under-estimating risk among women, the American Heart Association recommended lowering the cut-point for classifying women as “high risk” to 10% Global CVD FRS (7). This is expected to increase the number of women who would qualify for interventions, leading to an increase in health expenditures. All the above have posed significant challenges to patients, healthcare providers, and public health authorities in several countries, including Lebanon.
To quantitatively assess this problem, the present study measured the agreement level between guidelines, based on the FRS (CCS) versus Heart Score (ESC) systems, in recommending lipid lowering interventions in a seemingly healthy Lebanese cohort.
Methods
The data for this study were drawn from the nation-wide Nutrition and Non-Communicable Diseases Risk factors cross-sectional survey conducted in Lebanon between years 2008 and 2009 (8). The sampling was random, multistage (by governate) and based on the age-sex distribution of the Lebanese population [Living Conditions of households: The National Survey of Household Living Conditions 2004; Lebanese Republic Ministry of Social Affairs/Central Administration for Statistics/UNDP, pages 114-115]. Survey participants older than 18 years of age and with no chronic diseases were contacted to give blood samples (n=1,331). From those participants invited to participate, 316 subjects provided written consent and gave a blood sample (response rate: 24.3%). The protocol was approved by the Institutional Review Board of the American University of Beirut. All the analysis was done on a de-identified dataset. The details of the data collection have been described in previous studies (8,9). Since diabetes mellitus type 2 (DM II) patients are coronary artery disease equivalents (and therefore their risks are calculated differently), we elected to remove those with FBS ≥126 from our analysis.
For the purpose of this study, the Global CVD FRS was calculated using the established formula (3). Individuals with FRS <10% were considered to be at low risk; those with 10%≤ FRS <20% were categorized at intermediate risk and those with FRS ≥20% were considered to be at high risk. As per the CCS, individuals with FRS <10% and low density lipoprotein cholesterol (LDL-C) >5 mmol, or FRS 10-19% and [LDL-C >3.5 mmol or Total Cholesterol to high density lipoprotein (TC/HDL) ratio >5], or FRS ≥20% should be on lipid lowering therapy (to convert mg/dL to mmol division by 39 was performed). FRS was calculated as described, but was multiplied by 2 for individuals with positive family history of coronary artery disease.
As per the ESC 2011 Guidelines, the Heart Score equation for High Risk Countries was used to calculate the risk for the individuals for this cohort coming from the Mediterranean area (4). Individuals with Heart Score risk <1%, ≥1% to <5%, ≥5% to <10%, ≥10% were categorized as low, intermediate, high and very high risk, respectively. Low risk individuals with LDL<100 mg/dL, LDL 100 to <190 mg/dL, and LDL ≥190 mg/dL require no lipid lowering intervention, lifestyle intervention, lifestyle intervention and should consider drug therapy, respectively. Individuals at Intermediate risk with LDL <100 mg/dL, or LDL ≥100 mg/dL require lifestyle intervention only, and lifestyle intervention and should consider drug therapy, respectively. High risk individuals with LDL <100 mg/dL require lifestyle intervention and should consider drug therapy, while those with LDL ≥100 mg/dL require immediate drug therapy. Individuals at very high risk with LDL <70 mg/dL require lifestyle intervention and should consider drug therapy, while those with LDL ≥70 mg/dL require immediate drug therapy.
Statistical analysis
Data management and analyses were carried out using the Statistical Analysis Software (SAS), version 9.0. Descriptive analysis was carried out by calculating the mean and standard deviation (sd) for continuous variables, and number and percent for categorical variables. Bivariate analyses were carried out by student’s t-test and chi-square test for continuous and categorical variables, respectively. The association between the two scores was assessed by using simple linear regression analyses. Agreement between the ESC and CCS was assessed by calculating the Kappa coefficient. A Kappa value of 1 indicates complete agreement, whereas a Kappa value of 0 indicates agreement equivalent to chance. Moreover, association between the two scores was assessed by calculating the Pearson correlation coefficient. Statistical significance was considered at the P-value cut-off point of 0.05. Bland Altman analysis was used to compare the Heart Score with the FRS.
Results
In total, 316 subjects were studied, and the baseline demographics and clinical indices are listed in Table 1. Of the cohort 8.2% and 5.7% were found to have undiagnosed increased SBP and DBP, respectively. Also, 10.44% were diagnosed with DM II, 23.7% were obese (BMI >30), 51.9% were found to be smokers and 46.8% had family history of premature coronary artery disease.
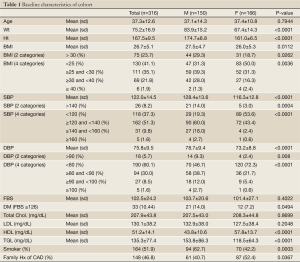
Full Table
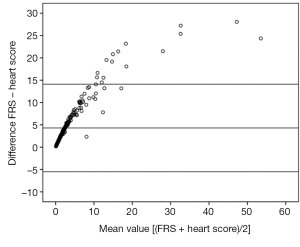
After excluding those found to be diabetic, the effect of use of various risk scoring systems and guidelines was investigated on the recommendations for lipid lowering interventions in this seemingly healthy cohort. FRS was found to have a significant linear relationship with Heart Score with a regression coefficient of 1.94 (Adj. R square 0.83, P<0.0001) for the overall cohort, 1.88 (Adj. R-Sq 0.85, P<0.0001) for Males, and 1.99 (Adj. R-Sq 0.48, P<0.0001) for Females. In the Bland Altman analysis, the majority of individuals had their Heart Score and FRS within the limits set for agreement (Figure 1). Upon categorizing the individuals, there were 85.5%, 9.2% and 4.6% low, intermediate and high risk persons as per FRS, respectively. As per the Heart Score there were 2.1%, 2.5%, 11.3%, and 84.1% very high risk, high, intermediate, and low risk as per the ESC categorization, respectively.
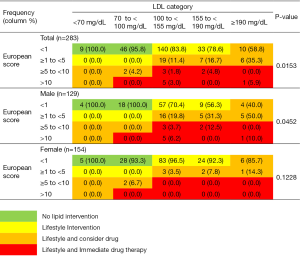
As per the ESC recommendations, 3.9% would warrant immediate drug therapy, 15.5% would be considered for drug therapy if they don’t respond to lifestyle measures and 61.1% would warrant lifestyle interventions. This means drug therapy is a choice in 19.4% of this cohort, and 80.1% require some form of intervention to lower LDL levels (Figure 2). In comparison, as per the CCS, 19.4% of seemingly health persons (70.9% were males) require medicinal intervention to lower lipids (Table 2).

Full Table
After combining those who require immediate therapy with those who may require drug therapy as per the ESC, the level of agreement between the ESC guidelines and the CCS guidelines on recommending medications to lower lipids was found to be good at Kappa =0.77 overall, 0.82 for males and 0.63 for females. The Kappa levels would become 0.80, 0.79 and 0.76 for the overall cohort, males and females respectively should the ESC risk be modulated for family history similar to the CCS recommendations (Table 3).
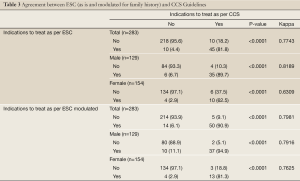
Full Table
Discussion
This study illustrated the differences arising from adopting the ESC versus the CCS CVD prevention guidelines in recommending lipid lowering interventions in a seemingly health sample from Lebanon. Around 80% of seemingly healthy persons would require interventions with 19.4% having drugs as a choice, either immediately or to be considered later, as per the ESC. As per the CCS, 19.1% would require drugs for intervention. The level of agreement between the ESC and CCS for recommending lipid lowering was moderate overall, and good in males, but lower limits of moderate for females with a Kappa level of 0.61. Modulation for family history seems to be the cause of this gender discrepancy as is suggested by an improved Kappa to 0.76 if risk modulation is performed in the ESC model. The high prevalence of family history of premature CAD in Lebanon, and the discrepancies arising from applying different guidelines in recommending lipid interventions prescription, to females specifically, pose important challenges to healthcare providers. Interventions to formulate standardized approaches based on rigorous applicability analyses are needed.
The very high prevalence of family history of CAD in Lebanon presents a unique challenge. Previous studies showed that family history of CAD accounts for near doubling of risk (10), thus absenting this risk factor in risk calculation is a source of error. In this cohort, the prevalence of this risk factor is around 46.8%. Hence, it is not surprising that incorporating this factor in the CCS algorithm to determine lipid lowering recommendations would differentiate it from the ESC algorithm. Understanding the reasons for this high prevalence of family history of CAD is beyond the scope of this study. However, these results are a call to pressure local healthcare authorities to put high on its priorities the need for locally validated risk scoring system that accounts for family history of CAD. The Reynold’s Risk Scoring system (5) does account for this factor, but this will come with the need for an added expense for measuring CRP.
The effect of excluding the modulatory influence of family history seems to particularly amplify the disagreement between the ESC and the CCS guidelines among women. Knowing that the predictive accuracy of risk scoring systems in women is questionable (11), a further hidden downstream effect arising from excluding family history might further weaken the overall guidelines’ impact on health outcomes. Similar to the CCS Guidelines, the ESC guidelines mention the need to modulate for family history in their text but, in contrary to the CCS, do not translate that into clear steps within the algorithm for downstream interventions. Reproducing this result in a larger sample size should alert adopters of the ESC guidelines to this shortcoming, and may urge the ESC guidelines committee to address this in a future update.
From an epidemiological standpoint, this study highlights that “seemingly healthy” masks significant CVD risk in individuals from Lebanon. The rising prevalence of obesity, undiagnosed HTN, DM II, family history of CAD and smoking is a threat and an opportunity. Efforts targeting combating the modifiable risk factors are well established to decrease unfavorable outcomes and cost of healthcare. The latter is an extremely important issue in a country with high debt, low wages, and relatively costly medications (12). In a previous study, our group showed that the cost of the cheapest original statin for primary prevention of 1 CV event in 5 years is at least 79,000 USD in comparison to a Gross National Income per Capita per year of 15,000 USD in Lebanon (13). The high need for intervention through lifestyle measures and therapeutics in 80.1% of the cohort, and with drugs being a choice in 19.4%, render access to medications a daily struggle to the Lebanese population not covered by insurance. Rightfully, this struggle amounts to be a public health problem. Strongly adopting the Best-Buys of the WHO to control non-communicable diseases is no longer a luxury but a compelling need to ensure the economic implications of non-communicable diseases are addressed (14).
Finally, adopting international CVD prevention guidelines without applicability analyses in each country is bound to lead to confusing the patients, the physicians, and local healthcare authorities/third party payers in Middle East. As shown from these results, disparities in recommending lipid lowering therapy will arise depending on (I) which risk scoring system is used (FRS vs. Heart Score); and (II) is family history modulated for in risk assessment. The latter seems to affect the decision of initiation of lipid interventions, more so in women. Considering the prevalence of CVD and their socioeconomic impact, the gravity of the situation is beyond the ability of local professional medical societies to manage and is a national issue qualifying for attention by the WHO.
This study has several limitations. The assumption that modulation for family history of premature CAD can be accomplished by multiplying CVD risk by 2 is a simplification. Previous studies have shown differences in this correcting factor whether this family history was in a sibling versus in parents (15), and also whether the individual whose risk is being calculated for is a male or female. Furthermore, as this factor was assessed for by self reporting, then it carries with it this method’s limitations. However, the factor of 2 for correction is the one recommended by the Canadian Cardiovascular Society for determining subsequent lipid interventions and is also highlighted in the European Society of Cardiology guidelines but indicating the factor to be 2 for males and 1.7 for females. Though performing a gender based calculation is possible, we opted not to hoping no further confusion is brought into the reader. Also, probably due to our sample size, the number of females that modulation affected their downstream recommendations was small; therefore, a repeat of this analysis on a larger sample size to reproduce our conclusion may be warranted. Including CV outcomes to verify risk stratification would have been ideal, however, outcomes for this cohort were not collected. The above limitations do not affect the final messages intended from this study which are: to (I) raise the awareness of physicians about the impact and significance of modulating for family history and (II) action is highly needed by healthcare authorities to address concerns which are at the fundamental basis of sound practice of prevention.
Learning points
- Agreement level between the ESC and CCS Guidelines in recommending lipid lowering is moderate overall, good in males and lower limits of moderate in females;/li>
- Including a modulating factor for Family History of CAD in the CCS algorithm and not in the ESC algorithm seems to lower the agreement levels between these guidelines;
- “Seemingly healthy” masks significant CVD burden in persons coming from Middle East Countries;
- 19.4% of seemingly healthy Lebanese require medical intervention for lipid lowering.
Acknowledgements
Disclosure: We wish to thank Dr. Anders Borglykke and Dr. M. Pencina for helping in the formulas for the Heart Score and Global FRS respectively. This was a secondary analysis from data collected from a study that was funded by the Training Programs in Epidemiology and Public Health Interventions Network (TEPHINET). Additional funds were contributed by the World Health Organization (WHO-Lebanon) and the Lebanese National Council for Scientific Research (LNCSR). Funders had no role in the study design, data collection, analysis, interpretation and reporting, and in the decision to submit the paper for publication.
References
- Genest J, McPherson R, Frohlich J, et al. 2009 Canadian Cardiovascular Society/Canadian guidelines for the diagnosis and treatment of dyslipidemia and prevention of cardiovascular disease in the adult - 2009 recommendations. Can J Cardiol 2009;25:567-79. [PubMed]
- Perk J, De Backer G, Gohlke H, et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur Heart J 2012;33:1635-701. [PubMed]
- D’Agostino RB Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 2008;117:743-53. [PubMed]
- HeartScore: cardiovascular disease (CVD) risk assessment and management. The interactive tool for predicting and managing the risk of heart attack and stroke in Europe.
- The Reynolds Risk Score: Preventing Stroke& Heart Disease in Women and Man. Available online: http://www.reynoldsriskscore.org
- Global Programme on Evidence for Health Policy Guidelines for WHO Guidelines. Geneva: World Health Organisation, 2003 (EIP/GPE/EQC/2003.1).
- Mosca L, Benjamin EJ, Berra K, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women--2011 update: a guideline from the american heart association. Circulation 2011;123:1243-62. [PubMed]
- Naja F, Nasreddine L, Itani L, et al. Dietary patterns and their association with obesity and sociodemographic factors in a national sample of Lebanese adults. Public Health Nutr 2011;14:1570-8. [PubMed]
- Naja F, Nasreddine L, Itani L, et al. Association between dietary patterns and the risk of metabolic syndrome among Lebanese adults. Eur J Nutr 2013;52:97-105. [PubMed]
- Lloyd-Jones DM, Nam BH, D’Agostino RB Sr, et al. Parental cardiovascular disease as a risk factor for cardiovascular disease in middle-aged adults: a prospective study of parents and offspring. JAMA 2004;291:2204-11. [PubMed]
- Brindle P, Beswick A, Fahey T, et al. Accuracy and impact of risk assessment in the primary prevention of cardiovascular disease: a systematic review. Heart 2006;92:1752-9. [PubMed]
- The World Bank, working for a world free of poverty. Available online: http://finances.worldbank.org/facet/countries/Lebanon
- Isma'eel H, Mohanna Z, Hamadeh G, et al. The public cost of 3 statins for primary prevention of cardiovascular events in 7 Middle East countries: not all of them can afford it. Int J Cardiol 2012;155:316-8. [PubMed]
- Bloom DE, Cafiero ET, Jané-Llopis E, et al. The Global Economic Burden of Noncommunicable Diseases. Geneva: World Economic Forum, 2011.
- Murabito JM, Pencina MJ, Nam BH, et al. Sibling cardiovascular disease as a risk factor for cardiovascular disease in middle-aged adults. JAMA 2005;294:3117-23. [PubMed]


