Doppler echocardiographic assessment of pulmonary artery pressure in children with sickle cell anaemia
Introduction
Sickle cell anemia (SCA) is the commonest form of sickle cell disease (SCD) which results from a homozygous state comprising two S genes (HbS) (1). This autosomal recessive genetic disorder results when there is an abnormal substitution of valine for glutamic acid at the position six of the β globin chain (1,2). It is one of the commonest genetic disorders occurring worldwide with a prevalence rate of 20–25 million worldwide, 12–15 million of which are in Sub-Saharan Africa (3). It is estimated that around 250,000 children worldwide are born with homozygous SCA every year (3,4). Approximately 6 million Nigerians are reported to be afflicted with the disease (4). It is a disease condition that carries significant risk of morbidity and mortality manifesting with complications in almost every organ of the body (1,5). One of such complications is an elevated pulmonary artery pressure (6,7).
Pulmonary artery hypertension (PAH) is defined as a mean pulmonary artery pressure (MPAP) of greater than or equal to (≥) 25 mmHg at rest or ≥30 mmHg during exercise as determined by cardiac catheterization irrespective of age except in infancy (8,9). It is a widely recognized complication of hereditary hemolytic anemia which includes SCA (10,11). PAH has been reported to occur in 32% of adult patients with SCD (12). However, limited data exists on the prevalence and natural history of PAH among children with SCA in Africa (13,14). PAH ultimately leads to straining of the right ventricle and increases the risk of heart failure in affected patients (11). The clinical presentation of PAH in the early stage is usually nonspecific (15). Its early symptoms are similar to those of many other diseases that present with dyspnoea on exertion, thus, delaying the diagnosis until the disease is far advanced (6,8). The gold standard of measurement of MPAP is by right sided heart catheterization but this method is highly invasive and not suitable for screening purposes (5). Measurement of the tricuspid regurgitant velocity (TRV) by Doppler echocardiography to estimate the peak right ventricular systolic pressure (RVSP), hence, pulmonary artery systolic pressure (PASP) has been found to be the most accurate noninvasive method to determine PAH and is almost always used (5,10,16). In the absence of structural obstruction to pulmonary blood flow, the peak RVSP is equal to PASP quantified by Doppler echocardiography (5). The TRV measured by Doppler echocardiography is further translated mathematically using the modified Bernoulli equation to quantify the peak RVSP. PASP is equal to the Bernoulli derived peak systolic right ventricular pressure in the absence of obstruction to pulmonary blood flow (5,13). TRV values of ≥2.5 m/s on Doppler echocardiography equating to PASP of 30 mmHg and above derived from the modified Bernoulli equation are correlated with PAH (17-20). Earlier studies reported the prevalence of PAH among children with SCD in steady state to be between 16% to 35% (5,17,21-24). This is identical to adult findings (12,25). Many of these studies were retrospective (17,22,23) and where prospective (5,21,24) only a small sample size was used. Moreover, very few researchers conducted studies on subjects within the first 3 years of life (14,26). It is possible that this complication takes its origin very early in childhood. PAH remains one of the leading causes of death in adults with SCA worldwide (27,28). While the prevalence of SCA is highest in sub-Saharan Africa (29), very little has been reported on the prevalence of PAH as well as risk factors for the development of this clinical condition among children with SCD in Nigeria (29). PAH confers a high risk of death with 2-year mortality rates as high as 40–50% even at modest elevation of pulmonary artery pressure (23,27,30). Median survival age after detection of the disease is said to be 25.6 months (18,19). Early detection of elevated pulmonary artery pressure in childhood and appropriate treatment with use of pharmacotherapy such as hydroxyurea (25), endothelin receptor antagonists (ERA) (31) and nitric oxide (NO) inhalation (31) may prevent the progression of this complication. The current study, in addition will help recommend the appropriate age to commence screening transthoracic Doppler echocardiography at the routine clinic evaluation programme for children with SCA.
The general aim of this study was to determine the pattern of pulmonary artery pressure in children with SCA aged 1 to 12 years in steady state and their healthy controls with hemoglobin AA genotype using Doppler echocardiography at LASUTH.
The specific objectives were to determine the prevalence of PAH in children with SCA aged one year to twelve years in their steady state, compare the mean pulmonary artery pressure of SCA patients in steady state with those of age and sex matched Hb AA controls as well as to determine the relationship between pulmonary artery pressure and age of subjects studied.
Methods
The study was carried out at the paediatric sickle cell as well as the Paediatric General Outpatient Clinics of the Lagos State University Teaching Hospital (LASUTH), situated at the central part of Ikeja in Lagos, South West Nigeria. It serves as a referral Centre for many primary health care hospitals as well as private hospitals in Lagos. Its Paediatric Unit has 80 beds (wards and emergency rooms inclusive). Its Paediatric Outpatient Clinics operate on a daily basis with an average of 100 patients attending both the General Outpatient as well as the Specialty Clinics daily. This study was an analytical, comparative, cross-sectional study which involved children with SCA as well as age and sex matched Hb AA genotype children as controls. The study period spanned over 7 months (31st August 2015 through 31st March 2016).
The study population comprised all SCA children aged 1 to 12 years attending the sickle cell clinic who were in steady state as well as age and sex matched children with documented genotype Hb AA attending the General Outpatient Clinics who met the inclusion criteria and whose parents/guardian gave a written informed consent. Steady state was defined as all of the following (32): history of an acute painful episode that required treatment in the emergency department or in the hospital for at least 4 consecutive weeks after a previous painful crisis, no history of blood transfusion during the previous 4 months of the point in time, no history of intercurrent illness such as infection, inflammation during the previous 4 weeks, no treatment with medications such as antibiotics that may affect blood counts during the previous 3 weeks.
The sample size was determined using the formula for case control studies (33).
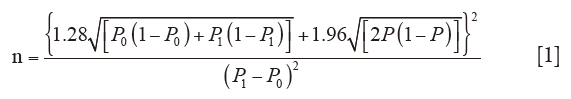
Where P0 = Proportion of controls with pulmonary artery hypertension derived from a Aliyu et al. study (29). P1 = Proportion of patients with sickle cell anaemia with pulmonary artery hypertension derived from Aliyu et al. study (29). P = Average proportions of controls and patients with sickle cell anaemia with pulmonary artery hypertension.

Where OR = odds ratio from a previous study (29)

P0 =7% (0.07), P1 =25% (0.25), therefore,
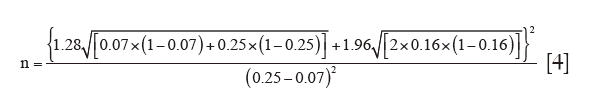
n=186. This value was rounded off to the nearest ten to give 190 subjects. For ease of stratification into groups, an additional 10 patients was added to the above sample size calculation to make a total of 200 subjects.
A total of 200 SCA patients were recruited. Using the stratified sampling method, patients were stratified into age brackets (1–3, 4–6, 7–9 and 10–12 years) with equal numbers of male and female subjects being represented for both SCA subjects as well as controls to avoid bias. A total number of 400 patients were recruited consecutively for this study.
Ethical approval was obtained from the Health Research and Ethics Committee of the Lagos State University Teaching Hospital. Parents/caregivers of subjects for the study were fully briefed on the research protocol in the language they understand after which a written informed consent was obtained. Social class was determined using Oyedeji social class stratification which takes into consideration the occupation as well as level of education of both parents (34). An assent form was filled by children who were 7 years of age and above. The cost of all investigations was borne by the investigator. The inclusion criteria for patients with SCD were; SCA aged 1 to 12 years who were in steady state. The exclusion criteria were children with pulmonary stenosis or any other structural heart defect children who were on chronic blood transfusion and children who were already on medications for pulmonary hypertension.
The inclusion criteria for children with haemoglobin AA Genotype were, children aged 1 to 12 years with documented Hb AA genotype attending the Paediatric General Outpatient Clinics who had recovered from acute illnesses attending the follow up clinics as well as those attending the dermatology clinic with no other co morbid conditions. Eligible subjects as stated above with packed cell volume (PCV) ≥30%. The weight of eligible children who were 2 years of age and below was measured with a First New England bassinet weighing scale model BB-21 while the height of children 2 years and below was measured using a Lincoln Mark England infant stadiometer code RGZ-160. The weight of children aged 2 years and above was measured using a Taylor glass digital weighing scale model 7906B. Body weight was recorded to the nearest 1 kg with subject’s barefoot and wearing light clothing. The height of children aged 2 years and above recruited for the present study was measured using a Seca portable stadiometer height rod code ESE213. The weight and height measured was used to calculate the BMI z score (BMIz) using the WHO anthro and anthro plus applications. Transcutaneous oxygen saturation levels were measured in all children recruited into the present study with the aid of a Concord Black Ox Pulse Oximeter Model SN 131226600721 with a paediatric sensor. The pulse oximeter was placed on each child’s thumb and oxygen saturation levels were read after two minutes of tight placement on the thumb. Temperature measurements were done with the aid of an Omron Digital Thermometer MC-246.
Echocardiography
The study employed the use of a Philips GE-Medical Systems Ultrasound, Vivid Q Doppler Echocardiography Machine REF 145021WP SN 2084 owned by Lagos State University Teaching Hospital. All eligible patients had a 2-dimensional Doppler echocardiography performed on them by the investigator inside the echocardiography room located just beside the outpatient clinics. Appropriate transthoracic transducer selection for subjects was made at the time of evaluation. All subjects had a complete echocardiography performed on them first to evaluate the structure and function of the heart using the 2-dimensional guided echocardiography machine before any cardiac measurements were taken. Cardiac measurements were performed according to the guidelines of the American Society of Echocardiography (35). Measurements of the TRV were done using four views (Apical 4-chamber, Parasternal long axis, Parasternal short axis and Subcostal views). An average of the measurements obtained in the different views was documented as the TRV value for the individual. The RVSP was calculated using the modified Bernoulli equation (4× TRV2) added to the estimated RAP, where TRV was the TRV measured. All the subjects recruited had a normal inferior vena cava size with collapse during inspiration >50%. Hence, the estimated right atrial pressure used for all subjects and controls was 5 mmHg (4). The pulmonary artery systolic pressure was then equal to the Bernoulli derived RVSP in the absence of obstruction to pulmonary blood flow.
Data analysis
Data collected was entered into Microsoft Excel spreadsheet of a personal computer. Analysis of data was done using the statistical package for Social Sciences (SPSS) version 20.0. Descriptive statistics were represented for continuous data using mean and standard deviation while frequency and percentage were used to represent categorical data. Continuous variables were assessed for normality using the Kolmogorov-Smirnov test. Independent t-test and Mann Whitney U test were used to compare clinical and laboratory parameters between the groups of TRV values (<2.5 and ≥2.5 m/s) while chi-square or Fisher exact test was used for categorical variables. Bar charts were used to represent the prevalence of PAH in controls and subjects as well as the trend of PAH in subjects.
Results
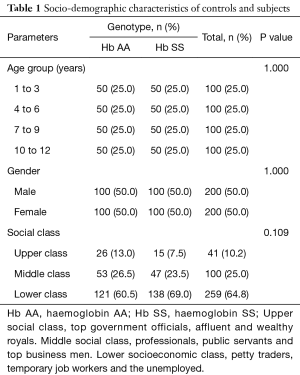
A total of 400 children aged one to twelve years were recruited. Out of this number, 200 were children with known hemoglobin genotype SS children (subjects) attending the Sickle Cell Clinic and 200 were known hemoglobin genotype AA children (controls) attending the Paediatric Follow-up as well as Outpatient clinics. Table 1 shows the sociodemographic distribution of subjects and controls. The mean age for controls was 6.51±3.39 years and the mean age for subjects was 6.59±3.30 years. The male to female ratio was 1:1. All the subjects were in steady state and were on routine oral drugs consisting of folic acid, proguanil and multivitamins. None of the subjects were on chronic transfusion, hydroxyurea or other anti-sickling drugs. Two hundred and fifty-nine (64.8%) of the total number of subjects and controls belonged to the lower socioeconomic class group.
Full table
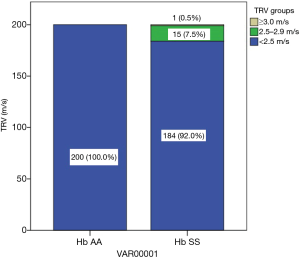
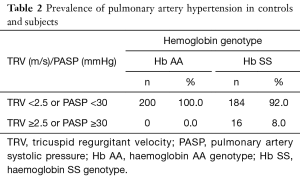
Figure 1 shows the prevalence of PAH in controls and subjects using a bar chart. None of the children with haemoglobin genotype AA had PAH while 8% of the children with sickle cell anaemia had PAH.
Table 2 shows TRV measurements in subjects and controls. All 200 children with haemoglobin AA genotype had TRV measurements <2.5 m/s which correlates with PASP <30 mmHg (normal pulmonary artery systolic pressure). Sixteen out of the 200 children with haemoglobin SS genotype studied had TRV ≥2.5 m/s giving a pulmonary artery hypertension rate of 8%. Out of this number, 15 (7.5%) had TRV measurements ≥2.5–2.99 m/s which correlates with PASP of ≥30–40 mmHg (mild PAH) and 1 (0.5%) had a TRV of ≥3.0 m/s which correlates with PASP of ≥41 mmHg (moderate PAH).
Full table
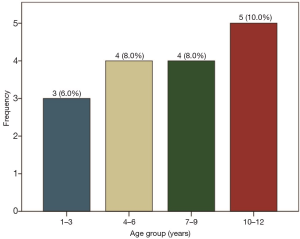
Figure 2 represents the trend of PAH across the stratified age brackets in controls. The frequency of PAH increases from 6% in the lowest age bracket to 10% in the lowest age bracket. This trend was, however, not statistically significant. Mantel Haenszel chi square for linear trend (−0.34, P=0.56).
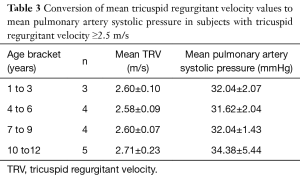
Conversion of TRV to systolic pulmonary artery pressure in subjects with TRV of 2.5 m/s or more using the Bernoulli equation is shown in Table 3. All the subjects (16) with tricuspid regurgitant jet velocity of 2.5 m/s or more had systolic pulmonary artery pressure equating to ≥30 mmHg using the Bernoulli equation. Fifteen of the subjects had mild pulmonary artery hypertension (range, 30.20–39.57 mmHg). One female Hb SS subject had moderate pulmonary artery hypertension with a systolic pulmonary artery pressure of 44.44 mmHg. The range of systolic pulmonary artery pressure found in controls was 6.96–29.20 mmHg (in all the controls pulmonary artery systolic pressure was <30 mmHg).
Full table
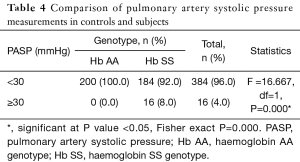
Table 4 shows the pulmonary artery systolic pressure measurements in controls and subjects. The mean pulmonary artery pressure of controls was 13.76±5.71 mmHg while, the mean pulmonary artery pressure of subjects was 18.54±5.81 mmHg. The mean pulmonary artery pressure measurement was significantly higher among subjects when compared with controls (P=0.000).
Full table
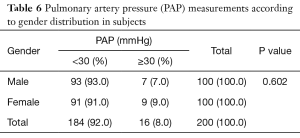
Table 5 shows the comparison of mean clinical parameters between controls and subjects. The mean values of heart rate and respiratory rate were significantly higher in subjects (P=0.006 and 0.003 respectively). While, the mean BMI z score and oxygen saturation values were significantly higher in controls (P=0.000 and 0.001 respectively). Thirty-one subjects versus five controls had hypoxemia defined as oxygen saturation values of 94% and below. Table 6 shows pulmonary artery pressure measurements according to gender distribution in subjects. Among the subjects, 9 (9%) out of the total number female subjects had pulmonary artery hypertension while 7 (7%) out of the total number of male subjects had pulmonary artery hypertension 2.5 m/s. More of the female subjects had pulmonary artery hypertension when compared with male subjects. Moderate to severe pulmonary artery hypertension was found in a 12-year-old female with SCA. The finding of pulmonary artery hypertension in more female subjects compared with male subjects was not statistically significant (P=0.602).

Full table
Full table
Discussion
The present study highlights the pattern of pulmonary artery pressure measurement in children with sickle cell anaemia in steady state in comparison with their healthy controls. The eight percent prevalence of pulmonary artery hypertension found in children with sickle cell anaemia in steady state in the present study is close to 11% found in another prospective study conducted by Minniti et al. (10) in the USA. The similarity in findings may be attributed to a similar study population used, an overlap in the age brackets of study as well as similarities in the techniques used to estimate pulmonary artery pressure by Doppler echocardiography. Other Nigerian studies have reported varying prevalence rates of 25% (29), 22.6% (36) and 3.6% (37) respectively. While the former two included adults (29) and older children (36) in their study population, the latter study (37) proposed that the low prevalence rate could be attributed to a relatively smaller sample size of subjects recruited for the study. Another similar study (5) conducted in the United States of America involving children as well as adults with SCA a prevalence of 30% was reported. Pulmonary artery hypertension is a widely recognized complication of chronic haemolytic anaemia which includes SCA (5,12,38). The age difference may explain the wide disparity in the prevalence rates of PAH in the present study when compared with that of prevalence rates of studies including the adult population. In addition, the present study group were a selection of patients attending the routine sickle cell clinic, were on routine drugs and are assumed to be better motivated for regular treatment in LASUTH, a tertiary hospital situated in the commercial center of the country with probably mild to moderate effect of haemolysis and associated clinical manifestations of SCA such as PAH. Routine care given to these SCA patients may have ameliorated the effect of haemolysis. Also, the Benin haplotype of SCD known to confer moderate disease is known to predominate in Nigeria (39) while the Bantu and Cameroon haplotypes of SCD which confer severe disease were known to predominate among subjects recruited into Pashankar et al. (5) study conducted in the USA. This prevalent haplotype in the present study region may have contributed to the lower prevalence of pulmonary artery hypertension found in the present study group relative to that found in similar studies conducted outside the country.
The overall mean pulmonary artery pressure derived from TRV measurements obtained on Doppler echocardiography of children with SCA was significantly higher than that of controls in the present study. This is comparable to figures obtained in previous studies (5,10) conducted on children and adults to determine the pattern of pulmonary artery pressure. This finding suggests a contributory effect of haemoglobin S genotype on PAH. The higher mean pulmonary artery pressure found in subjects with sickle cell anaemia in steady state can be explained by the compensatory increase in cardiac output secondary to chronic anaemia (40). The increase in cardiac output is favored by an increase in the pulmonary blood flow. An increased pulmonary blood flow requires that the precapillary pulmonary microcirculation vasodilate in order to accommodate for the increased flow of deoxygenated, sickled erythrocyte (41). In this high output state, any interference with vasodilation of the pulmonary artery by the high viscosity sickled blood cells will lead to an increase in pulmonary artery pressure (41). Also, it has been reported that the pulmonary artery pressure measurement by Doppler echocardiography tends to be higher in patients with SCA because of the seemingly thin habitus of some children with SCA and hyper dynamic cardiac output which favours an excellent Doppler image (29). The BMIz score was significantly lower in children with SCA in the present study when compared with controls.
The current study demonstrates that the prevalence of PAH increased with age in children with SCA in steady state. This is consistent with the findings of Minniti et al. (10), Colombatti et al. (14) and Dosunmu et al. (37) in the USA, Italy and Nigeria respectively in which the prevalence of PAH also increased with rising age. The finding however contrasts with another Nigerian study conducted on children and adults by Aliyu et al. (29) where the prevalence of pulmonary hypertension declined with rising age. Pashankar et al. (5) in the USA found no correlation between age and increasing TRV measurements in a study involving children and adults. Although, it is immediately difficult to explain the disparity in the age-related trend of prevalence of pulmonary artery hypertension between the present study and the earlier stated studies, it is speculated that the recent increase in survival of patients with SCA in our environment may be accompanied by a rise in the prevalence rates of SCA associated complications which includes pulmonary artery hypertension. It is therefore, understandable that the prevalence of pulmonary artery hypertension increased with age in children with SCA in the present study.
Pulmonary artery hypertension was found in a 2-year-old male child with SCA in the current study. This would be the youngest age documented in literature. Three years of age was the youngest age studied and at which pulmonary artery hypertension was found in previous studies (10,14). The current finding therefore suggests that pulmonary artery hypertension begins earlier than previously documented in literature.
It is speculated that PAH occurs more among females with SCA as it was noticed that more females with SCA had pulmonary artery hypertension (though not significant) compared with males in the present study. There are conflicting reports of male preponderance (42) and female preponderance (30) of PAH in literature. It is therefore not known for certain if gender has an influence on the development of pulmonary artery hypertension.
Doppler echocardiographic screening for PAH in SCD patients as early as the second year of life would help to identify patients with PAH early before complications set in. Pulmonary artery hypertension is known to progress unnoticed because there are usually no symptoms or signs until the disease have progressed far. Mortality rate is said to be as high as 40–50% if left untreated over 24 to 40 months (10) All the sixteen subjects with pulmonary artery hypertension in the present study were referred to the cardiology clinic. TRV measurements in subjects with PAH remained persistently elevated (≥2.5 m/s) on repeat Doppler echocardiography during clinic follow-up 3 months after initial visit. All the affected subjects (16) are currently being treated with bosentan an endothelin receptor antagonist and are being followed up at the haematology and Cardiology Clinics at LASUTH.
Conclusions
The prevalence of pulmonary hypertension in children with sickle cell anaemia in steady state (8%) is high.
This complication was noticed as early as in the second year of life in our study. This suggests that this complication begins very early in childhood among children with sickle cell anaemia.
The trend of pulmonary artery hypertension in children with sickle cell anaemia appears to increase with age within the age bracket studied.
The mean pulmonary artery pressure of children with sickle cell anaemia in steady state is significantly higher than the mean pulmonary artery pressure of children with haemoglobin AA genotype.
Recommendation
There is a need to incorporate routine Doppler Echocardiography measurement of tricuspid regurgitant jet velocity for the evaluation of pulmonary artery hypertension in children with SCA as early as the second year of life considering the early onset of pulmonary artery hypertension.
Limitation of study
The degree to which the different haplotypes of SCD influenced the prevalence of pulmonary artery hypertension in the present study environment is not known.
Acknowledgments
All patients and the caregivers that participated in this study. Mr. Clement Akinsola and Dr. Ayodeji Akinola for their support during the course of this study.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Ethical approval was obtained from the Health Research and Ethics Committee of the Lagos State University Teaching Hospital. Parents/caregivers of subjects for the study were fully briefed on the research protocol in the language they understand after which a written informed consent was obtained.
References
- Serjeant GR, Serjeant BS. Sickle cell disease. Oxford University Press, 2001.
- Pauling L, Itano HA, Singer SJ, et al. Sickle cell anemia, a molecular disease. Science 1949;110:543-48. [Crossref] [PubMed]
- WHO. Global distribution of haemoglobin disorders, in terms of births of affected infants. 2006. Available online: http://www.who.int/genomics/public/Maphaemoglobin.pdf
- Aliyu ZY, Kato G, Taylor J. Sickle cell disease and pulmonary hypertension in Africa: a global perspective and review of epidemiology, pathophysiology, and management. Am J Hematol 2008;83:63-70. [Crossref] [PubMed]
- Pashankar FD, Carbonella J. Prevalence and risk factors of elevated pulmonary artery pressures in children with sickle cell disease. Pediatrics 2008;121:777-82. [Crossref] [PubMed]
- Kato GJ, Onyekwere OC, Gladwin MT. Pulmonary hypertension in sickle cell disease: Relevance to children. Pediatr Hematol Oncol 2007;24:159-70. [Crossref] [PubMed]
- Zuckerman WA, Rosenzweig E. Pulmonary hypertension in children with sickle cell disease. Expert Rev Respir Med 2011;5:233-43. [Crossref] [PubMed]
- Barst RJ, Ertel S, Beghetti M, et al. Pulmonary arterial hypertension: a comparison between children and adults. Eur Respir J 2011;37:665-77. [Crossref] [PubMed]
- Abman SH, Hansmann G, Archer SL, et al. Pediatric Pulmonary Hypertension Guidelines From the American Heart Association Thoracic Society. Circulation 2015;132:2037-99. [Crossref] [PubMed]
- Minniti CP, Sable C, Campbell A, et al. Elevated tricuspid regurgitate pressure in children and adolescents with sickle cell disease: association with hemolysis and oxygen saturation. Haematologica 2009;94:340-7. [Crossref] [PubMed]
- Voskaridou E, Tsetsos G, Tsoutsias A, et al. Pulmonary hypertension in patients with sickle cell/ beta thalassemia: incidence and corellation with serum N-terminal pro-brain naturetic peptide concentrations. Haematologica 2007;92:738-43. [Crossref] [PubMed]
- Gladwin MT, Sachdev V, Jison M, et al. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N Engl J Med 2004;350:886-95. [Crossref] [PubMed]
- Liem RI, Young LT, Thompson AA. Tricuspid regurgitant jet velocity is associated with hemolysis in children and adults with sickle cell disease evaluated for pulmonary hypertension. Haematologica 2007;92:1549-52. [Crossref] [PubMed]
- Colombatti R, Maschietto N, Varotto E, et al. Pulmonary hypertension in sickle cell disease children under 10 years of age. Br J Haematol 2010;150:601-9. [Crossref] [PubMed]
- Brown LM, Chen H, Halpem S, et al. Delay in recognition of pulmonary arterial hypertension: factors identified from the REVEAL Registry. Chest 2011;140:19-26. [Crossref] [PubMed]
- Sedrak A, Rao SP, Miller ST, et al. A prospective appraisal of pulmonary hypertension in children with sickle cell disease. J Pediatr Hematol Oncol 2009;31:97-100. [Crossref] [PubMed]
- Ambrusko SJ, Gunawardena S, Sakara A, et al. Elevation of tricuspid regurgitant jet velociy, a marker for pulmonary hypertension in children with sickle cell disease. Pediatr. Blood Cancer 2006;47:907-13. [Crossref] [PubMed]
- Klings ES, Anton Bland D, Rosrman D, et al. Pulmonary arterial hypertension and left sided disease in sickle cell disease: Clinical characteristics and association with soluble adhesion molecule expression. Am J Hematol 2008;83:547-53. [Crossref] [PubMed]
- Bunn HF, Nathan DG, Dover GJ, et al. Pulmonary arterial hypertension and Nitric oxide depletion in sickle cell disease. Blood 2010;116:687-92. [Crossref] [PubMed]
- Klings ES. Pulmonary hypertension of sickle cell disease: more than just another lung disease. Am J Hematol 2008;83:4-5. [Crossref] [PubMed]
- Qureshi N, Joyce JJ, Qi N, et al. Right ventricular abnormalities in sickle cell anemia: evidence of a progressive increase in pulmonary vascular resistance. J Pediatr 2006;149:23-7. [Crossref] [PubMed]
- Suell MN, Benzold LI, Okcu MF, et al. Increased pulmonary artery pressure among adolescents with sickle cell disease. J Pediatr Hematol Oncol 2005;27:654-8. [Crossref] [PubMed]
- Minniti CP, Macahado RF, Coles WA, et al. Endothelin receptor antagonists for pulmonary hypertension in adult patients with sickle cell disease. Br J Haematol 2009;147:737-43. [Crossref] [PubMed]
- Dahoui HA, Hayek MN, Nietart PJ, et al. Pulmonary hypertension in children and young adults with sickle cell disease: evidence for familial clustering. Pediatr Blood Cancer 2010;54:398-402. [Crossref] [PubMed]
- Ataga KI, Sood N, Gent GD, et al. Pulmonary hypertension in sickle cell disease. Am J Med 2004;117:665-9. [Crossref] [PubMed]
- Gordeuk VR, Campbell A, Rana S, et al. Relationship of erythropoetin, fetal hemoglobin and hydroxyurea treatment to tricuspid regurgitation velocity in children with sickle cell disease. Blood 2009;114:4639-44. [Crossref] [PubMed]
- Lin EE, Rodgers GP, Gladwin MT. Hemolytic anaemia associated with pulmonary hypertension in sickle cell disease. Curr Hematol Rep 2005;4:117-25. [PubMed]
- Chaudry RA, Cikes M, Karu M, et al. Paediatric sickle cell disease: pulmonary hypertension but normal vascular resistance. Arch Dis Child 2011;96:131-6. [Crossref] [PubMed]
- Aliyu ZY, Gorduek V, Sachdev V, et al. Prevalence and risk factors for pulmonary systolic hypertension among sickle cell disease patients in Nigeria. Am J Hematol 2008;83:485-90. [Crossref] [PubMed]
- Lee MT, Rosenzweig EB, Cairo MS. Pulmonary hypertension in sickle cell disease. Clin Adv Hematol Oncol 2007;5:645-53. [PubMed]
- Humbert M, Sitbon O, Simonneau G. Treatment of Pulmonary Arterial Hypertension. N Engl J Med 2004;351:1425-36. [Crossref] [PubMed]
- Ballas SK. More definitions in sickle cell disease :steady state v baseline data. Am J Hematol 2012;87:338. [Crossref] [PubMed]
- Kirkwood BR, Sterne JA. Calculation of required sample size. 2nd ed. Oxford Blackwell, 2003:422-8.
- Oyedeji GA. Socio-economic and cultural background of hospitalized children in Ilesha. Nig J Paediatr 1985;12:111-7.
- Lai WW, Geva T, Shirali GS, et al. Guidelines and standards for performance of a pediatric echocardiogram: a report from the Task Force of the Paediatric Council of the American Society of Echocardiography; Pediatric Council of the American Society of Echocardiography. J Am Soc Echocardiogr 2006;19:1413-30. [Crossref] [PubMed]
- Sokunbi OJ, Ekure E, Temiye E, et al. Pulmonary Hypertension among 5 to 18 year children with sickle cell anaemia in Nigeria. PLoS One 2017;12:e0184287. [Crossref] [PubMed]
- Dosunmu AO, Balogun TM, Adeyeye OO, et al. Prevalence of pulmonary hypertension in sickle cell anaemia patients of a tertiary hospital in Nigeria. Niger Med J 2014;55:161-5. [Crossref] [PubMed]
- Sutton LL, Castro O, Cross DJ, et al. Pulmonary hypertension in sickle cell disease. Am J Cardiol 1994;74:626-8. [Crossref] [PubMed]
- Powars DR, Meiselman H, Fisher T, et al. Beta-S Gene Cluster Haplotypes Modulate Hematologic and Hemorheologic Expression in Sickle Cell Anaemia. Am J Pediatr Hematol Oncol 1994;16:55-61. [PubMed]
- Peacock JA, Naeije R, Rubin LJ. Pulmonary circulation disease and their treatment. 3rd ed. Boca Raton, Florida: CRC Press, 2011.
- Clarke ME. Pulmonary hypertension in sickle cell disease. Medscape. 2006. Available online: https://www.medscape.org/viewarticle/547201
- Nelson SC, Adade BB, Mc Donough EA, et al. High prevalence of pulmonary hypertension in children with sickle cell disease. J Pediatr Hematol Oncol 2007;29:334-7. [Crossref] [PubMed]