
Telerehabilitation with live-feed biomedical sensor signals for patients with heart failure: a pilot study
Introduction
Heart failure (HF) is a complex syndrome, defined by a weakness of the heart’s pumping action. Typical signs of HF are weak cardiac output, pulmonary or systemic congestion, and an increased risk of developing such conditions (1). Frequent clinical symptoms such as dyspnea, oedema, fatigue, and orthopnea lead to a reduced quality of life for patients suffering from this condition (2). In Canada, around 500,000 people live with HF and 50,000 diagnoses are made each year (3). One in 5 Canadians aged 40 years and over will be affected by HF, with a 23% mortality rate at 1 year. Hospitalization rates due to HF should triple by 2050, leading to subsequent increased healthcare costs (4). In 2005, international recommendations concerning HF treatment included drugs and a global care plan involving diet control, regular physical activity, and education (5).
Cardiac rehabilitation (CR) supports the global care initiative, adding a psychological and social aspect to its care plan (6). In preventing deconditioning, CR gives tools to patients with HF to better manage their symptoms and to reduce future hospitalizations and associated costs (7-11). Despite the proven benefits of CR, only 34% of eligible patients are directed to this program (12-14) and 20% of them actually complete the program (15). Factors associated with underutilization of CR include difficult access to CR, availability, travelling issues caused by lack of transportation, poor health (16), financial costs, and other personal barriers (17).
Telerehabilitation is one of the proposed solutions to meet the increasing demand and improve access to HF care programs. This method of healthcare service delivery uses telecommunication technologies allowing the transmission of real-time audio and video data over the Internet. This approach allows the patient to receive interventions at home while the healthcare professionals remain at their work location (18). Telerehabilitation has already proven to be effective in many patient populations, including patients burdened by strokes (19), multiple sclerosis (20,21), or knee replacements (22-24). However, only a few telerehabilitation studies exist in the cardiorespiratory population.
In CR, Hwang et al. (25) conducted a non-inferiority RCT with 53 patients with chronic HF. Patients received a 12-week, real-time exercise/education intervention program, either using online group-based videoconferencing software (experimental) or a traditional hospital outpatient-based program (control). Results showed no significant difference in clinical outcomes between the groups, and observed significantly higher attendance rates in the telerehabilitation group. The telerehabilitation intervention group demonstrated non-inferiority over traditional methods despite the lack of real-time monitoring of vital signs with participants’ vitals only reported once verbally, at the beginning of each intervention session. However, this lack of simultaneous monitoring of vital signs could have had an impact on the personalization and the intensity of the program, as the exercises could not be adjusted to the patient’s condition while they were being executed. In such a fragile population, real-time data could also prove useful as a safety feature to detect and prevent cardiac problems that could occur during the sessions.
In this context, sensors were included in our telerehabilitation platform, allowing real-time transmission to the clinician of the electrocardiogram (ECG) signal, oxygen saturation, and heart rate through the system for the entire length of each exercise session. These clinical data could help optimize the exercise prescription and make timely adjustments to the patient’s condition, if needed, to ensure the patient’s safety.
To our knowledge, no studies exist on the telerehabilitation of patients with HF using a videoconferencing system, including live-feed sensors to monitor real-time vital signs. Thus, this pilot study’s focus is to demonstrate the feasibility of the use of real-time ECG signals in the rehabilitation of HF patients with the aim of improving their functional capacity and quality of life.
Methods
Design
This pilot study used a pre-/post-test device without a control group. Assessments were performed before the treatment (T1) and one month after the end of treatment (T2), as shown in Figure 1.
Sample
Patients with HF were recruited by a cardiologist from the Centre intégré universitaire de santé et de services sociaux de l’Estrie - Centre hospitalier universitaire de Sherbrooke (CIUSSS de l’Estrie-CHUS). If the participant was interested in participating in the study, a research agent reached him/her by phone to further explain the study, verified admissibility criteria, and scheduled the T1 assessment. Inclusion criteria were to have: (I) a HF diagnosis with a left ventricular ejection fraction (LVEF) ≤40%, which is in accordance with the definition of HF; (II) a score of I, II or III on the New York Heart Association (NYHA) scale (26); (III) >18 years old; (IV) medical stability 4 weeks prior to T1; (V) no CR in the past 12 months; (VI) physician’s approval to do exercises; (VII) a sufficient understanding of verbal and written instructions to follow a remote treatment; (VIII) access to a high-speed Internet connection. Exclusion criteria were to have: (I) inability to give informed consent; (II) a rheumatic, articular, or neuromuscular pathology preventing use of a stationary bicycle; (III) an unstable cardiac condition; (IV) oxygen dependency; (V) a LVEF ≤35% without pacemaker/defibrillator, for security reasons.
The study was previously approved by the Research Ethics Board of the CIUSSS de l’Estrie-CHUS (No. 2016-1175) and the informed consent was obtained from all patients.
Telerehabilitation platform
The telerehabilitation platform that was used in this study is presented in Figure 2. A clinician system was installed on a dedicated computer at the clinic. A speaker, microphone and a pan-tilt-zoom (PTZ) camera were connected to the computer. A similar system was installed at each participant’s home. On the software side, a solution developed by the research team, Vigil2, allowed the clinician to execute the telerehabilitation sessions and provided remote control on both cameras (local and remote), allowed for live bi-directional audio and video communication with a secure connection over the Internet, allowed for live sensor data streaming and provided an easy to use experience for both the clinician and the patient. Work is now in progress to open-source that software for general use and to facilitate the distribution of the platform.
As an innovative device used in cardiac telerehabilitation, the patient had two commercial biomedical sensors wirelessly transmitting a real-time ECG signal (180° eMotion Faros device), oxygen saturation, and heart rate (Nonin WristOx2 3150 device) to the clinician’s system. The ECG signal was validated remotely in real-time by a nurse clinician to detect abnormal cardiac function during the exercises. The cardiology team trained the physiotherapist to detect any critical events.
Tele-treatment
Each session lasted approximately one hour, beginning with a quick screening of HF symptoms to evaluate for any contraindications to exercise: dyspnea, dizziness, ankle and belly oedema, fatigue or weakness, and sudden weight gain. The program included a warm up, cardiovascular exercises on a stationary bicycle, strengthening exercises, flexibility exercises, and a cool down. Exercise intensity was established depending on the clinical data (heart rate, ECG and oxygen saturation) transmitted to the clinician by platform-integrated sensors. The rehabilitation program lasted 12 weeks, with 3 sessions per week. For the first 2 weeks, the 3 sessions were done with supervision over telerehabilitation. For the weeks 3 to 7, 2 sessions were supervised and 1 was performed without supervision by the patient at home. Finally, for weeks 8 to 12, only 1 session was supervised and 2 were performed without supervision. Decreasing the supervised sessions to the benefit of unsupervised sessions was done to encourage patient responsibility to maintain an active lifestyle, and be aware of their limits. To help them, participants had to fill out a journal for the duration of the program regarding the exercises done, rate of perceived exertion on the Borg scale, saturation, and heart rate. Exercise progression was personalized, based on each patient’s response to the entire program: execution of the exercises, respect of the target cardiac rate, of the Borg scale, and the ECG.
Outcome measures
Feasibility of using live-feed ECG was assessed by the rate of non-valid ECG signal during the 45 minutes of training. Phone calls made to the technician to solve problems (log book) were collected.
Efficacy of the telerehabilitation intervention was established by the change of physiological and functional variables between T2 and T1. The primary outcome of the study was the functional capacity change between T1 and T2 as measured by Cardiopulmonary Exercise Testing (CPX) with simultaneous monitoring of respiratory gases (27). The patient had to pedal on a stationary bicycle in a dynamic and progressive way in order to observe functional adaptations and to link them to symptoms. On average, the duration of the test ranged between 10 and 12 minutes (28). Two variables from the CPX were analyzed: (I) the VO2 peak, and (II) the ventilatory adaptation threshold (SV1). The VO2 peak indicates heart function and the maximal oxygen uptake and the SV1 indicates aerobic capacity. These variables are good markers of CR improvement (29,30).
Two other physical outcomes were measured: the 6-minute walk test (6MWT) and the sit to stand test (STST). The 6MWT requires a 100-foot hallway, and it measures the distance that a patient can quickly walk on a flat and hard surface, for 6 minutes (31,32). The 6MWT provides information regarding functional capacity, response to therapy, and prognosis across a range of chronic cardiopulmonary conditions. Heart rate and saturation were taken both before and after the 6MWT to measure the patient’s response to an endurance exercise and recovery. A distance of less than 350 meters is associated with increased mortality in COPD, chronic HF, and pulmonary arterial hypertension (31,32). The distance of 54 meters is the minimal detectable change for patients with COPD (95% confidence interval: 37–71 m) (31). The sit-to-stand (STS) (33) measures the general lower extremity strength by recording the time required to complete 5 successive “sit-to-stand” repetitions (33,34). The test-retest reliability of the STS is high (ICC =0.89).
To measure quality of life, the Kansas City Cardiomyopathy Questionnaire (KCCQ) was used. This test is valid, reproducible and sensitive for patients with HF. It includes 15 questions on the influence of HF on quality of life and contains 23 items subdivided in 7 domains: “Physical Limitation (6 items), Symptom Stability (1 item), Symptom Frequency (4 items), Symptom Burden (3 items), Self-Efficacy (2 items), Quality of Life (3 items) and Social Limitations (4 items)” (35). Each item is auto-administered by the patient, and quoted on a numeric scale for a total score between 0 and 100. A high score shows a better health status (35). Two subscores can be measured: (I) overall summary, which regroups the mean of the physical limitation, total symptoms, quality of life, and social limitation, and (II) clinical summary that is the mean of the physical limitation and total symptoms. For both subscores, a significant change is defined as an increase of more than 5 points (36).
Data collection procedures
Outcomes were measured twice, once before the intervention (T1) and 1-month post-intervention (T2). An independent research clinician carried out both evaluations. The first evaluation was split over 2 days.
On the first day, patients signed the consent form and then answered the KCCQ and performed physical tests with a trained member of the research team. On the second day, the cardiac clinical team (cardiologist, nurse, kinesiologist) performed the evaluation. First, an ECG was used to confirm HF. Then, the cardiologist validated the patient’s medical stability and optimized the co-morbidity treatments before executing the CPX. Following this meeting, a nurse clinician and a kinesiologist met with the patient to educate him/her on his/her medical condition. They established maximal heart rate goal during exercise and training intensity using the perceived exertion on the Borg scale.
The second evaluation (T2) was identical to T1, except that there was no meeting with the nurse or kinesiologist.
Results
Participants
Five participants were recruited to take part in this study. However, one of them was excluded after initial assessment (T1) because he was hospitalized due to problems with his pacemaker and an unstable cardiac condition with fluid retention. From the four remaining participants, one (participant 1) completed only ten weeks of the program before having to stop for an unrelated hip surgery. Data of T1 and T2 evaluations were completed nonetheless, and will be considered in the final results, except for the CPX.
Sociodemographic characteristics of the remaining four participants are detailed in Table 1.

Full table
Feasibility of using live-feed ECG
Clinical signs (heart rate, ECG, and oxygen saturation) were transmitted to the clinician in real-time at each session. Physiotherapists reported no loss of ECG signal that could have had an impact on monitoring the intensity of the exercises. No phone call for assistance was addressed to the technician during the session, due to difficulties with the Internet connection or sensor signals. Clinicians and the cardiologist involved in the study were satisfied with the quality of signals, as they were good enough to corroborate their clinical judgement concerning patient fatigue. Thus, sensors led to a real-time adaptation of exercise intensity, according to each participant’s current condition. No HF decompensation was detected during training sessions.
Effect of telerehabilitation with live-feed ECG on physiological variables
The results obtained from the CPX, such as the VO2 peak and the SV1, are shown in Table 2. Participant 1 was unable to perform the CPX at T2 because of his hip surgery. Participant 4’s data is not shown in Table 2, because his performance on the CPX at T1 was submaximal, due to severe knee pain, and he has not yet taken the test again at T2. For reasons unknown, the patient did not show up for his appointment for CPX at T2.
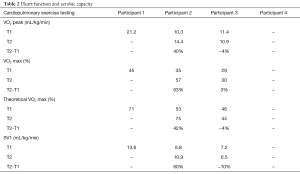
Full table
After 12 weeks of a telerehabilitation program, both participants who had completed T1 and T2 improved their VO2 max. However, participant 2 improved his aerobic capacity to 4.1 mL/kg/min and participant 3 experienced a decrease in his aerobic capacity of 0.5 mL/kg/min.
Effect of telerehabilitation with live-feed ECG on physical variables
At T2 compared to T1, all participants except one experienced an improvement at the 6MWT (see Table 3). The mean variation of walking distance was 44 meters (T2: 405 m; T1: 362 m). Participants perceived exertion after the test, which was similar after both 6MWT evaluations, with a Borg score ranging from 1 to 6. Moreover, the number of repetitions done in the STST was improved for 3 out of the 4 participants.

Full table
KCCQ overall and clinical summary scores improved post-intervention for all participants, as shown in Table 4. Moreover, for participants 2 and 3, a significant clinical change was observed on the overall score (difference of 17 and 6 points, respectively) and for participants 1 and 3, a significant clinical change was observed in the clinical summary score (9 and 8 points, respectively).

Full table
Discussion
The objective of this study was to evaluate the feasibility and the usefulness of real-time biomedical sensors in telerehabilitation with patients with HF. Moreover, gains in functional capacity, quality of life, and satisfaction towards this type of service delivery were also investigated.
First, our study demonstrated that real-time biomedical sensors can be safely used in a valid way by clinicians during a telerehabilitation session. These sensors were useful and helped to properly adjust the level of intensity of the exercises expected from each HF patient. Second, the results of this study demonstrated a trend for general improvement in the participants’ functional capacity.
On the 6MWT, 3 of the 4 participants walked a greater distance at T2, which demonstrated an increase in functional capacity, as well as a good response to exercise (42, 49 and 40 m). The use of real-time biomedical sensors has probably helped in the improvements noticed by optimizing the training while making it safe, but their contribution to such improvements remains to be further tested in other studies. Previous telerehabilitation studies (25,37) comparing this delivery method with standard CR (SCR) have shown similar results to ours. One of Piotrowicz’s studies (37) was done with HF patients (n=152), and demonstrated significant improvements in the 6MWT and VO2 peak in both telerehabilitation and SCR groups with an 8-week program. An increase of 11% of the 6MWT was noted for the telerehabilitation group, and 16% for the SCR group, which is similar to the results obtained in our study (improvement ranging from 9% to 16%). In the non-inferiority RCT conducted by Hwang et al. (25) results obtained showed no significant difference between groups on the 6MWT gains (mean difference of 15 m, 95% CI: –28 to 59).
Third, our study showed an increase in the quality of life for all the participants. Three participants out of four even experienced a significant improvement in one or both KCCQ categories, since their T2 score increased by over 5 points (36). Finally, there was high level of satisfaction for all participants regarding the healthcare services received, and their telerehabilitation experience is congruent with the satisfaction rate obtained in previous studies on telerehabilitation (38,39).
Clinical implication of the study
Our study shows that it’s feasible to integrate real-time ECG (cardiac rhythm and oxygen saturation) in telerehabilitation platforms during a remote CR program in a HF population.
As demonstrated in the literature (12-17), accessibility of CR is largely challenging, particularly outside ultra-specialized centers. In this context, it may possible to consider telerehabilitation as a compromise to increase access to CR in other centers. Furthermore, some patients having access to CR in ultra-specialized centers cannot get to the services: local transportation, caregiver availability, fatigue, etc. In both situations, telerehabilitation may be a solution. However, the cost of usual CR in groups was a weakness in telerehabilitation. The number of 4 participants at a time versus 15 is a cost-efficiency challenge against the implementation of CR telerehabilitation. We will consider this in the clinical trial: standard CR in gyms should be encouraged, but for more unstable patients who require a more specific follow-up during CR or for those who cannot attend the CR group; these patients could be directed to telerehabilitation.
Limitations
The small number of participants is a weakness and a reality in pilot studies: no generalization is allowed. However, the feasibility of using live-feed ECG is not disputed. This pilot study justified the need for a clinical trial: who could benefit from CR rehabilitation? What is the social imputability of offering an alternative to usual care to increase accessibility and at what cost? Our team will address these important research questions.
Conclusions
There is a need to enhance access to HF care programs. Telerehabilitation is an alternative to outpatient CR for people with HF who face transportation and accessibility problems. Our study not only proves the feasibility of cardiac telerehabilitation, including biomedical sensors from a technological point of view, but also demonstrates a high level of satisfaction towards the services received. As well, our study’s use of sensors creates a safe environment for the patient with a personalized level of exercise training intensity.
Future research should concentrate on comparing the effectiveness of telerehabilitation in patients with HF, and the results gained in a normal setting of CR. It would increase this alternative’s credibility as another possibility of CR in regions with reduced access to such services.
Acknowledgments
The authors would like to thank the CR team of the CIUSSS de l’Estrie-CHUS, in particularly Carl Fortier and Marie-Christine Noiseux, for their help in participant recruitment and evaluation. This research was supported by funding from the Research Chair in Telerehabilitation.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was previously approved by the Research Ethics Board of the CIUSSS de l’Estrie-CHUS (No. 2016-1175) and the informed consent was obtained from all patients.
References
- Arnold JM, Liu P, Demers C, et al. Canadian Cardiovascular Society consensus conference recommendations on heart failure 2006: diagnosis and management. Can J Cardiol 2006;22:23-45. [Crossref] [PubMed]
- Riley J. Practical issues in acute heart failure management. Br J Cardiac Nurs 2014;9:18-24. [Crossref]
- Ross H, Howlett J, Arnold JM, et al. Treating the right patient at the right time: access to heart failure care. Can J Cardiol 2006;22:749-54. [Crossref] [PubMed]
- O'Meara E, Thibodeau-Jarry N, Ducharme A, et al. The epidemic of heart failure: a lucid approach to stemming the rising tide. Can J Cardiol 2014;30:S442-54. [Crossref] [PubMed]
- Hunt SA. American College of Cardiology. American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure). ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure). J Am Coll Cardiol 2005;46:e1-82. [Crossref] [PubMed]
- Monpère C. Recommandations de la société francaise de cardiologie concernant la pratique de la réadaptation cardiovasculaire chez l'adulte. Arch Mal Coeur 2002;95:963-97.
- Gibbs CR, Jackson G, Lip GY. ABC of heart failure. Non-drug management. BMJ 2000;320:366-9. [Crossref] [PubMed]
- Ducharme A, Doyon O, White M, et al. Impact of care at a multidisciplinary congestive heart failure clinic: a randomized trial. CMAJ 2005;173:40-5. [Crossref] [PubMed]
- McAlister FA, Stewart S, Ferrua S, et al. Multidisciplinary strategies for the management of heart failure patients at high risk for admission: a systematic review of randomized trials. J Am Coll Cardiol 2004;44:810-9. [PubMed]
- Belardinelli R, Georgiou D, Cianci G, et al. Randomized, controlled trial of long-term moderate exercise training in chronic heart failure: effects on functional capacity, quality of life, and clinical outcome. Circulation 1999;99:1173-82. [Crossref] [PubMed]
- Rich MW. Office management of heart failure in the elderly. Am J Med 2005;118:342-8. [Crossref] [PubMed]
- Dafoe W, Arthur H, Stokes H, et al. Universal access: but when? Treating the right patient at the right time: access to cardiac rehabilitation. Can J Cardiol 2006;22:905-11. [Crossref] [PubMed]
- Witt BJ, Jacobsen SJ, Weston SA, et al. Cardiac rehabilitation after myocardial infarction in the community. J Am Coll Cardiol 2004;44:988-96. [Crossref] [PubMed]
- Candido E, Richards JA, Oh P, et al. The relationship between need and capacity for multidisciplinary cardiovascular risk-reduction programs in Ontario. Can J Cardiol 2011;27:200-7. [Crossref] [PubMed]
- Suaya JA, Shepard DS, Normand SL, et al. Use of cardiac rehabilitation by Medicare beneficiaries after myocardial infarction or coronary bypass surgery. Circulation 2007;116:1653-62. [Crossref] [PubMed]
- Mampuya WM. Cardiac rehabilitation past, present and future: an overview. Cardiovasc Diagn Ther 2012;2:38-49. [PubMed]
- Neubeck L, Freedman SB, Clark AM, et al. Participating in cardiac rehabilitation: a systematic review and meta-synthesis of qualitative data. Eur J Prev Cardiol 2012;19:494-503. [Crossref] [PubMed]
- Ricker JH, Rosenthal M, Garay E, et al. Telerehabilitation needs: a survey of persons with acquired brain injury. J Head Trauma Rehabil 2002;17:242-50. [Crossref] [PubMed]
- Johansson T, Wild C. Telerehabilitation in stroke care--a systematic review. J Telemed Telecare 2011;17:1-6. [Crossref] [PubMed]
- Finkelstein J, Lapshin O, Castro H, et al. Home-based physical telerehabilitation in patients with multiple sclerosis: a pilot study. J Rehabil Res Dev 2008;45:1361-73. [Crossref] [PubMed]
- Gutiérrez RO, Galan Del Rio F, Cano de la Cuerda R, et al. A telerehabilitation program by virtual reality-video games improves balance and postural control in multiple sclerosis patients. NeuroRehabilitation 2013;33:545-54. [PubMed]
- Piqueras M, Marco E, Coll M, et al. Effectiveness of an interactive virtual telerehabilitation system in patients after total knee arthoplasty: a randomized controlled trial. J Rehabil Med 2013;45:392-6. [Crossref] [PubMed]
- Moffet H, Tousignant M, Nadeau S, et al. In-Home Telerehabilitation Compared with Face-to-Face Rehabilitation After Total Knee Arthroplasty: A Noninferiority Randomized Controlled Trial. J Bone Joint Surg Am 2015;97:1129-41. [Crossref] [PubMed]
- Tousignant M, Moffet H, Boissy P, et al. A randomized controlled trial of home telerehabilitation for post-knee arthroplasty. J Telemed Telecare 2011;17:195-8. [Crossref] [PubMed]
- Hwang R, Bruning J, Morris NR, et al. Home-based telerehabilitation is not inferior to a centre-based program in patients with chronic heart failure: a randomised trial. J Physiother 2017;63:101-7. [Crossref] [PubMed]
- Bennett JA, Riegel B, Bittner V, et al. Validity and reliability of the NYHA classes for measuring research outcomes in patients with cardiac disease. Heart Lung 2002;31:262-70. [Crossref] [PubMed]
- Opasich C, Pinna GD, Bobbio M, et al. Peak exercise oxygen consumption in chronic heart failure: toward efficient use in the individual patient. J Am Coll Cardiol 1998;31:766-75. [Crossref] [PubMed]
- Aguilaniu B, Richard R, Costes F, et al. Méthodologie et Pratique de l’Exploration Fonctionnelle à l’eXercice (EFX). Rev Mal Respir 2007;24:114-60. [Crossref]
- Piña IL, Apstein CS, Balady GJ, et al. Exercise and heart failure: A statement from the American Heart Association Committee on exercise, rehabilitation, and prevention. Circulation 2003;107:1210-25. [Crossref] [PubMed]
- Experience from controlled trials of physical training in chronic heart failure. Protocol and patient factors in effectiveness in the improvement in exercise tolerance. European Heart Failure Training Group. Eur Heart J 1998;19:466-75. [Crossref] [PubMed]
- Rasekaba T, Lee AL, Naughton MT, et al. The six-minute walk test: a useful metric for the cardiopulmonary patient. Intern Med J 2009;39:495-501. [Crossref] [PubMed]
- Bittner V, Weiner DH, Yusuf S, et al. Prediction of mortality and morbidity with a 6-minute walk test in patients with left ventricular dysfunction. SOLVD Investigators. JAMA 1993;270:1702-7. [Crossref] [PubMed]
- Bohannon RW. Sit-to-stand test for measuring performance of lower extremity muscles. Percept Mot Skills 1995;80:163-6. [Crossref] [PubMed]
- Csuka M, McCarty DJ. Simple method for measurement of lower extremity muscle strength. Am J Med 1985;78:77-81. [Crossref] [PubMed]
- Spertus JA, Jones PG. Development and Validation of a Short Version of the Kansas City Cardiomyopathy Questionnaire. Circ Cardiovasc Qual Outcomes 2015;8:469-76. [Crossref] [PubMed]
- Spertus J, Peterson E, Conard MW, et al. Monitoring clinical changes in patients with heart failure: a comparison of methods. Am Heart J 2005;150:707-15. [Crossref] [PubMed]
- Piotrowicz E, Baranowski R, Bilinska M, et al. A new model of home-based telemonitored cardiac rehabilitation in patients with heart failure: effectiveness, quality of life, and adherence. Eur J Heart Fail 2010;12:164-71. [Crossref] [PubMed]
- Kairy D, Tousignant M, Leclerc N, et al. The patient's perspective of in-home telerehabilitation physiotherapy services following total knee arthroplasty. Int J Environ Res Public Health 2013;10:3998-4011. [Crossref] [PubMed]
- Tousignant M, Boissy P, Moffet H, et al. Patients' satisfaction of healthcare services and perception with in-home telerehabilitation and physiotherapists' satisfaction toward technology for post-knee arthroplasty: an embedded study in a randomized trial. Telemed J E Health 2011;17:376-82. [Crossref] [PubMed]




