
Vascular magnetic resonance angiography techniques
Introduction
With the increasing size of the aging population and high prevalence of atherosclerotic disease, the need for examining the vasculature continues to rise and the demand for vascular imaging ever higher. While catheter angiography remains the gold standard, its invasive nature, ionizing radiation exposure, and risk for serious complications such as plaque disruption/embolization has prompted an emergence of noninvasive modalities. In the last two decades, advancement in the field of magnetic resonance angiography (MRA) has made it an ever-attractive choice. In particular, the availability of non-contrast MRA techniques denotes a unique niche for patients with poor renal function, which is substantially prevalent with significant vasculopathy.
Owing to the growing options of imaging protocols and complexity of advanced methods, choosing an appropriate imaging strategy is becoming more difficult. Here, we provide an overview and considerations of the available technical options.
MRA techniques
We should note that classically MRA techniques haven been categorized into “dark blood” and “bright blood” techniques. While dark-blood techniques remain invaluable in specific clinical scenarios, such as vessel wall imaging, cardiac imaging, and susceptibility venography, it plays limited role in the routine evaluation of the peripheral vasculature, wherein the imaging is tailored to address course, caliber, patency, and degree of stenosis. Further, many between-vendor differences exist, and many sequences are proprietary in nature. Instead, here we focus on main mechanisms underlying bright blood techniques commonly available. Vendor-specific names for the various techniques are shown in Table 1.

Full table
Time-of-flight (TOF) MRA
TOF MRA is the conventional workhorse of vascular imaging. Leveraging flow related enhancement exhibited by incoming blood, images of the vascular system are obtained with drastic contrast from the background without administration of gadolinium.
In TOF MRA, repetitive RF pulses saturate the spin magnetization of the stationary structures in the imaging field, resulting in background signal suppression. As moving blood lacks the same degree of spin saturation, relative hyperintense signal is observed (1). Typically, a gradient echo-based sequence is used in reducing flow related signal loss often seen with spin echo-based sequences. A sample simplified pulse sequence diagram is shown in Figure 1. Moderate to high flip angles increase signal of the flowing blood. Very short TE value minimizes T2* dephasing. To selectively augment visualization to only arterial signal, a saturation pulse is placed downstream from the region of interest, nulling the venous inflow. The addition of fat suppression counters bright signals from intrinsic fast spin recovery of fat. Cardiac gating with full image acquisition or central k-space filling during the systolic phase can accentuate the TOF effect and reduce pulsation artifacts. The capability to repeat the imaging sequence to adjust the field of view or lessen certain artifacts is an important but underappreciated advantage of non-contrast techniques.
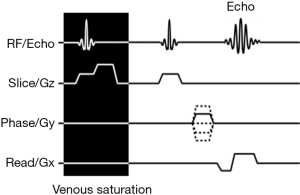
Both 2D (slice) and 3D (slab) acquisitions are possible with TOF. 2D imaging offers shorter scan time at the expense of imaging resolution, and 3D the opposite. The longer the blood stays in the imaging field, the lower the resultant signal. Consequently, 2D acquisition has low sensitivity to in-plane flow (in-plane saturation). Similarly, in 3D acquisition, the overall size of the imaging slab is limited to roughly a few centimeters, and the more distal aspect of the slab may demonstrate less intense flow signal. Ramped flip angles have been used to reduce this effect with some success (2). Multi-slab acquisition allows for larger field of view by sequential acquisition of small 3D slabs that are then merged by post-processing. Venetian blind artifacts represent manifestations of the within-slab saturation effects. Compared to the other methods, a relatively lengthy scan time is the least favorable characteristic of TOF MRA.
Phase contrast (PC) MRA
PC MRA exploits the phase shift following application of bipolar gradients on moving blood in not only generating an angiographic image but also allowing for velocity and pressure gradient quantification in stenotic lesions.
In PC MRA, sets of balanced motion-sensitizing bipolar gradients are added to an imaging sequence. A sample simplified pulse sequence diagram is shown in Figure 2. Because precession frequency is dependent on magnetic field strength, spins subjected to the added gradients precess in a slightly altered frequency. With time, the spins accumulate phase difference that is dependent on the length of gradient exposure and gradient magnitude/directionality. In stationary tissues, exposure to both positive and negative gradients results in no net phase change. In moving spins, however, unbalanced phase exposure leads to a measurable precession phase difference. By analyzing the phase data, both presence and velocity of flow can then be determined (3). Accurate velocity estimation depends on successful balance between avoiding phase aliasing and detection of slow flow in vessels from background noise. This balance is accomplished via adjustment in the strength of the bipolar gradients to accommodate the expected velocity range, which is termed velocity encoding (4). Traditionally performed in 2D mode, 3D velocity encoding may be performed by application of the motion-sensitizing bipolar gradients along multiple directions (3,5).
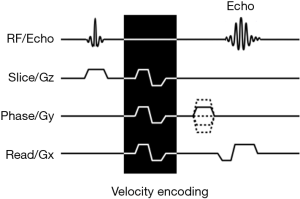
Due to the long acquisition time required, routine use of PC MRA is often limited to clinical scenarios where both anatomy and hemodynamic assessments are warranted, such as in the case of suspected renal artery stenosis. Its relative insensitivity to T1 shine-through effects faced by other MRA techniques make it valuable in evaluating vessels near large amount of fat or hematomas. Unfortunately, the presence of non-laminar flow hampers interpretation and deconvolution of the phase data, making PC MRA ineffective in quantitative evaluation of known aneurysm or significant narrowing. However, the presence of this artifact often signals the presence of hemodynamically significant stenosis and may itself be helpful (6).
Balanced steady-state free precession (bSSFP) MRA
In contradistinction to TOF and PC MRAs that hinge on the effects of blood flow to create contrast, the intrinsic signal characteristics of blood generate angiographic details in bSSFP MRA.
In bSSFP, a gradient echo sequence is made nearly completely balanced by using symmetric gradients in slice selection, frequency encoding, and phase encoding directions prior to and following the echo signal with TE at half of TR. A sample simplified pulse sequence diagram is shown in Figure 3. In doing so, the free induction decay and stimulated spin echo coalesce into a single signal with each and subsequent excitation. The sequence is functionally of spin echo characteristic with T2/T1 weighing (7). Interestingly, due to the intrinsically high T2/T1 ratio of blood, the resulting image provides angiographic intensity weighing. Flow independent signal means higher sensitivity to lower flow velocity regardless of flow direction. An inversion pulse is used to suppress background signal. Short scan time and high signal-to-noise ratio are key advantages of this technique (8).
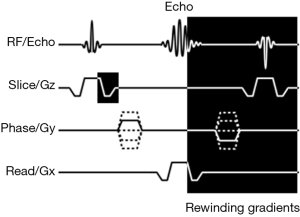
Due to the high T2/T1 ratio of fluid and fat, bSSFP is challenging when evaluating vessels in close proximity to fluid and fat, including the mesenteric vasculatures. Fat suppression partially alleviates this issue. Venous contamination remains a problem despite available venous suppression methods. Lastly, bSSFP is technically demanding and requires precise control of magnetic field homogeneity/stringent shimming. Frequently, artifactual distortions plague image quality at least at the image margins (Moiré Fringes).
Fast spin echo (FSE) MRA
FSE MRA, also known as fresh blood imaging (FBI), is a cardiac phase-specific subtraction technique that utilizes both the intrinsic high T2 signal of the blood substance and flow-related signal loss in generating the angiogram (9).
Flow related signal loss is a phenomenon that occurs largely with spin echo techniques due to time lapse between the initial excitation pulse and the refocusing pulse encountered by fast flowing blood- spins that exit the imaging field prior to the refocusing pulse manifest in a “flow void” (10,11). In 3D FSE, the signal loss is further exacerbated by the repetitive readout gradients and incomplete refocusing flip angles (12), which are phenomena beyond the scope of this review. Because of the faster arterial flow velocity, the signal loss is much more pronounced in systole. In diastole, the arterial blood remains bright due to its intrinsic long T2 relaxation. With cardiac gating and subtraction of systole and diastole image sets, a nearly pure arteriogram is obtained (13,14). To confine acquisition to specific cardiac phases, some form of acceleration is typically used, such as partial or half k-space sampling and/or parallel imaging.
The key advantage of this technique lies in its lack of venous or nonvascular tissue contamination. Venous structures do not typically demonstrate significant flow related signal loss due to slower and less phasic flow. The inability to appropriately depict high flow (where signal loss occurs in diastole), slow flow (where signal loss does not occur in systole), or hyperdynamic states constitutes major weakness of this technique. Proper timing and execution of cardiac gating may be problematic in select patient populations.
Hybrid MRA
Given the increasing number of MRA methods available, it is unsurprising that some newer techniques now take advantage of a combination of the above-mentioned methods. Quiescent interval single-shot (QISS, Siemen’s Healthcare, Erlangen, Germany) MRA is one vendor’s example that operates with both TOF and bSSFP principles.
In QISS MRA, a saturation pulse nulls the signal of the background tissue, similar in form to TOF. A following short delay (quiescent interval) allows for entry of unsaturated blood. Instead of obtaining images with gradient echo sequence as in TOF, however, bSSFP sequence is then used to obtain the angiographic image. This technique benefits from arterial signal augmentation by both TOF effect and the intrinsic high signal of blood in bSSFP. Venous signals are suppressed by additional saturation pulses. Cardiac gating is used to coincide the quiescent period with the time of systole, maximizing inflow of unsaturated blood. High sensitivity (92%) and specificity (95%) are reported in the detection of peripheral vascular disease related stenosis with this method (15), offering benefits over computed tomography angiography that is limited by blooming artifacts in heavily calcified arterial segments (16).
This technique suffers from some drawbacks of TOF and bSSFP. Mainly, areas of vessel tortuosity and in-plane flow may not allow for adequate washout of saturated spin. Vigorous shimming is required to achieve adequate field homogeneity, and precise cardiac gating may not be possible with some patients. In general, hybrid techniques demonstrate augmented flow visualization but share similar setbacks of the parent methods. As many newer hybrid techniques enter the commercial market, it may be worth noting the main principles in which the new sequence is derived in order to understand its limitations.
Contrast enhanced (CE) MRA
CE MRA is a conceptually straightforward technique that employs intravascular gadolinium agents in generating high vessel-to-background contrast-to-noise ratio.
Gadolinium, a paramagnetic substance, shortens the T1 relaxation time of the adjacent tissue in vivo. Consequently, presence of intravascular gadolinium contrast agents allows for visualization of the vessel in great conspicuity on T1-weighted images (17). Because the signal arises from altered relaxation of blood, flow related issues faced by other techniques are infrequently problematic. Typically, venous access is secured at a site away from the vessel of interest or non-selectively through the right upper extremity due to the short distance to the heart. Along with application of a post-contrast saline flush, appropriate selection of the injection site avoids streak artifacts arising from venous contrast pooling. TE and TR are minimized to produce T1-weighing and shorten time scan time. Gradient echo-based sequences are favored over spin echo sequences to avoid flow related signal loss. Subtraction of pre-contrast images from post-contrast images improves visualization. Fat suppression further enhances signal-to-background contrast.
The use of gadolinium contrast agent precludes CE MRA in some vulnerable patient population, representing the biggest weakness of the technique. Precise timing is a critical issue in CE MRA. Imaging preferably coincides with first pass peak vessel enhancement. Poor vessel visualization occurs with premature image acquisition, and delayed imaging leads to venous contamination. Traditionally, single time point acquisition is used with a time delay, which may be set manually, determined by testing with a small contrast bolus, or via real-time “fluoroscopic” triggering.
Large field imaging (such as lower extremity run-off) represents another complicating issue of CE MRA. Due to the limitation on field-of-view set by hardware constraints, a large imaging field necessitates separation into smaller “stations”, i.e., aortoiliac, femoropopliteal, and infrapopliteal segments. With non-contrast techniques, this segmentation is of little consequence. Yet, manual adjustment of table positioning to catch the arterial contrast is not possible in CE MRA due to speed of blood flow. “Bolus-chasing” or “moving-table technique” may thus be performed, in which automatic table movement is coupled to image acquisition by use of specially designed hardware (18). Acceleration techniques and 3D imaging help to provide adequate field coverage and shorten scan time. Alternatively, some centers employ multiple lower volume injections in accomplishing multi-station imaging. Application of tourniquet induced venous compression has been shown to retard venous enhancement and lengthen the imaging window (19). Recently “blood pool” contrast agents were developed that remain intravascular for extended time by binding to macromolecules such as albumin, further lengthening the imaging window (20). Unfortunately, the first and only agent that received FDA approval, gadofosveset, is no longer in production in the US due to small market niche. The commonly available gadobenate dimeglumine (MultiHance) demonstrates weak albumin binding and mildly increased vascular persistence (21), although the practical benefit of this subtle difference remains to be debated. Ferumoxytol, a non-gadolinium superparamagnetic iron oxide contrast agent, demonstrates a prolonged vascular residence time (22) and is a promising vascular imaging agent especially in patient with a contraindication to gadolinium such as renal failure (23). However, it is currently considered off-label use by the FDA. Non-trivial incidence of anaphylaxis/anaphylactoid reactions, hypersensitivity, and hypotension have been reported in iron therapy (24).
Time resolved MRA
In time resolved (4D) MRA, a series of image acquisitions is performed following intravenous administration of contrast to ensure capture of the arterial contrast bolus, made possible by keyhole imaging.
A fundamental characteristic of the frequency domain (“k-space”, where image data are naturally acquired) is the frequency specific arrangement of data- lower frequency data in the central k-space and higher frequency peripherally. As higher frequency data correspond to smaller wavelengths that depict edges and fine structures, the peripheral k-space is composed of predominantly resolution information. Conversely, the central k-space contains low frequency data that correspond to larger structural information that is bulk of the image contrast. Accordingly with contrast inflow, the central portions of the frequency domain vary with arriving of the contrast bolus while the peripheral data remain grossly unchanged (25). In 4D keyhole imaging, a full acquisition is first performed, forming the initial data set. Subsequent acquisitions following contrast administration are then aimed at only preferentially replacing the central component of k-space, drastically reducing the imaging time (26). The resultant images are in effect a hybrid with changing contrast information of the later scans and image resolution provided from the initial full acquisition. Typically, a 3D gradient echo-based sequence is used. Concurrent use of other acceleration techniques is the norm in further improving temporal resolution.
The timing insensitivity and flow invariant visualization make time resolved angiography a popular choice in the evaluation of vascular malformations/anomalies, in which the timing of the contrast bolus can be difficult to predict. The nature of keyhole imaging makes it vulnerable to motion artifacts. Determining the size of the central k-space remains a difficult challenge in balancing temporal resolution and true image contrast. Lastly, the sheer number of images obtained with this technique is a nontrivial issue in terms of processing technical demands and interpretation.
Post-processing
Several post-processing methods aid in displaying the angiographic data. Multiplanar reformation (MPR) allows for display of the acquired images in various planes, providing more advantageous viewing angulation of the vessels. Near isotropic voxels with minimal between-slice gaps help in generating reformats with little artifactual distortion. Maximum intensity projection (MIP) uses an algorithm that creates projections by displaying the highest signal intensity along the user-defined line of sight. Projections obtained from small regular angular intervals may be displayed in a cine-like fashion, which appear like rotating 3D objects. Lastly, volumetric rendering (VR) permits display of angiographic data in a virtual 3D display. With the aid of thresholding, segmentation, coloring, and shading, a life-like representation of the vessel of interest may be obtained. While diagnostically VR is infrequently helpful, it is nevertheless an invaluable tool in conveying the imaging findings to the non-radiology referring colleagues.
Special considerations
Specific indications
Thoracic outlet syndrome
Thoracic outlet syndrome arises from neurovascular bundle compression at the thoracic outlet with neurogenic, arterial, and venous forms. While the diagnosis is clinical, imaging plays a vital role in supporting the diagnosis of vascular compression. Because the affected patient is often young, MRA is the preferred evaluation tool in imaging dynamic vascular compression and/or thrombosis due to its lack of ionizing radiation (27). With the preferred blood pool agent gadofosveset no longer commercially available, CE MRA with split bolus injection at 2 mL/sec via the contralateral arm is typically employed. Acquisition is performed at both arterial and delayed venous phases and in both neutral and external rotation positions (28). Diameter change by >30% for subclavian artery and >50% for subclavian vein between the neutral and abducted positions is considered positive for thoracic outlet compression (29). Breath-held 3D volume-interpolated GRE is helpful for delineating anatomic structures. In case with suspected distal embolization, additional evaluation of the forearm or hand may be useful.
Median arcuate ligament syndrome
Median arcuate ligament syndrome (celiac artery compression syndrome, Dunbar syndrome) is characterized by postprandial pain and weight loss due to celiac trunk compression by the median arcuate ligament. The compression is accentuated by expiration with superior movement of the median arcuate ligament and relieved by inspiration with its descent (30). Although this is a diagnosis of exclusion, imaging can be supportive. Traditional evaluation by ultrasound can be limited due to operator dependent nature of the technique. CE MRA using split bolus technique in inspiration and expiration mitigates operator dependence and is thus useful. Because it is common to see celiac artery compression by the median arcuate ligament with expiration, persistence of superior hook-like narrowing of the celiac trunk in the appropriate clinical context is needed to suggest the diagnosis (31). Additionally, abnormal thickness of the median arcuate ligament (>4 mm) can also serve as a diagnostic clue (32).
Popliteal artery entrapment
Popliteal artery entrapment is an uncommon cause of lower limb claudication, usually seen in young athletes, caused by entrapment of the popliteal artery between the medial femoral condyle and the medial head of the gastrocnemius muscle. While the compression is dynamic, repeated injury can lead to permanent vessel damage, occlusion, or embolization (33). MRA allows for evaluation of dynamic occlusion and enables anatomical evaluation of the popliteal fossa. Before scanning, maneuvers that reproduce the symptoms need to be elicited from the patient. Commonly, hyperextension at the knee, dorsiflexion of the foot, or alternating dorsiflexion/plantarflexion at the ankle against resistance is provocative. Dual contrast injection 4D MRA is used with the patient at rest followed by the position that reproduces the symptoms. If there is a contraindication for contrast administration, bSSFP MRA can serve as an alternative, performed in rest and provocative positions (34).
Perforator flap imaging
Perforator flap imaging is a novel MRA method in increasing demand due to rising popularity for breast surgery reconstruction and microvascular lymphedema surgery. Although lower in spatial resolution compared to CTA, MRA has higher contrast resolution, enabling more accurate localization of intra-muscular perforators (35) while avoiding ionizing radiation. Artifacts from breathing/motion, time-consuming nature, and expertise/technical demand constitute the main limitations of perforator flap MRA. Patients with tissue expanders represent another issue due to safety concerns, although not necessarily contraindicated (36). Imaging in the prone position with phase encoding in the transverse direction helps to avoid overlapping artifacts from respiratory motion. For surface marking, a vitamin E capsule can be placed at surface landmarks to assist in calculating perforator locations, most commonly umbilicus for deep inferior epigastric perforator flap, superior gluteal cleft for gluteal artery free flap, and inferior gluteal crease for profunda artery perforator flap. Because spoiled GRE sequences help suppress background and non-vascular signal, CE MRA with 3D spoiled GRE backbone (VIBE/LAVA/THRIVE/3D QUICK/TIGRE) is typically used.
Contrast agent safety
Allergic reaction, nephrogenic systemic fibrosis (NSF), and gadolinium deposition represent the major potential complications of gadolinium contrast use. Immediate allergic-like reaction is seen in a minority of patients, estimated at 9 events/10,000 administrations (0.5 events considered severe) by a meta-analytic study (37). Allergic-like reaction is more common with agents that have high ionicity, high protein binding, and macrocyclic structure. Prior history of allergic-like reaction to gadolinium contrast increases the risk of subsequent allergic-like reaction nearly eight times (38). NSF is a known but rare adverse effect of gadolinium contrast administration that was described in 2000 (39), characterized by thickening and hardening of large areas of the skin. Seen mostly in patients with severely impaired renal function and more commonly with older/linear gadolinium agents (40), the true incidence of NSF is likely very low with the use of newer agents and avoidance of its use in patients with poor renal clearance. Recently, it was shown that gadolinium contrast is not fully excreted from the body regardless of renal function (41). Although highly variable, organ deposition of gadolinium is noted to be reduced but not eliminated by use of macrocyclic agents (42). The clinical significance of gadolinium retention is currently unknown and under investigation. In 2015, the FDA’s safety announcement indicates that gadolinium contrast use should be limited to clinical circumstances in which contrast administration provides important additional information.
Gadolinium contrast in special populations
Pregnancy
Currently, there are no known harmful effects of MRI to fetuses (43). However, the available studies are limited in number. While pregnancy is not a risk factor for NSF, an association between gadolinium contrast exposure during pregnancy and an increased incidence of stillbirth or neonatal deaths was reported previously (43). Because of unknown fetal effects, the American College of Radiology recommends that gadolinium contrast be administered only if its use is critical and the benefits of the study justify the possible but unclear fetal risks (38). If gadolinium contrast is used, one of the agents believed to be at low risk for NSF should be used at the lowest possible dose. Obtaining informed consent is also recommended.
Breastfeeding
In patients with normal renal function, the plasma half-life for most gadolinium contrasts is estimated at 2 hours with bloodstream clearance in 24 hours. Most of the contrast excreted in breast milk is in stable and chelated form. A minimal proportion of the excreted gadolinium is absorbed by the infant’s gastrointestinal tract (44). The American College of Radiology states that it is safe for both mother and infant to continue breastfeeding after receiving gadolinium contrast, although an informed decision should be made with the lactating mother (38). If there is concern for unknown potential harmful effects, breastfeeding may be suspended for 12–24 hours, and breast milk expressed and discarded. There is no value for halting breastfeeding beyond 24 hours.
Pediatric patients
The use of gadolinium contrast agents in children is considered off-label, and none of the commercially available agents is FDA approved in all age groups. The American College of Radiology recommendations for gadolinium contrast are similar in both pediatric and adult populations (38). The fluid-shifts associated with high osmolality and viscosity contrast is of less concern in MRI as compared to iodinated CT contrast due to the smaller contrast volume. The data on allergic-like reactions to gadolinium contrast in the pediatric population remain limited. In a study on 15,706 administrations in children, the incidence of allergic-like reactions was found to be 0.05% (45). These reactions are treated similar to reactions to iodinated contrast agents. Similar to adults, the use of gadolinium contrast is best avoided in children with severely impaired renal function.
Conclusions
The advent of magnetic resonance techniques makes MRA an indispensable tool in the evaluation of the vasculature. In particular, its noninvasive nature, freedom from ionizing radiation, and versatility suggest MRA will play an increasing role in the management of vascular patients. Understanding the technical underpinnings of the various MRA methods helps in recognition of common imaging issues and artifacts in rendering clinically relevant interpretations.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Laub GA. Time-of-flight method of MR angiography. Magn Reson Imaging Clin N Am 1995;3:391-8. [PubMed]
- Atkinson D, Brant-Zawadzki M, Gillan G, et al. Improved MR angiography: magnetization transfer suppression with variable flip angle excitation and increased resolution. Radiology 1994;190:890-4. [Crossref] [PubMed]
- Dumoulin CL, Souza SP, Walker MF, et al. Three-dimensional phase contrast angiography. Magn Reson Med 1989;9:139-49. [Crossref] [PubMed]
- Reimer P, Boos M. Phase-contrast MR angiography of peripheral arteries: technique and clinical application. Eur Radiol 1999;9:122-7. [Crossref] [PubMed]
- Lee AT, Bruce Pike G, Pelc NJ. Three-Point Phase-Contrast Velocity Measurements with Increased Velocity-to-Noise Ratio. Magn Reson Med 1995;33:122-6. [Crossref] [PubMed]
- Wasser MN, Westenberg J, van der Hulst VP, et al. Hemodynamic significance of renal artery stenosis: digital subtraction angiography versus systolically gated three-dimensional phase-contrast MR angiography. Radiology 1997;202:333-8. [Crossref] [PubMed]
- Scheffler K, Lehnhardt S. Principles and applications of balanced SSFP techniques. Eur Radiol 2003;13:2409-18. [Crossref] [PubMed]
- Fuchs F, Laub G, Othomo K. TrueFISP—technical considerations and cardiovascular applications. Eur J Radiol 2003;46:28-32. [Crossref] [PubMed]
- Hoey ETD, Ganeshan A, Puni R, et al. Fresh Blood Imaging of the Peripheral Vasculature: An Emerging Unenhanced MR Technique. AJR Am J Roentgenol 2010;195:1444-8. [Crossref] [PubMed]
- Jara H, Yu BC, Caruthers SD, et al. Voxel sensitivity function description of flow-induced signal loss in MR imaging: Implications for black-blood MR angiography with turbo spin-echo sequences. Magn Reson Med 1999;41:575-90. [Crossref] [PubMed]
- Meuli RA, Wedeen VJ, Geller SC, et al. MR gated subtraction angiography: evaluation of lower extremities. Radiology 1986;159:411-8. [Crossref] [PubMed]
- Wheaton AJ, Miyazaki M. Non-contrast enhanced MR angiography: Physical principles. J Magn Reson Imaging 2012;36:286-304. [Crossref] [PubMed]
- Lanzman R, Blondin D, Schmitt P, et al. Non-Enhanced 3D MR Angiography of the Lower Extremity using ECG-Gated TSE Imaging with Non-Selective Refocusing Pulses - Initial Experience. RöFo - Fortschritte auf dem Gebiet der Röntgenstrahlen und der Bildgeb Verfahren 2010;182:861-7.
- Miyazaki M, Sugiura S, Tateishi F, et al. Non-contrast-enhanced MR angiography using 3D ECG-synchronized half-Fourier fast spin echo. J Magn Reson Imaging 2000;12:776-83. [Crossref] [PubMed]
- Edelman RR, Sheehan JJ, Dunkle E, et al. Quiescent-interval single-shot unenhanced magnetic resonance angiography of peripheral vascular disease: Technical considerations and clinical feasibility. Magn Reson Med 2010;63:951-8. [Crossref] [PubMed]
- Wu G, Yang J, Zhang T, et al. The diagnostic value of non-contrast enhanced quiescent interval single shot (QISS) magnetic resonance angiography at 3T for lower extremity peripheral arterial disease, in comparison to CT angiography. J Cardiovasc Magn Reson 2016;18:71. [Crossref] [PubMed]
- Prince MR. Gadolinium-enhanced MR aortography. Radiology 1994;191:155-64. [Crossref] [PubMed]
- Ho KY, Leiner T, de Haan MW, et al. Peripheral vascular tree stenoses: evaluation with moving-bed infusion-tracking MR angiography. Radiology 1998;206:683-92. [Crossref] [PubMed]
- Vogt FM, Ajaj W, Hunold P, et al. Venous Compression at High-Spatial-Resolution Three-dimensional MR Angiography of Peripheral Arteries. Radiology 2004;233:913-20. [Crossref] [PubMed]
- Saeed M, Wendland MF, Higgins CB. Blood pool MR contrast agents for cardiovascular imaging. J Magn Reson Imaging 2000;12:890-8. [Crossref] [PubMed]
- Lohr HA, Froehlich JM, Pfyffer M, et al. Comparison of Gd-BOPTA and Gd-DOTA for Peripheral CE-MRA: A Double-Blind Clinical Study. Acad Radiol 2002;9:S421-4. [Crossref] [PubMed]
- Li W, Salanitri J, Tutton S, et al. Lower Extremity Deep Venous Thrombosis: Evaluation with Ferumoxytol-enhanced MR Imaging and Dual-Contrast Mechanism—Preliminary Experience. Radiology 2007;242:873-81. [Crossref] [PubMed]
- Oliveira IS, Hedgire SS, Li W, et al. Blood pool contrast agents for venous magnetic resonance imaging. Cardiovasc Diagn Ther 2016;6:508-18. [Crossref] [PubMed]
- Lu M, Cohen MH, Rieves D, et al. FDA report: Ferumoxytol for intravenous iron therapy in adult patients with chronic kidney disease. Am J Hematol 2010;85:315-9. [PubMed]
- Van Vaals JJ, Brummer ME, Thomas Dixon W, et al. “Keyhole” method for accelerating imaging of contrast agent uptake. J Magn Reson Imaging 1993;3:671-5. [Crossref] [PubMed]
- Willinek WA, Hadizadeh DR, von Falkenhausen M, et al. 4D time-resolved MR angiography with keyhole (4D-TRAK): More than 60 times accelerated MRA using a combination of CENTRA, keyhole, and SENSE at 3.0T. J Magn Reson Imaging 2008;27:1455-60. [Crossref] [PubMed]
- Chavhan GB, Batmanabane V, Muthusami P, et al. MRI of thoracic outlet syndrome in children. Pediatr Radiol 2017;47:1222-34. [Crossref] [PubMed]
- Raptis CA, Sridhar S, Thompson RW, et al. Imaging of the Patient with Thoracic Outlet Syndrome. Radiographics 2016;36:984-1000. [Crossref] [PubMed]
- Nagpal P, Maller V, Garg G, et al. Upper Extremity Runoff: Pearls and Pitfalls in Computed Tomography Angiography and Magnetic Resonance Angiography. Curr Probl Diagn Radiol 2017;46:115-29. [Crossref] [PubMed]
- Reuter SR, Bernstein EF. The anatomic basis for respiratory variation in median arcuate ligament compression of the celiac artery. Surgery 1973;73:381-5. [PubMed]
- Lee VS, Morgan JN, Tan AGS, et al. Celiac artery compression by the median arcuate ligament: a pitfall of end-expiratory MR imaging. Radiology 2003;228:437-42. [Crossref] [PubMed]
- Eliahou R, Sosna J, Bloom AI. Between a rock and a hard place: clinical and imaging features of vascular compression syndromes. Radiographics 2012;32:E33-49. [Crossref] [PubMed]
- Ersoy H, Rybicki FJ. MR angiography of the lower extremities. AJR Am J Roentgenol 2008;190:1675-84. [Crossref] [PubMed]
- Williams C, Kennedy D, Bastian-Jordan M, et al. A new diagnostic approach to popliteal artery entrapment syndrome. J Med Radiat Sci 2015;62:226-9. [Crossref] [PubMed]
- Agrawal MD, Thimmappa ND, Vasile JV, et al. Autologous breast reconstruction: preoperative magnetic resonance angiography for perforator flap vessel mapping. J Reconstr Microsurg 2015;31:1-11. [PubMed]
- Thimmappa ND, Prince MR, Colen KL, et al. Breast Tissue Expanders with Magnetic Ports: Clinical Experience at 1.5 T. Plast Reconstr Surg 2016;138:1171-8. [Crossref] [PubMed]
- Behzadi AH, Zhao Y, Farooq Z, et al. Immediate Allergic Reactions to Gadolinium-based Contrast Agents: A Systematic Review and Meta-Analysis. Radiology 2018;286:471-82. [Crossref] [PubMed]
- ACR Committee on Drugs and Contrast Media. ACR Manual on Contrast Media Version 10.3. 11th ed. Reston, VA 2018.
- Cowper SE, Robin HS, Steinberg SM, et al. Scleromyxoedema-like cutaneous diseases in renal-dialysis patients. Lancet 2000;356:1000-1. [Crossref] [PubMed]
- Beam AS, Moore KG, Gillis SN, et al. GBCAs and Risk for Nephrogenic Systemic Fibrosis: A Literature Review. Radiol Technol 2017;88:583-9. [PubMed]
- McDonald RJ, McDonald JS, Kallmes DF, et al. Intracranial Gadolinium Deposition after Contrast-enhanced MR Imaging. Radiology 2015;275:772-82. [Crossref] [PubMed]
- McDonald RJ, McDonald JS, Dai D, et al. Comparison of Gadolinium Concentrations within Multiple Rat Organs after Intravenous Administration of Linear versus Macrocyclic Gadolinium Chelates. Radiology 2017;285:536-45. [Crossref] [PubMed]
- Ray JG, Vermeulen MJ, Bharatha A, et al. Association Between MRI Exposure During Pregnancy and Fetal and Childhood Outcomes. JAMA 2016;316:952-61. [Crossref] [PubMed]
- Kubik-Huch RA, Gottstein-Aalame NM, Frenzel T, et al. Gadopentetate dimeglumine excretion into human breast milk during lactation. Radiology 2000;216:555-8. [Crossref] [PubMed]
- Davenport MS, Dillman JR, Cohan RH, et al. Effect of abrupt substitution of gadobenate dimeglumine for gadopentetate dimeglumine on rate of allergic-like reactions. Radiology 2013;266:773-82. [Crossref] [PubMed]


