
Shaping the future of renal denervation-the relevance of sham-controlled randomized trials and recent meta-analyses
The overall prevalence of hypertension in adults globally is estimated to be 30–45% with even higher rates of >60% in people aged above 60 years (1). It is expected that the number of people with hypertension will further grow by 15% to 20% and reach ~1.5 billion in 2025 (2). A systolic blood pressure (BP) ≥140 mmHg contributes substantially to the mortality and disability burden (70%), mostly related to ischemic and hemorrhagic stroke (1.5 and 2 million, respectively), and ischemic heart disease (4.9 million) (3). While lifestyle modification and antihypertensive (AH) pharmacotherapy are highly effective in reducing elevated BP, many patients remain uncontrolled due to a variety of reasons including non-adherence and non-compliance, intolerance to prescribed drugs, or true treatment resistance. Some of these patients may benefit from novel interventional procedures such as catheter-based renal denervation (RDN) as a suitable alternative.
Indeed, initial proof-of-concept studies and randomized controlled clinical trials (Symplicity HTN-1 and HTN-2) demonstrated significant BP-lowering efficacy as add on therapy to concomitant drug therapy (4,5). However, the randomized, blinded, sham-controlled Symplicity HTN-3 trial (6) failed to demonstrate the superiority of RDN in BP-lowering compared to a sham control group at 6 months post procedure. The unexpected results of the Symplicity HTN-3 trial have been extensively discussed and attributed to some possible confounding factors (7) which were taken into account in the design of studies in the post-Symplicity HTN-3 era.
A decade after the publication of the original proof-of-concept RDN study (4) recent evidence from appropriately designed trials have resulted in a renewed interest in RDN. These include the DENERHTN trial (8), the SPYRAL HTN-OFF MED (9) and RADIANCE-HTN SOLO (10) trials, both in drug-naïve hypertensive patients, as well as the SPYRAL HTN-ON MED trial (11) in hypertensive patients on concomitant AH therapy. All of these studies demonstrated a significant and clinically relevant reduction in ambulatory BP compared to respective control groups. Evidence is, therefore, now available from a number of properly designed, randomized, sham-controlled trials confirming the BP-lowering efficacy of a catheter-based RDN approach (12). Based on findings from recent large scale outcome studies a decrease in office BP of around 10 mmHg, as achieved in these RDN trials, if maintained in the long-term, would likely be associated with a reduction in cardiovascular (CV) events by ~25%.
Very recently, an updated study-level meta-analysis of all published sham-controlled randomized trials evaluated the effect of RDN on BP in uncontrolled hypertensive subjects (13). Six trials (Table 1) that met the inclusion and exclusion criteria were identified by the authors. These trials involved a total of 977 participants (582 randomized to RDN and 395 to sham). Four out of 6 trials allowed the maintenance of stable optimal medical therapy in both groups, while two trials enrolled individuals who were off AH drugs for at least 3–4 weeks prior to randomization. The three trials applying second-generation RDN devices—SPYRAL HTN-ON MED, SPYRAL HTN-OFF MED, and RADIANCE-HTN SOLO, were designed and performed RDN with more attention to procedural techniques, the number of ablations, monitoring of adherence in some, and appropriate patient selection. The Symplicity HTN-3 trial provided ~55% of all patients included in this meta-analysis. Mean patients age ranged from ~53 to 65 years, 54–87% were male, and median follow-up ranged from 2 to 6 months. Five trials used radiofrequency (RF) energy and 1 used ultrasound for RDN (Table 1).
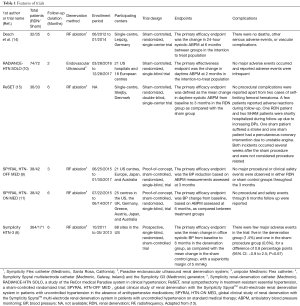
Full table
Importantly, all studies used ambulatory BP measurements as the primary endpoint, which has been shown to be superior to office measurements at predicting CV events (16,17). The meta-analysis revealed that reductions in 24-h ambulatory systolic blood pressure (ASBP) were significantly greater with RDN than sham procedures (weighted mean differences: WMD −3.65 mmHg, 95% CI: −5.33 to −1.98 mmHg; P<0.0001; I2=0%) (Figure 1A). RDN was also associated with a significant decrease in 24-h ambulatory diastolic blood pressure (ADBP) compared with the sham group (WMD −1.71 mmHg, 95% CI: −3.06 to −0.35 mmHg; P=0.01; I2=38%) (Figure 1B) (13). In addition, both daytime ASBP (WMD −4.07 mmHg, 95% CI: −6.46 to −1.68 mmHg; P<0.001; I2=31%) and daytime ADBP (WMD −1.57 mmHg, 95% CI: −2.73 to −0.42 mmHg; P=0.008; I2=0%) were substantially decreased by RDN in comparison to sham procedures. Changes in nigh-time ASBP and ADBP were similar between RDN and sham procedures.
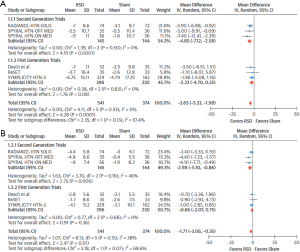
The RDN office systolic (WMD −5.53 mmHg, 95% CI: −8.18 to −2.87 mmHg; P<0.001; I2=0%) and diastolic (WMD −3.37 mmHg, 95% CI: −4.86 to −1.88 mmHg; P<0.001; I2=0%) BP-lowering effect was also superior in comparison to sham procedures.
The ASBP fall caused by RDN was consistent regardless of whether AH drugs were present. Compared with first-generation trials, a significantly more significant reduction of daytime ASBP was observed with RDN in second-generation trials (6.12 vs. 2.14 mmHg; P interaction =0.04), but no interaction was described for 24-h ASBP, night-time ASBP or office BP. The ADBP reduction achieved by RDN was statistically significant only in second-generation trials (WMD −2.98 mmHg, 95% CI: −5.10 to −0.86 mmHg; P=0.006).
No significant difference in the changes from baseline in estimated glomerular filtration rate between the RDN and sham procedure groups in first- or second-generation trials was demonstrated. No major periprocedural adverse events were reported in either group in 5 trials. Symplicity HTN-3 reported significant adverse events in 1.4% of the RDN group and 0.6% of the sham-controlled group. Meta-regression with multiple covariates did not detect any confounding factors/effect modifiers for changes in ASBP.
To put these findings into context, it is worthwhile to compare the BP-lowering effect of RDN with those of commonly used AH drugs in placebo-controlled trials. Indeed, a recent meta-analysis of 52 placebo-controlled studies, including 9,500 patients found that a variety of AH drug regimens reduced ASBP and office SBP by 1.4 and 4.6 mmHg, respectively (18). While perhaps not directly comparable, findings from these two meta-analyses comparing RDN vs. AH drug treatment with their relevant controls (sham and placebo, respectively) do indicate that the ASBP-lowering effect of RDN may be superior to that of a single AH drug (~2.5 times the effect size). Assuming that the BP-lowering effect of RDN is consistently observed and durable, this approach may offer several benefits over time and overcome the inherent limitations of AH drug therapy including drug intolerance, non-adherence, and variability in BP control due to trough levels (11). AH medications have produced less pronounced effects on BP in placebo-controlled when compared with non-placebo controlled single-arm studies. Likewise, RDN demonstrated a more pronounced reduction in BP in single-arm studies, which evaluated pre- and post-RDN treatment effects (19,20).
An obvious question in this context is whether RDN is ready for more widespread clinical use. The latest RDN trials have been designed in collaboration with the US Food and Drug Administration and are still considered proof-of-concept studies to be extended into pivotal trials as currently ongoing. The results presented in the aforementioned meta-analysis, however, reinforce the safety and efficacy of RDN for BP reduction and emphasize the importance of incorporating relevant modifications into trials design (e.g., randomized sham-controlled trials, selection of patients with combined systolic and diastolic hypertension rather than isolated systolic hypertension (21), procedural techniques employed, AH drugs regimen prescribed, highly experienced operators, endpoint ascertainment, and others). Longer-term follow up will be required to ultimately determine the vascular safety of RDN. The ongoing pivotal studies have incorporated these features and will provide more robust and much-needed evidence to inform several remaining questions and will allow appropriate positioning of RDN as an alternative approach to lower BP in clinical medicine.
Acknowledgments
None.
Footnote
Conflicts of Interest: Markus P. Schlaich is supported by an NHMRC Research Fellowship and has received consulting fees, and/or travel and research support from Medtronic, Abbott, Novartis, Servier, Pfizer, and Boehringer-Ingelheim. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Chow CK, Teo KK, Rangarajan S, et al. Prevalence, awareness, treatment, and control of hypertension in rural and urban communities in high-, middle-, and low-income countries. JAMA 2013;310:959-68. [Crossref] [PubMed]
- Kearney PM, Whelton M, Reynolds K, et al. Global burden of hypertension: analysis of worldwide data. Lancet 2005;365:217-23. [Crossref] [PubMed]
- Forouzanfar MH, Liu P, Roth GA, et al. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mmHg, 1990-2015. JAMA 2017;317:165-82. [Crossref] [PubMed]
- Krum H, Schlaich M, Whitbourn R, et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet 2009;373:1275-81. [Crossref] [PubMed]
- Symplicity HTN-2 Investigators , Esler MD, Krum H, et al. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet 2010;376:1903-9. [Crossref] [PubMed]
- Bhatt DL, Kandzari DE, O'Neill WW, et al. A controlled trial of renal denervation for resistant hypertension. N Engl J Med 2014;370:1393-401. [Crossref] [PubMed]
- Kandzari DE, Bhatt DL, Brar S, et al. Predictors of blood pressure response in the SYMPLICITY HTN-3 trial. Eur Heart J 2015;36:219-27. [Crossref] [PubMed]
- Azizi M, Sapoval M, Gosse P, et al. Optimum and stepped care standardised antihypertensive treatment with or without renal denervation for resistant hypertension (DENERHTN): a multicentre, open-label, randomised controlled trial. Lancet 2015;385:1957-65. [Crossref] [PubMed]
- Townsend RR, Mahfoud F, Kandzari DE, et al. Catheter-based renal denervation in patients with uncontrolled hypertension in the absence of antihypertensive medications (SPYRAL HTN-OFF MED): a randomised, sham-controlled, proof-of-concept trial. Lancet 2017;390:2160-70. [Crossref] [PubMed]
- Azizi M, Schmieder RE, Mahfoud F, et al. Endovascular ultrasound renal denervation to treat hypertension (RADIANCE-HTN SOLO): a multicentre, international, single-blind, randomised, sham-controlled trial. Lancet 2018;391:2335-45. [Crossref] [PubMed]
- Kandzari DE, Böhm M, Mahfoud F, et al. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. Lancet 2018;391:2346-55. [Crossref] [PubMed]
- Schlaich MP, Kiuchi MG, Esler MD. Renal Denervation-Ready for Prime Time!? The Steep SPYRAL Stairs to RADIANCE in Hypertension Treatment. Hypertension 2018;72:287-90. [Crossref] [PubMed]
- Sardar P, Bhatt DL, Kirtane AJ, et al. Sham-Controlled Randomized Trials of Catheter-Based Renal Denervation in Patients With Hypertension. J Am Coll Cardiol 2019;73:1633-42. [Crossref] [PubMed]
- Desch S, Okon T, Heinemann D, et al. Randomized sham-controlled trial of renal sympathetic denervation in mild resistant hypertension. Hypertension 2015;65:1202-8. [Crossref] [PubMed]
- Mathiassen ON, Vase H, Bech JN, et al. Renal denervation in treatment-resistant essential hypertension. A randomized, SHAM-controlled, double-blinded 24-h blood pressure-based trial. J Hypertens 2016;34:1639-47. [Crossref] [PubMed]
- Dolan E, Stanton A, Thijs L, et al. Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: the Dublin outcome study. Hypertension 2005;46:156-61. [Crossref] [PubMed]
- Clement DL, De Buyzere ML, De Bacquer DA, et al. Prognostic value of ambulatory blood-pressure recordings in patients with treated hypertension. N Engl J Med 2003;348:2407-15. [Crossref] [PubMed]
- Soranna D, Zambon A, Corrao G, et al. Different effects of antihypertensive treatment on office and ambulatory blood pressure: a meta-analysis. J Hypertens 2019;37:467-75. [Crossref] [PubMed]
- Fadl Elmula FEM, Feng YM, Jacobs L, et al. Sham or no sham control: that is the question in trials of renal denervation for resistant hypertension. A systematic meta-analysis. Blood Press 2017;26:195-203. [Crossref] [PubMed]
- Coppolino G, Pisano A, Rivoli L, et al. Renal denervation for resistant hypertension. Cochrane Database Syst Rev 2017;2:CD011499. [PubMed]
- Ewen S, Ukena C, Linz D, et al. Reduced effect of percutaneous renal denervation on blood pressure in patients with isolated systolic hypertension. Hypertension 2015;65:193-9. [Crossref] [PubMed]


