Screening of extra-coronary arteriopathy with magnetic resonance angiography in patients with spontaneous coronary artery dissection: a single-centre experience
Introduction
Spontaneous coronary artery dissection (SCAD) represents an important cause of acute coronary syndromes, particularly in young and middle-aged women. The condition appears to arise from an apparent frailty of the coronary arteries leading to a separation (i.e., dissection) within the arterial wall layers, which may or may not be precipitated by an identifiable stressor (emotion, exertion, drugs) (1,2). The pathophysiology underlying this vessel frailty remains unclear and is currently matter of research. Recently, extra-coronary arterial abnormalities found in the screening of some SCAD cohorts has led to the proposal that a systemic arterial disorder may underlie SCAD (2-4). Especially noteworthy was the finding of fibromuscular dysplasia (FMD), a non-inflammatory non-atherosclerotic systemic arteriopathy that manifests with stenosis, dilatations and dissections of medium-calibre vessels, commonly affecting renal, carotid and iliofemoral arterial territories (5,6). The prevalence of FMD varies across studies: in two North-American SCAD cohorts, screening with computed tomography angiography (CTA) or invasive catheterization has revealed FMD in 45% and 63% of the patients with SCAD (7,8). Other studies, with possibly less protocolised screening approaches, have shown lower prevalences (13–37%) (9-11). Importantly, intracranial aneurysms have been detected in 7–9% of SCAD patients from the two North American cohorts (7,8). Although screening for extra-coronary arteriopathies is currently encouraged by expert consensus statements (2,3), its clinical value remains to be clearly determined. The current rationale for extended vascular imaging is particularly driven by the reported finding of a higher than expected proportion of intracranial aneurysms, with the potential, albeit unconfirmed in this population, adverse associated risk (12,13). Conversely, detecting concomitant FMD or other abnormalities, although of potential interest for research, has uncertain clinical implications for the individual patient, especially when these abnormalities are mild. Given the paucity of prospective studies on this topic, we sought to investigate extra-coronary arterial abnormalities in all patients with SCAD diagnosed at our centre. We opted for using a non-invasive non-ionising technique, magnetic resonance angiography (MRA). We here report our experience undertaking this approach.
Methods
Study population
The Interventional Cardiology unit at Hospital Clínico San Carlos, a tertiary university hospital, has a dedicated clinic for patients with SCAD. Consecutive patients diagnosed with SCAD were eligible for recruitment. Prospective recruitment started in November 2016 and finished in November 2017. Historical diagnoses of SCAD from 2009 were also assessed for eligibility and invited to participate. Diagnosis of non-atherosclerotic SCAD was corroborated by at least two interventional cardiologists based on angiography +/− intracoronary imaging findings, adhering to published diagnostic criteria (4). All recruited patients signed a dedicated informed consent form. Specific magnetic resonance exclusion criteria were established: (I) glomerular filtration rate less than 30 mL/min/1.73 m2, (II) severe claustrophobia and absence of consent for conscious sedation (patients showing some degree of anxiety or propensity to mild claustrophobia at the moment of eligibility assessment were offered the option of receiving oral lorazepam 1 mg before the procedure was performed), (III) non-compatible metallic devices. The study was approved by the Institutional Review Board and research ethics committee.
Magnetic resonance angiography protocol
To optimise the diagnostic yield of the screening protocol, MRA examination was limited to cervical and abdominopelvic territories (see an example of the acquisition in Figure S1). We opted to exclude the thoracic region was owing to: (I) limited thoracic aortic pathology in reported studies on SCAD patients (8,14); (II) rare involvement of thoracic aorta in FMD (15); and (III) the ascending aorta and arch are routinely imaged with echocardiography in all patients at our centre.
Cervical and abdominopelvic MRA examinations were performed using a Signa 1.5T Excite (General Electric, Boston, Massachusetts, USA). The study was fully acquired in one only patient visit. The protocol was divided in two phases corresponding to the abdominal and cervical-cranial regions. Abdominopelvic acquisition (feet first, supine position) were obtained using an 8-element phased-array abdominal coil (General Electric. A two-phases postcontrast coronal MRA were acquired (from the diaphragm to the pelvis, including femoral arteries 36 cm of FOV, 2.6 mm thickness and 384×224 matrix) after intravenous administration of gadoterate meglumine (Dotarem, Guerbet, Villepinte, France) with 0.1 mmol per kilogram of body weight at flow rates of 2.0 mL/sec followed by a 30 mL saline flush at a flow rate of 1.5 mL/sec, launched using smart prep when the contrast reached the abdominal aorta. Afterwards, the cervical study was performed with an 8-element phased-array cervical vascular coil. A postcontrast coronal MR angiography; from the aortic arch to the brain vessels distal from circle of Willis (thickness of 1.4 mm, 320×224 matrix and 32 cm of FOV) after intravenous administration of gadoterate meglumine (Dotarem, Guerbet, Villepinte, France) with 0.1 mmol per kilogram of body weight at flow rates of 2.5 mL/sec followed by a 30 mL saline flush at a flow rate of 1.5 mL/sec, launched using smart prep when the contrast reached aortic arch.
Image-based diagnosis of fibromuscular dysplasia
Magnetic resonance angiograms were analysed independently by two board-certified radiologists blinded to patient outcomes and symptoms. In cases of disagreement, the case was brought to a radiology meeting to reach a consensus. FMD was diagnosed following European consensus criteria: multifocal FMD was defined as beading or string-of-beads appearance in at least one medium-calibre arterial territory; whereas focal FMD is defined as at least one arterial stenosis found in a young patient (<40 years) in the absence of clinically-evident atherosclerotic disease, multiple vascular risk factors, inflammatory syndrome or vascular thickening, and familial or syndromic disease (5). Arterial tortuosity was a subjective diagnosis adjudicated when the assessed artery had a redundant anatomical course and had more curves than normal.
Statistical analysis
Quantitative variables were summarised using mean and standard deviation or median and interquartile range according to distribution type, whereas number of cases and percentages were used to describe categorical variables. Saphiro-Wilk test and standardized normal probability plots were used to assess normality of the distributions. To compare subgroups of patients, Chi square or Fisher exact tests and Student’s T or Wilcoxon-Mann-Whitney tests were used as appropriate. All analyses were performed with Stata 13 software (StataCorp LP, College Station, TX, USA) SPSS statistics, version 21 (IBM Corp., Armonk, NY, USA). A P value <0.05 was considered statistically significant.
Results
Patients and procedures
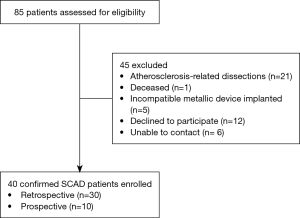
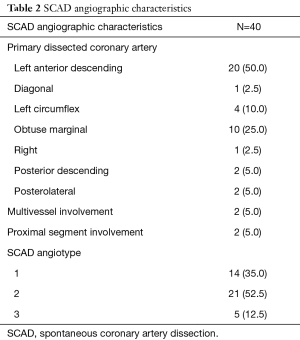
From January 2009 to November 2017, 85 patients with spontaneous coronary dissection were assessed for eligibility. On grounds of angiography and/or intracoronary imaging, 21 patients were excluded because of having significant atherosclerotic disease at the site of the dissection. Of the remaining 64 confirmed non-atherosclerotic SCAD, considered for the protocol, 24 (37.5%) were also excluded due to various reasons (Figure 1), which resulted in a final study population of 40 patients. Baseline and angiographic characteristics at the moment of SCAD diagnosis are summarised in Tables 1,2, respectively. Eleven patients (27.5%) were 45 years old or less. Cardiovascular risk burden was low except from smoking, as half of the patients were active smokers at the moment of the event. The left anterior descending artery was the most commonly dissected coronary artery, and angiotype 2 (smooth vessel tapering) was the most frequent angiographic presentation [see angiographic classification of SCAD elsewhere (16)]. MRI angiography was successfully performed in all 40 patients. Ten patients (25%) requested oral sedation (Lorazepam 1mg) to prevent anxiety. No further sedation was required and there were no complications whatsoever related to the procedure.

Full table
Full table
Radiologic findings
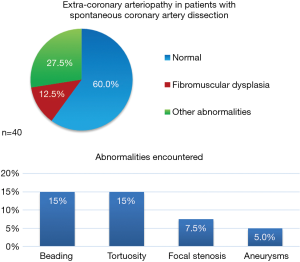
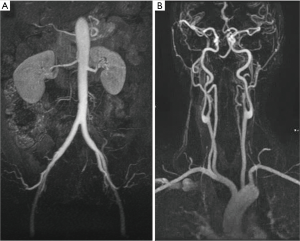
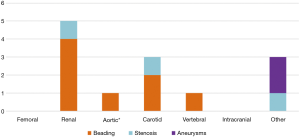
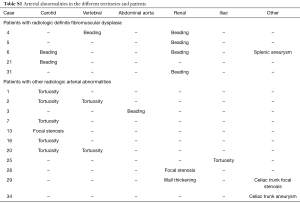
The overall prevalence of extracoronary arteriopathy investigated with MRA in our study population is shown in Figure 2. A total of 16 patients (40%) had at least one pathological finding: 5 patients (12.5%) were diagnosed of multifocal FMD, 6 patients (15%) showed arterial tortuosity, 3 (7.5%) had focal stenoses (not meeting criteria for focal FMD), and 2 patients (5%) were found to have aneurysms, at the celiac trunk (11 mm) and the splenic artery (15 mm) respectively (Figure 3). One case that presented a beading-like pattern in abdominal aorta was not adjudicated FMD diagnosis [previously reported (17)]. None of the patients was found to have intracranial aneurysms. Figure 4 shows the anatomical distribution of the findings with potential prognostic implications i.e., dysplasia (beading), focal stenoses and aneurysms. Arterial beading was more often seen in the renal artery, but also in the carotid, vertebral and abdominal aorta (Figure 4). Iliac-femoral involvement was not detected. A more detailed breakdown of the findings is shown in Table S1 in supplementary material.
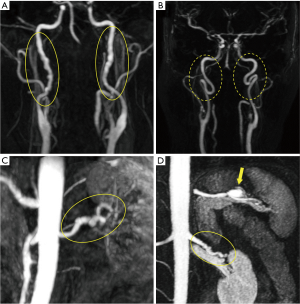
Full table
Management of arteriopathy
The five patients with multifocal FMD were counselled to quit tobacco and control blood pressure (prioritising betablockers if any drug needed), as well as maintaining aspirin life-long in the absence of contraindications, in keeping with the available expert guidance for medical management of FMD (5,6). The two patients with visceral aneurysms were scheduled for imaging surveillance, with no further action was recommended given the small size of them.
Outcomes in patients with and without arterial abnormalities
Patients with vascular abnormalities (n=16) were significantly older than those without (n=24) (54.8±8.1 vs. 48.3±8.1 years, P=0.02). Patients diagnosed of FMD (n=5) were all women and received percutaneous coronary intervention (PCI) more frequently [3/5 (60%)] than the non-FMD group [5/35 (14%), P=0.046].
Furthermore, the presentation of the three patients with FMD that required PCI was particularly challenging: one case was an extensive and severe dissection from proximal to distal left anterior descending coronary artery (LAD) and the other two were LAD dissections that both suffered iatrogenic dissections of the left main stem, one during planned angioplasty and the other one after wiring for investigation with intracoronary imaging. The 5 patients without FMD underwent uneventful PCI for circumflex coronary (n=2) and LAD (n=3) spontaneous dissections.
Regarding outcomes, at a mean follow-up duration of 4±3 years from the date of diagnosis of SCAD, no deaths or strokes occurred. Two patients presented with SCAD recurrence (5%). One case was a 53-year-old lady who had a first SCAD in a posterolateral branch of the right coronary artery and 10 years later had another SCAD in the middle segment of the left anterior descending artery. This patient had FMD in the renal and carotid arteries. The other case was a 46-year-old lady who had a SCAD in the obtuse marginal which was treated with balloon-only angioplasty; a recurrent SCAD in the same vessel and segment occurred 7 years later, in this occasion it was managed conservatively. This latter patient only had carotid tortuosity.
Discussion
In this prospective study, magnetic resonance angiography identified extra-coronary arteriopathy in 40% of patients with SCAD confirmed diagnosis, of which 12.5% were definite FMD and 5% extra-cranial arterial aneurysms.
Rationale of using MRA for the screening
Selection of a diagnostic technique should be based on a thorough appraisal of the potential risks of the technique and the expected benefits derived from the diagnostic test. In this regard, it should be kept in mind that, despite rather firm recommendations on performing extended vascular imaging in SCAD patients (2,3), no compelling evidence supports such practise, in that there is currently no data to show that screening can identify patients at risk of future vascular events or that any such events can be prevented in this population.
Considering all these facts, we opted for MRA in our patient management protocol. MRA is a non-ionising technique capable of detecting clinically-relevant abnormalities that would benefit from treatment or surveillance. Despite the finding of magnetic resonance signal changes in deep nuclei of the brain with repeated administration of gadolinium-based contrast agents, no associated adverse clinical sequelae have been demonstrated to date (18). Moreover, the absence of radiation is especially important in the SCAD population, which is mainly young to middle-aged women with a rather long lifespan, of which, a considerable proportion are in child-bearing age at the moment of SCAD (2,3).
MRA screening also constitutes a feasible non-invasive systematic approach that can be provided universally to SCAD survivors during the convalescence phase; except to those with severe renal impairment (due to the risk of nephrogenic systemic fibrosis) or metallic incompatible devices, which represent a tiny minority in this population. We did not find claustrophobia to be a significant impediment to MRA in our study if patients reporting previous claustrophobia were given oral benzodiazepines before the acquisition. Potential obstacles or limitations to the wider adoption of MRA include costs, waiting list and longer procedural time.
Sensitivity of MRA
MRA is endorsed by groups of experts as an adequate modality for the diagnosis of FMD (5,6). Its diagnostic performance was assessed in a study by Willoteaux et al. in which 1.5-T contrast-enhanced MRA was compared with invasive angiography for diagnosing FMD, yielding a sensitivity of 93% and specificity of 97% (19). Nonetheless, some authors have expressed their concerns regarding its lower spatial resolution compared to CTA and invasive catherization (3,20). This possibility cannot be ruled out, especially considering the findings of this study report a lower prevalence of remote arteriopathies than other reported CTA studies in SCAD cohorts (Table 3). Additionally, MRA is inadequate to evaluate vascular calcium and therefore it may be limited for distinguishing between focal FMD and atherosclerosis, although atherosclerotic disease is reported to be unusual in the SCAD population, a finding recently supported by genetic evidence (24). Although the overall sensitivity of MRA for detecting mild FMD might be lower than that of CTA (19,25,26), as discussed above, the clinical implications of detecting non-occlusive FMD in these patients is unclear. A comparator study of CTA and MRA will be needed to definitively address this question. In the meantime, a careful optimised acquisition technique is paramount to obtain good quality images and maximise the performance of the screening with MRA.
Full table
The detection of intracranial aneurysms, depending on their size and other characteristics, may have a clearer impact in the prognosis and management of patients with SCAD (12,13,27). The use of 1.5-T MRA for this purpose seems adequate, as it is sensitive and reproducible in detecting intracranial aneurysms of significant size (27-30). Of note, in our study the voxel size for cervical-cranial acquisition was 1×1×1.4 mm3, below the 3–4 mm threshold for unruptured intracranial aneurysms; therefore, small aneurysms (below 1.5 mm) would not be consistently detectible.
Prevalence of extra-coronary arteriopathy in SCAD
Previous studies have reported variably in the prevalence of FMD and intracranial aneurysms in patients with SCAD (Table 3). The heterogeneity in the reported figures may arise from variations in study populations, imaging modalities and the protocols and definitions used. In addition, the two largest series were reported by quaternary referral centres with dedicated SCAD units (7,8) and are subject to potential referral bias.
The true prevalence of FMD in general population is not known: the only reference available, derived from angiographic screening of potential kidney donors, has shown a prevalence of renal FMD 4% (6). Our study, demonstrated a prevalence of any extra-coronary arterial abnormality and definite FMD of 40% and 12.5%, respectively. This provides further support to the existence of an association between SCAD and extra-coronary arteriopathy. Moreover, regarding FMD as an underlying aetiology potentially accounting for vessel frailty in SCAD, it is interesting that anecdotally patients with FMD in our study had rather complex presentations and were more prone to receive PCI. This observation requires further confirmation in larger observational studies.
Importantly, no intracranial aneurysms were detected in the 40 patients screened in our study. These figures are in contrast with those reported by Saw and Prasad: 9% (29/327) and 8% (9/115) respectively (7,8). In these studies, the use of CTA could have accounted for a higher pickup rate, but this could have been at the expense of small aneurysms of uncertain clinical significance (size not reported).
For the time being, the main value of diagnosing FMD in SCAD is stratifying vascular risk (5,6), but this scenario may change in the future. Recent research suggests that the use of betablockers in patients with SCAD may decrease the incidence of future vascular events (7). Should it be clarified that the benefit of any medication or extraordinary intervention is higher in patients with SCAD and extra-coronary arteriopathy, a more directed recommendation of extra-coronary screening in patients with SCAD might follow. Likewise, a more comprehensive evaluation of sensitivity and utility among the different imaging modalities may be warranted. Meanwhile, MRA appears as a good option for screening clinically-relevant extra-coronary arteriopathy in patients with SCAD.
Limitations
Due to the rarity of the condition, although this is the largest cohort of SCAD patients screened with MRA to date, the sample size is still limited. A significant attrition rate from the initially targeted sample occurred, partially related to retrospective enrolment of most patients (75%). The relatively small sample and the limited follow-up time prevents conclusive data with regards to the impact of this practise on stratifying prognosis and facilitating prevention. In fact, the two patients with recurrences were among the ones with the longest follow-up times. The sample size may also limit detection of patients with intracranial aneurysms, which is the most feared abnormality and supposed to make the difference in prognosis. Moreover, a control reference imaging technique to evaluate sensitivity of MRA was missing within this study, namely CTA; however, ethical concerns precluded the inclusion of an additional imaging modality with radiation in the protocol.
Conclusions
This single-centre study demonstrates the feasibility of contrast-enhanced magnetic resonance angiography for the screening of extra-coronary arteriopathy in patients with SCAD. The use of this radiation-free screening protocol should be considered as an alternative to CT or angiography in a condition that affects young and middle-age women. The coexistence of SCAD and systemic arteriopathy deserves further investigation.
Acknowledgments
This work is supported by Fundación Interhospitalaria de Investigación Cardiovascular (Madrid. Spain), which took over extraordinary expenses regarding the execution of this project, including processing charges of scientific articles derived from it. The first author of this article has received a grant from this research foundation. The authors are grateful to the members of the research division Silvia Mera, Aranzazu Ortega and Esther Bernardo. David Adlam acknowledges the leadership of the ESC-ACCA SCAD Study Group and funding from the British Heart Foundation, the NIHR Rare Diseases Translational Collaboration and Beat SCAD.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study protocol and corresponding informed consent were approved by the Clinical Research Ethics Committee of Hospital Clínico San Carlos, Madrid, Spain. The internal registration number for the approved study protocol is 17/043-E. Every patient that have partaken in the present study has provided signed consent. The informed consent of this study included a statement declaring the possibility of publishing anonymised data and/or images of the patients enrolled in it.
References
- Vrints CJM. Spontaneous coronary artery dissection. Heart 2010;96:801-8. [Crossref] [PubMed]
- Adlam D, Alfonso F, Maas A, et al. European Society of Cardiology, acute cardiovascular care association, SCAD study group: a position paper on spontaneous coronary artery dissection. Eur Heart J 2018;39:3353-68. [Crossref] [PubMed]
- Hayes SN, Kim ESH, Saw J, et al. Spontaneous Coronary Artery Dissection: Current State of the Science: A Scientific Statement From the American Heart Association. Circulation 2018;137:e523-57. [Crossref] [PubMed]
- Macaya F, Salinas P, Gonzalo N, et al. Spontaneous coronary artery dissection: contemporary aspects of diagnosis and patient management. Open Heart 2018;5:e000884. [Crossref] [PubMed]
- Persu A, Giavarini A, Touzé E, et al. European consensus on the diagnosis and management of fibromuscular dysplasia J Hypertens 2014;32:1367-78. [Crossref] [PubMed]
- Olin JW, Gornik HL, Bacharach JM, et al. Fibromuscular Dysplasia: State of the Science and Critical Unanswered Questions: A Scientific Statement From the American Heart Association. Circulation 2014;129:1048-78. [Crossref] [PubMed]
- Saw J, Humphries K, Aymong E, et al. Spontaneous Coronary Artery Dissection. J Am Coll Cardiol 2017;70:1148-58. [Crossref] [PubMed]
- Prasad M, Tweet MS, Hayes SN, et al. Prevalence of Extracoronary Vascular Abnormalities and Fibromuscular Dysplasia in Patients With Spontaneous Coronary Artery Dissection. Am J Cardiol 2015;115:1672-7. [Crossref] [PubMed]
- Rogowski S, Maeder MT, Weilenmann D, et al. Spontaneous Coronary Artery Dissection: Angiographic Follow-Up and Long-Term Clinical Outcome in a Predominantly Medically Treated Population. Catheter Cardiovasc Interv 2017;89:59-68. [Crossref] [PubMed]
- McGrath-Cadell L, McKenzie P, Emmanuel S, et al. Outcomes of patients with spontaneous coronary artery dissection. Open Heart 2016;3:e000491. [Crossref] [PubMed]
- Nakashima T, Noguchi T, Haruta S, et al. Prognostic impact of spontaneous coronary artery dissection in young female patients with acute myocardial infarction: A report from the Angina Pectoris?Myocardial Infarction Multicenter Investigators in Japan. Int J Cardiol 2016;207:341-8. [Crossref] [PubMed]
- Wiebers DO, Whisnant JP, Huston J, et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet 2003;362:103-10. [Crossref] [PubMed]
- UCAS Japan Investigators, Morita A, Kirino T, et al. The natural course of unruptured cerebral aneurysms in a Japanese cohort. N Engl J Med 2012;366:2474-82. [Crossref] [PubMed]
- García-Arribas D, Macaya F, Vilacosta I, et al. Coexistence of spontaneous coronary artery dissection and ascending aortic aneurysm. Ann Thorac Surg 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Kadian-Dodov D, Gornik HL, Gu X, et al. Dissection and Aneurysm in Patients With Fibromuscular Dysplasia. J Am Coll Cardiol 2016;68:176-85. [Crossref] [PubMed]
- Yip A, Saw J. Spontaneous coronary artery dissection—A review. Cardiovasc Diagn Ther 2015;5:37-48. [PubMed]
- Macaya F, Aldazábal A, Moreu M, et al. Screening of systemic arteriopathy in patients with spontaneous coronary artery dissection. Eur Heart J Cardiovasc Imaging 2018;19:357. [Crossref] [PubMed]
- Gulani V, Calamante F, Shellock FG, et al. International Society for Magnetic Resonance in Medicine. Gadolinium deposition in the brain: summary of evidence and recommendations. Lancet Neurol 2017;16:564-70. [Crossref] [PubMed]
- Willoteaux S, Faivre-Pierret M, Moranne O, et al. Fibromuscular Dysplasia of the Main Renal Arteries: Comparison of Contrast-enhanced MR Angiography with Digital Subtraction Angiography. Radiology 2006;241:922-9. [Crossref] [PubMed]
- Tweet MS, Gulati R, Williamson EE, et al. Multimodality Imaging for Spontaneous Coronary Artery Dissection in Women. JACC Cardiovasc. Imaging 2016;9:436-50. [Crossref] [PubMed]
- Toggweiler S, Puck M, Thalhammer C, et al. Associated vascular lesions in patients with spontaneous coronary artery dissection. Swiss Med Wkly 2012;142:w13538. [PubMed]
- Rashid HNZ, Wong DTL, Wijesekera H, et al. Incidence and characterisation of spontaneous coronary artery dissection as a cause of acute coronary syndrome — A single-centre Australian experience. Int J Cardiol 2016;202:336-8. [Crossref] [PubMed]
- Bastante T, Rivero F, Cuesta J, et al. Association of Spontaneous Coronary Artery Dissection With Fibromuscular Dysplasia. Rev Esp Cardiol (Engl Ed) 2015;68:719-20. [Crossref] [PubMed]
- Adlam D, Olson TM, Combaret N, et al. Association of the PHACTR1/EDN1 Genetic Locus With Spontaneous Coronary Artery Dissection. J Am Coll Cardiol 2019;73:58-66. [Crossref] [PubMed]
- Vasbinder GBC, Nelemans PJ, Kessels AGH, et al. Accuracy of computed tomographic angiography and magnetic resonance angiography for diagnosing renal artery stenosis. Ann Intern Med 2004;141:674-82. [Crossref] [PubMed]
- Netuka D, Belšán T, Broulíková K, et al. Detection of carotid artery stenosis using histological specimens: a comparison of CT angiography, magnetic resonance angiography, digital subtraction angiography and Doppler ultrasonography. Acta Neurochir 2016;158:1505-14. [Crossref] [PubMed]
- Thompson BG, Brown RD, Amin-Hanjani S, et al. Guidelines for the Management of Patients With Unruptured Intracranial Aneurysms: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2015;46:2368-400. [Crossref] [PubMed]
- Okahara M, Kiyosue H, Yamashita M, et al. Diagnostic accuracy of magnetic resonance angiography for cerebral aneurysms in correlation with 3D-digital subtraction angiographic images: a study of 133 aneurysms. Stroke 2002;33:1803-8. [Crossref] [PubMed]
- Walkoff L, Brinjikji W, Rouchaud A, et al. Comparing magnetic resonance angiography (MRA) and computed tomography angiography (CTA) with conventional angiography in the detection of distal territory cerebral mycotic and oncotic aneurysms. Interv Neuroradiol 2016;22:524-8. [Crossref] [PubMed]
- Kim HJ, Yoon DY, Kim ES, et al. Intraobserver and interobserver variability in CT angiography and MR angiography measurements of the size of cerebral aneurysms. Neuroradiology 2017;59:491-7. [Crossref] [PubMed]