
Pulmonary hypertension in low- and middle-income countries with focus on sub-Saharan Africa
Introduction
Pulmonary hypertension (PH) is a devastating, progressive disease with increasingly debilitating symptoms and usually shortened overall life expectancy due to narrowing of the pulmonary vasculature and consecutive right heart failure (RHF). PH is defined by an increase in mean pulmonary arterial pressure (mPAP) >25 mmHg at rest assessed by right heart catheterization (1,2) or by echocardiography with a right ventricular systolic pressure (RVSP) >35 mmHg, absence of pulmonary stenosis and acute RHF, and usually accompanied by shortness of breath, fatigue, peripheral edema and other cardiovascular symptoms (3). PH can be caused by a multitude of conditions and co-morbidities highly prevalent in low- and middle-income countries (LMICs) and may complicate the majority of respiratory and cardiovascular diseases leading to excess morbidity and mortality in these multi-morbid patients.
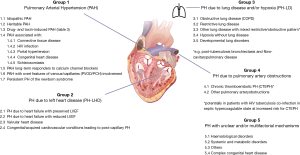
Figure 1 illustrates the updated clinical classification of PH with emphasis on risk factors and co-morbidities prevalent in LMICs according to the 6th World Symposium on Pulmonary Hypertension (WSPH) task force published by Simonneau et al. (4). Group 1 (pulmonary arterial hypertension, PAH) includes idiopathic, heritable, and drug-induced PAH and is associated with conditions such as human immunodeficiency virus (HIV), schistosomiasis, and congenital heart disease (CHD). Group 2 includes PH due to left heart disease (PH-LHD) related to systolic dysfunction, diastolic dysfunction, valvular disease, or a combination of these conditions. PH resulting from lung diseases and hypoxemia, or both, comprises Group 3 PH-LD and is associated with chronic obstructive pulmonary disease (COPD), interstitial lung disease, pulmonary diseases with a mixed restrictive and obstructive pattern. Post tuberculosis (TB) bronchiectasis and fibrotic-cavitary pulmonary disease is another major risk factor for the development of Group 3 PH. Chronic thromboembolic pulmonary hypertension (CTEPH) corresponds to Group 4, and Group 5 includes a heterogeneous group of disorders that may cause PH by unclear or multiple mechanisms, or both (1,2,4,5).
In the last decades, significant progresses in the diagnosis and management of PH have gradually moved this condition from an orphan disease to major global health problem (6). It is estimated that about 80% of the global burden of the disease is in LMICs where PH is known to be highly associated with CHD, rheumatic heart disease, schistosomiasis and HIV (6,7). Despite these risk factors for PH, left heart disease (LHD) still remains the most common etiology for PH in both LMICs and high-income countries (HICs) (8-10). Contrary to what is observed in HICs where PH is predominant in the elderly above the ages of 65 years, it is very common in younger individuals in LMICs (7).
LMICs are known to have limited resources for the diagnosis and treatment of several diseases including PH. PH has debilitating symptoms and if left untreated, it has poor survival and reduced life expectancy (11,12). Another well-known factor that has accounted for high mortality rates in sub-Saharan Africa (SSA) is the fact that patients in these settings usually present to the hospital at late stages of the disease (WHO functional class III and IV) (3,8,13). This is mainly due to two reasons: (I) accessibility to health care, and (II) non-specific nature of PH symptoms such as shortness of breath and fatigue. In addition to the above, there is lack of adequate diagnostic tools at primary care, with most cases in SSA and other LMICs are usually misdiagnosed as heart failure, other cardio-respiratory conditions, or even TB (12,14).
Recent advances have been made in the development of effective treatments, especially for PAH and CTEPH (15,16). Vasodilators, endothelin receptor antagonists, phosphodiesterase (PDE) inhibitors (e.g., sildenafil) and high-dose calcium channel blockers (e.g., amlodipine) are the most commonly prescribed drug, with the latter two also available in SSA and other LIMC (17). CTEPH is a potentially curable disease when treated with pulmonary thromboendarterectomy (PTE).
Nonetheless of all advances in the management of PH, there is still limited data on the epidemiology of PH in LMICs. The pan African pulmonary hypertension cohort (PAPUCO) study provides the most comprehensive data on the prevalence, etiologies, classification, morbidity and mortality of PH on the African continent (3,17).
Epidemiology of PH
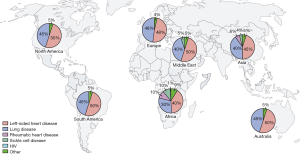
The exact global burden of PH remains unknows, and most likely largely underestimated due to the above-mentioned circumstances in LMICs. It is estimated that about 80% of patients with PH live in LMICs and disproportionately affecting younger people at an age <65 years (7). Overall, LHD still remains the dominant risk factor for PH (PH-LHD), followed by chronic lung disease and hypoxia (PH-LD), while PAH remains rare even in high HIV prevalent settings in SSA (Figure 2), where also untreated streptococcal infections leading to rheumatic fever rheumatic heart disease and sickle cell disease are endemic (3,6,7,18). As of 2015, it was estimated that up to 50–70 million individuals were affected by PH globally, which is expected to rise as global population and life expectancy increases (7,19). About 30 million individuals are estimated to have heart failure associated PH, 25 million estimated to have COPD associated PH, 150,000 individuals estimated to have HIV-associated PH (HIV-PH), 3.75 million individuals estimated to suffer rheumatic heart disease associated PH, and 2 million individuals estimated to have sickle cell disease associated PH (7).
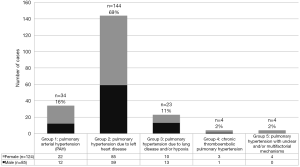
Mortality of PH is high in both LMICs and HICs (20-24). A 5-year mortality rate of 26% was recorded in a Swiss cohort of PAH, CTEPH and PH-LD patients (11). Similar mortality rates have been recorded in SSA in a short observational period of only 6 months (3,8). The PAPUCO study described the causes, treatment and outcome of PH in Africa in both adult and pediatric populations (3). According to this continent wide study, 69% of PH-LHD, 16% due to PAH, 11% of PH-LD, 2% due to CTEPH, and 2% due to multifactorial/unclear mechanism (Figure 3).
In PAPUCO, we found a high 6-month mortality of 21% in adults associated with functional limitations at time of presentation. To our knowledge, PAPUCO remains the only study that has classified PH according to the current guidelines. In a recent review on PH in Africa, findings showed that the prevalence of PH varies widely across different population: 9.8% in patients presenting with cardio-respiratory complaints, 10% in HIV-infected patients, 32.9% in patients with heart failure, 23.2% in patients on hemodialysis, 12.9% in patients with RHD, 37% to 53% in patients with sickle cell disease (9,22,25,26).
The high prevalence of PH-LHD in LMICs is expected due to a higher prevalence of uncontrolled hypertension which is also a principal cause of heart failure according to the sub-Saharan acute heart failure (THESUS-HF) study, a study conducted in nine African countries (27). Lung disease and hypoxia is known to be the second leading cause of PH after LHD. Studies from SSA suggest a relationship between indoor exposure to smoke from burning biomass and PH, but the actual risk has not been fully explored (3,8). A study from Egypt reported a prevalence of 63% among patients with COPD (28). Another study in Libya showed a prevalence of 8% among patients with pulmonary disease (29). The high prevalence of COPD across all age groups may be linked to occupation risk such as mining, direct and passive smoking, urban pollution, and indoor pollution from domestic fires for cooking and heating, as well as to endemic levels of TB leading to post-TB bronchiectasis and fibro-cavitary pulmonary disease with COPD type pattern (TB obstructive pulmonary disease, TOPD) (3). Ahmed et al. published a case series of 14 Sudanese patients with PH after successful completion of treatment for TB. The group reported further different severity and grades of PH with a median time from completion of treatment to TB to diagnosis of PH of nine years, suggesting an underestimated burden of persistent morbidity in TB globally in patients defined as “cured” by microbiologic means (30).
A South African study identified PH as one of the commonest causes of death accounting for 31% of total cardiovascular deaths (31). A study in Cameroon reported 6-month mortality rates of 28% (8). The reasons for high mortality rates in LMICs are lack of access to health care, infrastructure and equipment to diagnose PH within the health care system, and availability of specific treatment for PH. In Mexico a study involving patients with CTEPH reported that only one patient out of 50 underwent pulmonary endarterectomy despite many patients being eligible. Reasons identified were lack of insurance coverage for patients eligible for surgery and lack of infrastructure for post-operative care after the procedure (32).
People living with HIV have an increased risk of developing PH. With HIV being more prevalent in LMICs and endemic in SSA it is expected that there will be a higher burden of PAH in this region. To date, no systematic studies have been carried out to report the incidence of PH among HIV patients in SSA. On a global scale, the prevalence of PH in HIV-infected individuals varies between 0.5% and 5.0% with HIV being recognized as an independent risk factor for the development of PH. The prevalence of HIV-PH in developed countries in the era of combined antiretroviral therapy (cART) is 0.5%, while 5.5% of patients with no symptoms of PH may be at risk for PH; 1,000-fold higher than in the general population. Studies conducted in Africa found evidence of PH by echocardiographic measurements in 0.6–5% of HIV patients in Nigeria, Burkina Faso, and Zimbabwe. Estimated survival rates in developed countries in the pre-cART era were about 70% at 1 year and 50% at 3 years compared with about 90% at 1 year and 70% at 3 years after the advent of cART. Even though these data seem convincing a direct benefit of cART on the incidence and survival of HIV-PH could not be demonstrated. Combination antiretroviral therapy alone without specific treatment for PH does not result in an improved cardiac function. Also, no association between HIV-PH and CD4 count, viral load, or stage of disease could be identified. Thus, the prevalence of PH among HIV patients in the African context varies widely. Studies also report a slight female predominance (33). It would be relevant to investigate in a large cohort study the true incidence and factors that affect the occurrence of HIV-PH.
Schistosomiasis has been identified to be one of the major causes of PH as a result of host immune response to the parasite antigen. Worldwide, 200 million people are infected by Schistosomiasis, with majority of these living in LMICs especially in Africa, parts of Asia and South America. About 1% of people with schistosomiasis develop PH (34,35). Sickle cell disease is another identified risk factor that has been associated with PH. In Nigeria, a case-control study among patients with sickle cell disease revealed a prevalence of 22.9% in patients with hemoglobin SS as compared to 2.3% in patients with hemoglobin AA (36). In another echocardiography study, PH was detected in 23.9% of adults with sickle cell disease (37), with reported higher mortality in these group of patients (38).
Pathogenesis of PH
The pathogenesis of PH is different for every clinical WHO group and the underlying risk factors, diseases and co-morbidities contribute to pathophysiological changes in the heart, lungs and pulmonary arteries. PH presents as a clinical syndrome characterized by shortness of breath and loss of exercise capacity that is related to increased PAP and pulmonary vascular resistance (PVR), with vasoconstriction, proliferation, thrombosis, and re-modelling of the pulmonary vessels (4). Vasoconstriction occurs in early disease, followed by micro-thrombotic embolism. Re-modelling of the small and middle size pulmonary arteries is the critical factor leading to disease progression with increasing morbidity and mortality.
Management PH in LMICs
A number of guidelines exist for the diagnosis and treatment of PH, but the joined European Society of Cardiology (ESC) and the European Respiratory Society (ERS) guidelines for the diagnosis and treatment of PH are the most comprehensively used, regularly updated and widely recognized in PH. The 2015 guidelines provide some changes from the 2009 guidelines, including but not limited to simplified content structure, new parameters for the hemodynamic definition of post-capillary PH, the inclusion PVR in the hemodynamic definition of PAH, and updated diagnostic and treatment algorithms (1). With limitations in clinical and hemodynamic characterization of PH based on a resting value of mean pulmonary artery pressure alone, recent suggestions have been made to include a PVR ≥3 Wood Units in the definition of all forms of pre-capillary PH and a cut-off point of mPAP ≥20 mmHg (1,4).
According to ESC/ERS guidelines, the standard procedures in a patient with suspected PH are complex investigations inclusive, but not limited to doppler echocardiography (doppler echo), pulmonary angiography, right heart and left heart catheterization, high-resolution computed tomography, lung function testing, and ventilation/perfusion scan (V/Q scan) (1,2). The above diagnostic techniques require high skilled operational personnel, continuous technical maintenance or even radionucleotides, mostly not readily available in resource limited settings, or only available in large urban centers such as university hospitals.
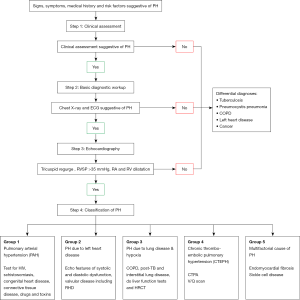
Doppler echo has been shown to be highly specific and sensitive in the detection of PH, if in the hands of a well-trained cardiologist or technician (39). In the context of the previously described limitations in LMICs and with PH-LHD being the most frequent form of PH, our group recently provided a simplified diagnostic algorithm (Figure 4) for the diagnosis of PH-LHD in resource-limited settings (40). This four-step diagnostic approach includes in step (I) a clinical evaluation to detect predisposing conditions for PH-LHD such as hypertension, and signs including pitting edema, raised jugular venous pressure, nail clubbing; step (II) assessment with chest X-ray and electrocardiogram to uncover the presence of PH-LHD such as cardiomegaly with enlarged right atrium and pulmonary arteries, prominent pulmonary outflow tract, and pruning of peripheral pulmonary vessels on chest X-ray, and sinus tachycardia with right-axis deviation, right atrial enlargement, ventricular hypertrophy and strain, and broadening of the QRS complex on electrocardiogram; step (III) confirmation of the presence of PH-LHD using doppler echo, and step (IV) investigation of differential etiologies and classification of the clinical WHO group of PH.
Despite advances in treatment of various forms of PH, specific PH drugs are still largely unavailable in LMICs. Specific drugs for PH such as vasodilators, endothelin receptor antagonists, phosphodiesterase PDE inhibitors (e.g., sildenafil) and high-dose calcium channel blockers (e.g., amlodipine) are the most common prescribed drug, with the latter two at least available in some LIMC. In most regions of the world, drugs used to manage all forms of PH remain those for the management of heart failure including diuretics, calcium channel blockers, angiotensin-converting-enzyme (ACE) inhibitor, angiotensin II receptor blockers (ARB), beta blockers, digitalis, and anti-coagulants (3,41).
Conclusions
PH is a common clinical syndrome in SSA and other LMICs and many of the identified risk factors for PH are hyperendemic in those regions. These include LHD, lung disease & TB, rheumatic heart disease, HIV and schistosomiasis. The intersection of communicable and non-communicable diseases in SSA with, generally, late presenting patients of the two entities contributing to a broad range of PH in the region. Pathways leading to PH need to be identified early and managed accordingly. Treatment options are limited often to heart failure management. More comprehensive data on the epidemiology of PH from LMICs is desperately needed to plan for health interventions and the previously described algorithm to diagnose PH in LMICs needs to be validated as a screening tool for PH.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Ntobeko A. B. Ntusi) for the series “Cardiovascular Diseases in Low-and Middle-Income Countries” published in Cardiovascular Diagnosis and Therapy. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: The series “Cardiovascular Diseases in Low- and Middle-Income Countries” was commissioned by the editorial office without any funding or sponsorship. The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 2015;46:903-75. [Crossref] [PubMed]
- Hoeper MM, Bogaard HJ, Condliffe R, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol 2013;62:D42-50. [Crossref] [PubMed]
- Thienemann F, Dzudie A, Mocumbi AO, et al. The causes, treatment, and outcome of pulmonary hypertension in Africa: insights from the pan African pulmonary hypertension cohort (PAPUCO) Registry. Int J Cardiol 2016;221:205-11. [Crossref] [PubMed]
- Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019;53. [Crossref] [PubMed]
- Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013;62:D34-41. [Crossref] [PubMed]
- Mocumbi AO, Thienemann F, Sliwa K. A global perspective on the epidemiology of pulmonary hypertension. Can J Cardiol 2015;31:375-81. [Crossref] [PubMed]
- Hoeper MM, Humbert M, Souza R, et al. A global view of pulmonary hypertension. Lancet Respir Med 2016;4:306-22. [Crossref] [PubMed]
- Dzudie A, Dzekem BS, Tchoumi CT, et al. Pulmonary hypertension as seen in a rural area in sub-Saharan Africa: high prevalence, late clinical presentation and a high short-term mortality rate during follow up. Cardiovasc J Afr 2018;29:208-12. [Crossref] [PubMed]
- Bigna JJ, Noubiap JJ, Nansseu JR, et al. Prevalence and etiologies of pulmonary hypertension in Africa: a systematic review and meta-analysis. BMC Pulm Med 2017;17:183. [Crossref] [PubMed]
- Strange G, Playford D, Stewart S, et al. Pulmonary hypertension: prevalence and mortality in the Armadale echocardiography cohort. Heart 2012;98:1805-11. [Crossref] [PubMed]
- Tueller C, Stricker H, Soccal P, et al. Epidemiology of pulmonary hypertension: new data from the Swiss registry. Swiss Med Wkly 2008;138:379-84. [PubMed]
- Dzudie A, Kengne AP, Thienemann F, et al. Predictors of hospitalisations for heart failure and mortality in patients with pulmonary hypertension associated with left heart disease: a systematic review. BMJ Open 2014;4:e004843. [Crossref] [PubMed]
- Mocumbi AO, Lameira E, Yaksh A, et al. Challenges on the management of congenital heart disease in developing countries. Int J Cardiol 2011;148:285-8. [Crossref] [PubMed]
- Brown LM, Chen H, Halpern S, et al. Delay in recognition of pulmonary arterial hypertension: factors identified from the REVEAL Registry. Chest 2011;140:19-26. [Crossref] [PubMed]
- Hoeper MM, Madani MM, Nakanishi N, et al. Chronic thromboembolic pulmonary hypertension. Lancet Respir Med 2014;2:573-82. [Crossref] [PubMed]
- Humbert M, Lau EM, Montani D, et al. Advances in therapeutic interventions for patients with pulmonary arterial hypertension. Circulation 2014;130:2189-208. [Crossref] [PubMed]
- Thienemann F, Dzudie A, Mocumbi AO, et al. Rationale and design of the pan African pulmonary hypertension cohort (PAPUCO) study: implementing a contemporary registry on pulmonary hypertension in Africa. BMJ Open 2014;4:e005950. [Crossref] [PubMed]
- Satoh T. Current practice for pulmonary hypertension. Chin Med J (Engl) 2014;127:3491-5. [PubMed]
- Halbert RJ, Natoli JL, Gano A, et al. Global burden of COPD: systematic review and meta-analysis. Eur Respir J 2006;28:523-32. [Crossref] [PubMed]
- D'Alonzo GE, Barst RJ, Ayres SM, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med 1991;115:343-9. [Crossref] [PubMed]
- Benza RL, Miller DP, Barst RJ, et al. An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL Registry. Chest 2012;142:448-56. [Crossref] [PubMed]
- Karaye KM, Saidu H, Bala MS, et al. Prevalence, clinical characteristics and outcome of pulmonary hypertension among admitted heart failure patients. Ann Afr Med 2013;12:197-204. [Crossref] [PubMed]
- Shah SJ. Pulmonary hypertension. JAMA 2012;308:1366-74. [Crossref] [PubMed]
- Adir Y, Amir O. Pulmonary hypertension associated with left heart disease. Semin Respir Crit Care Med 2013;34:665-80. [Crossref] [PubMed]
- Faqih SA, Noto-Kadou-Kaza B, Abouamrane LM, et al. Pulmonary hypertension: prevalence and risk factors. Int J Cardiol Heart Vasc 2016;11:87-9. [Crossref] [PubMed]
- Ondze Kafata LI, Ngolet L, Letomo KN, et al. 0422: Echocardiographic aspects of Congolese sickle cell disease heart. Arch Cardiovasc Dis Suppl 2016;8:43.
- Damasceno A, Mayosi BM, Sani M, et al. The causes, treatment, and outcome of acute heart failure in 1006 Africans from 9 countries. Arch Intern Med 2012;172:1386-94. [Crossref] [PubMed]
- Soliman M, Heshmat H, Amen Y, et al. Detection of right sided heart changes and pulmonary hypertension in COPD patients. Egypt J Chest Dis Tuberc 2015;64:335-41. [Crossref]
- Gaber K, Elfaitouri O, Hassi S, et al. Prevalence of Pulmonary Hypertension in Patients Attending Echocardiolgy Clinic in the Eastern Part of Libya. Chest 2014;145:516A. [Crossref]
- Ahmed AE, Ibrahim AS, Elshafie SM. Pulmonary hypertension in patients with treated pulmonary tuberculosis: analysis of 14 consecutive cases. Clin Med Insights Circ Respir Pulm Med 2011;5:1-5. [Crossref] [PubMed]
- Steenekamp JH, Simson IW, Theron W. Cardiovascular causes of death at Tshepong Hospital in 1 year, 1989-1990. A necropsy study. S Afr Med J 1992;81:142-6. [PubMed]
- Al-Naamani N, Espitia HG, Velazquez-Moreno H, et al. Chronic Thromboembolic Pulmonary Hypertension: Experience from a Single Center in Mexico. Lung 2016;194:315-23. [Crossref] [PubMed]
- Aminde LN, Dzudie A, Kengne AP, et al. Gender disparities in pulmonary hypertension at a tertiary centre in Cameroon. S Afr Med J 2017;107:892-9. [Crossref] [PubMed]
- Graham BB, Bandeira AP, Morrell NW, et al. Schistosomiasis-associated pulmonary hypertension: pulmonary vascular disease: the global perspective. Chest 2010;137:20S-29S. [Crossref] [PubMed]
- Papamatheakis DG, Mocumbi AO, Kim NH, et al. Schistosomiasis-associated pulmonary hypertension. Pulm Circ 2014;4:596-611. [Crossref] [PubMed]
- Sokunbi OJ, Ekure EN, Temiye EO, et al. Pulmonary hypertension among 5 to 18-year-old children with sickle cell anaemia in Nigeria. PLoS One 2017;12:e0184287. [Crossref] [PubMed]
- Amadi VN, Balogun MO, Akinola NO, et al. Pulmonary hypertension in Nigerian adults with sickle cell anemia. Vasc Health Risk Manag 2017;13:153-60. [Crossref] [PubMed]
- Aliyu ZY, Kato GJ, Taylor J 6th, et al. Sickle cell disease and pulmonary hypertension in Africa: a global perspective and review of epidemiology, pathophysiology, and management. Am J Hematol 2008;83:63-70. [Crossref] [PubMed]
- Naing P, Kuppusamy H, Scalia G, et al. Non-invasive assessment of pulmonary vascular resistance in pulmonary hypertension: current knowledge and future direction. Heart Lung Circ 2017;26:323-30. [Crossref] [PubMed]
- Dzudie A, Kengne AP, Lamont K, et al. A diagnostic algorithm for pulmonary hypertension due to left heart disease in resource-limited settings: can busy clinicians adopt a simple, practical approach? Cardiovasc J Afr 2019;30:61-7. [Crossref] [PubMed]
- Gidwani S, Nair A. The burden of pulmonary hypertension in resource-limited settings. Glob Heart 2014;9:297-310. [Crossref] [PubMed]


